Abstract
The changes in depressive symptomatology during the first year following one-anastomosis gastric bypass (OAGB) were evaluated and its association with uric acid (sUA). Fifty patients were included in this analysis. Beck Depression Inventory (BDI) for measuring depressive symptomatology, blood samples, and anthropometric measurements were assessed before (T0), at 6 (T6), and 12 months (T12) after surgery. There was a significant reduction in BDI total score at T6 (− 5.6 (95% CI − 2.1, − 9.1) points; p = 0.001) and at T12 (− 4.3 (95% CI − 0.9, − 7.9) points; p = 0.011). BMI loss was unrelated to depressive symptomatology. Patients with moderate to severe depressive symptomatology presented lower sUA levels than patients with none or minimal to mild (p = 0.028). ROC analysis revealed that sUA levels below 5.0 at T6 and 4.5 mg/dl at T12 had a prognostic accuracy for depression severity. Furthermore, delta sUA was significantly associated with delta BMI (β = 0.473; p = 0.012) and delta waist circumference (β = 0.531; p = 0.003). These findings support an improvement in depressive symptomatology in the first year postoperatively, however, without relation to BMI loss. Patients with moderate to severe depressive symptomatology presented with lower sUA levels over time. Therefore, sUA could be useful to predict moderate to severe depressive symptomatology in patients undergoing OAGB in clinical practice.
Similar content being viewed by others
Introduction
Presently, bariatric surgery is the most effective weight loss method in adults with morbid obesity, who have been unsuccessful with nonsurgical interventions1,2.
Obesity and major depressive disorder are important public health problems connected to high disease burden, health care expenses and present great challenges for the upcoming decades3,4. Depressive symptoms are highly prevalent co-morbidities occurring in patients with severe obesity5. Various studies have reported a correlation between obesity and depressive symptoms compared to controls6,7,8. Furthermore, detecting depression in candidates for bariatric surgery is important as the presence of depressive symptoms prior to surgery has been associated with poor outcomes and regaining the lost weight9,10. However, up to date, no study has investigated the course of depressive symptoms following one-anastomosis gastric bypass (OAGB). OAGB is the third most common bariatric procedure worldwide11.
Additionally, major depressive disorder has been found associated with oxidative stress pathways and altered purinergic metabolism12. This is of significance as increasing evidence shows the important role of the purinergic system in mood regulation, motor activity, cognitive function, sleep, and behaviour13 and its impairment seems to be implicated in the pathophysiological mechanisms of depressive symptoms14. As serum uric acid (sUA) is the end product of purine metabolism15, patients with major depressive disorder have been found to have lower levels of sUA compared to those without major depressive disorder12. Moreover, recent evidence suggests that sUA concentrations were strongly associated with body weight in adults16.
Utilizing data from the LOAD (“Link between Obesity And Vitamin D”) study, a six-months double-blind, placebo-controlled, randomized trial of vitamin D supplementation in OAGB patients, the aims of the current analysis are: (1) to describe changes in depressive symptomatology during the first year following surgery; (2) to examine whether depressive symptomatology changes in parallel with total body weight loss; (3) to determine the association between depressive symptomatology and sUA and (4) to examine whether decreases in sUA levels would correlate with weight change.
Materials and methods
Study design
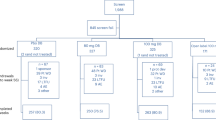
This paper presents a secondary analysis of the data from a six-months double-blind, placebo-controlled, randomized trial of vitamin D supplementation: “Link between Obesity And Vitamin D”17. The study protocol was previously published18. In the first six months after OAGB surgery, the intervention group received the following vitamin D dosing regimen: Up to three oral loading doses of each 100,000 IU in the first month postoperatively followed by maintenance dose of 3420 IU/day; and the control group received conventional supplementation (placebo with following maintenance doses of 3420 IU/day). Afterwards, both groups were recommended to continue the vitamin D3 supplementation until the follow-up visit at 12 months. At baseline, no participant took any vitamin D supplementation, which was an inclusion criterion for this trial. Inclusion criteria were; planned OAGB surgery, above 18 years old, 25-hydroxy-vitamin D (25(OH)D) serum concentrations of < 75 nmol/L, and a body weight < 140 kg (due to body weight limitation of the dual energy X-ray absorptiometry). Specific exclusion criteria included any other planned form of bariatric surgery than OAGB, hypo- and hypercalcemia, renal insufficiency, or primary hyperparathyroidism. The details on design, the used materials and methods, as well as the sample size calculation of the study have been previously published18.
All OAGB procedures were performed at the General Hospital Vienna, Medical University of Vienna by the same surgical team using a laparoscopic approach. It is a simplified procedure that consists of a unique gastrojejunal anastomosis between a 30 to 40 ml sleeve gastric pouch and a jejunal omega-loop of approximately 200 cm19. The study methods are in accordance with the CONSORT (Consolidated Standards Of Reporting Trials) guidelines for reporting randomized trials20.
This study was approved by the local Ethics Committee of the Medical University of Vienna (No° 1899/2013), by the Austrian Competent Authority (No° LCM-718280-0001), registered at clinicaltrials.gov (Identifier: NCT02092376) and EudraCT (Identifier: 2013-003546-16), complies with the Declaration of Helsinki21, and conducted from April 2014 to June 2016 at the Medical University of Vienna (Austria). The study participants were scheduled for OAGB surgery and all of them gave written informed consent preoperatively.
Assessment of variables
Data were assessed before surgery (T0), at 6 months (T6), and at follow-up visit at 12 months postoperatively (T12). At T0, age, sex, medical history (e.g. comorbidities, prescribed medication) were collected as previously described18. Clinically diagnosed depression, was defined whether the patients were ever diagnosed with depression by a health professional e.g. psychologist or had taken antidepressant medications. The following set of evaluations was obtained for each participant at the three time points: height and body weight (measured with the calibrated scale Seca mBCA 515) and waist circumference measured with an inelastic tape at the narrowest point between the lower costal border and the top of the iliac crest in accordance with the International Standards for Anthropometric Assessment (ISAK)22.
Blood samples were collected and serum uric acid (sUA), using uricase-PAP-method, was used for this analysis.
Beck Depression Inventory
The Beck Depression Inventory (BDI)23 is a 21-question multiple-choice self-report inventory for measuring the severity of depressive symptomatology. Symptoms of depression over the past one week were assessed using the BDI version 123,24. Because many patients are advised to lose weight in preparation for surgery, no points were assigned to the BDI item, “I have lost more than 5 lb,” for participants who indicated purposefully trying to lose weight. The following cut-offs were used for the total score: 0–9, 10–18, 19–29 and 30–63, reflecting none or minimal, mild, moderate and severe symptomatology, respectively25.
Somatic and physical items of the BDI, such as distortion of body image, work inhibition, sleep disturbance, fatigability, loss of appetite, somatic preoccupation, loss of libido, and weight loss can overlap with the symptoms of obesity rather than being due to depressive symptomatology26,27. Previously published studies reported that patients with obesity or patients undergoing bariatric surgery endorsed the somatic-vegetative items more than the cognitive affective items of the BDI28,29. The initial validation study by Steer et al. with adult psychiatric outpatients using both exploratory and confirmatory factor analyses found two-factor solutions including somatic-affective and cognitive factors31. Therefore, the items of the BDI have been divided into two subscales: the cognitive-affective subscale which assesses the mental aspect of depression (BDI items 1–13) and the somatic-vegetative subscale which measures vegetative and somatic symptoms (BDI items 14–21). This was also used in the study by Jorde et al.30.
Statistical analysis
The results are expressed as mean (standard deviation) for continuous and as percentages for categorical variables. In order to test for normal distribution, a visual test (histograms and box plots) was used and the Kolmogorov–Smirnov test was applied in addition.
The internal consistency of the BDI items was determined by a reliability analysis (Cronbach’s alpha) and showed a Cronbach’s alpha of 0.895, which shows that the questionnaire is reliable in this population.
The main outcome of interest was the change in BDI scores in the first postoperative year. In this secondary analysis, there was no significant effect of vitamin D supplementation (intervention or control group) nor of vitamin D status (25(OH)D) on Beck Depression Inventory (BDI) score. Therefore, the entire sample for this analysis was used. Repeated-measures analysis of covariance was applied to assess the effect of time, by using different covariance structure models as appropriate and were adjusted for age, sex, season, and baseline values to supply an unbiased estimate of the mean difference31. A post-hoc analysis with Bonferroni correction was used. Additionally, linear mixed models were performed to assess associations between changes in body weight and BMI and the depressive symptomatology.
Estimates of the prevalence of depressive symptomatology over time were calculated using generalized estimating equation (GEE) with a logit link function for binary outcomes and unstructured covariance matrices. With this approach, effects with time as repeated factor with depressive symptomatology as dependent variable was examined, adjusted for age, sex, season, and baseline values.
Moreover, to examine the effects of time (from baseline to 6 and 12 months), group (depressive symptomatology), and their interaction on sUA, GEEs were used. Linear link function and an unstructured correlation matrix were used. Significance tests were performed with Wald χ2 (α = 0.05). The models were adjusted for age, sex, and season. Baseline data and none or minimal to mild depressive symptomatology were the reference. For detection the optimal cut-off in sUA levels for predicting moderate to severe depressive symptomatology at different time points, receiver operator characteristic curves (ROC) were used and described as area under the curve (AUC) with standard errors, the negative (NPV) and positive predictive value (PPV).
The statistical association between sUA concentrations and BDI scores with anthropometric parameters such as weight, BMI and waist circumference was examined by multiple linear regression analysis.
Means were compared unadjusted without imputation of missing data. All statistical analyses were performed with IBM SPSS Statistics for Windows, Version 23 software (IBM Corporation, Armonk, New York, USA). P-values < 0.05 were considered statistically significant and all tests were two-sided.
Statements regarding ethics and consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Results
Patients’ characteristics
Out of 67 eligible patients, 17 declined to participate (25%). The remaining 50 patients entered the randomized controlled trial17 with 39 patients who completed the BDI questionnaire at baseline. Baseline characteristics are demonstrated in Table 1. Before surgery, 23% of the patients had a clinically diagnosed depression, 20% among them were using antidepressant medications. However, 56% of the patients showed moderate to severe depressive symptomatology as measured by the BDI.
Changes in depressive symptomatology in the first postoperative year
Table 2 shows the change in weight and depressive symptomatology over time. As expected, there was a significant BMI change (p < 0.001) and total body weight loss (p < 0.001) over time. There was also a significant reduction in BDI total score between baseline and 6 months (21.1 ± 9.6 vs. 14.0 ± 7.7 points; p = 0.001), and baseline and 12 months (21.1 ± 9.6 vs. 16.0 ± 11.3 points; p = 0.011). The analysis at 6 and 12 months showed a significant improvement of the score in the subscale somatic-vegetative (T6 p < 0.001, T12 p = 0.003), as well as for the items “fatigability” (T6 p = 0.001, T12 p = 0.007), “loss of appetite” (T6 p = 0.018, T12 p = 0.015), and “somatic preoccupation” (T6 p < 0.001, T12 p < 0.001). The cognitive-affective subscale analysis had also a significant improvement at 6 months (p = 0.001) but the change between baseline and 12 months was not significant (p = 0.283). The items of the cognitive-affective subscale showed a significant improvement in “guilty feeling” (T6 p = 0.014), “sense of punishment” (T12 p = 0.048), “indecisiveness” (T6 p = 0.002, T12 p = 0.021), and “distortion of body image” (T6 p = 0.016, T12 p = 0.030). The complete analysis with the subscales can be observed in Table 3.
Figure 1 shows the percentage change in depressive symptomatology over time. The prevalence of moderate to severe depressive symptomatology decreased from 56% at baseline to 26% at 6 months and increased slightly to 35% at 12 months. The prevalence of none or minimal to mild depressive symptomatology increased from 44% at baseline to 74% at 6 months and to 65% at 12 months (p = 0.045). The adjusted OR for moderate to severe depressive symptomatology at 6 months was 0.28 (95% CI 0.12, 0.62; p = 0.002), and at 12 months 0.37 (95% CI 0.14, 1.0; p = 0.051) compared to baseline.
Change in depressive symptomatology over time. P values were based on Generalized Estimating Equations to examine the effects of time (from baseline to 6 and 12 months) with the prevalence of depressive symptomatology as the dependent variable. Logit link function and an unstructured correlation matrix were used. Significance tests were performed with Wald χ2 (α = 0.05). The models were adjusted for age, sex, and season. Baseline data was the reference.
Depressive symptomatology and body weight loss
The baseline weight, BMI, and waist circumference parameters of patients with no or minimal to mild vs. moderate to severe depressive symptomatology were similar with 124.0 (17.0) kg vs. 120.5 (13.2) kg (p = 0.940), 44.9 (4.6) kg/m2 vs. 44.1 (4.1) kg/m2 (p = 0.800), and 124.5 (11.5) vs. 129.9 (9.8) cm (p = 0.086), respectively.
Furthermore, in the longitudinal course, patients with no or minimal to mild depressive symptomatology showed a 29.8 (6.6) % and 36.5 (9.5) % Total Body Weight Loss (TBWL) and patients with moderate to severe depressive symptomatology showed a 26.2 (5.4) % and 33.9 (6.3) % TBWL at 6 and 12 months (p = 0.367), respectively. Moreover, there were no significant associations between BMI loss and the change of BDI total score at 6 (β = 0.103; p = 0.641) and 12 months (β = 0.032; p = 0.886) after surgery.
Depressive symptomatology and sUA
Patients undergoing OAGB with moderate to severe depressive symptomatology presented with lower uric acid levels than patients with no or minimal to mild depressive symptomatology over time (Fig. 2).
Change in serum uric acid according to depressive symptomatology over time. P values were based on Generalized Estimating Equations to examine the effects of time (from baseline to 6 and 12 months), group (depressive symptomatology), and their interaction on sUA as the dependent variable. Linear link function and an unstructured correlation matrix were used. Significance tests were performed with Wald χ2 (α = 0.05). The models were adjusted for age, sex, and season. Baseline data and the none/minimal to mild depressive symptomatology group were the reference.
ROC analysis revealed that sUA levels had a moderate to good prognostic accuracy in the total sample (at baseline: AUC 0.57, 95% CI 0.40–0.73; at 6 months: AUC 0.69, 95% CI 0.49–0.85; at 12 months: AUC 0.57, 95% CI 0.37–0.76).
The optimal cut-off levels for predicting moderate to severe depressive symptomatology in patients undergoing OAGB were 5.0 mg/dl (PPV of 71%; sensitivity: 46%; specificity: 77%) at baseline, 5.2 mg/dl (PPV of 71%; sensitivity: 88%; specificity: 53%) at 6 months, and 4.5 mg/dl (PPV of 71%; sensitivity: 78%; specificity: 50%) at 12 months (calculated by the Youden Index method).
sUA levels and weight change
To investigate whether changes in sUA concentrations (delta sUA) were associated with anthropometric parameters, changes of weight, BMI and waist circumference to delta sUA levels were correlated. The changes were calculated as 12 months follow-up minus baseline. Furthermore, at 12 months, delta sUA was significantly correlated with delta weight (β = 0.379; p = 0.003), delta BMI (β = 0.473; p = 0.012), and delta waist circumference (β = 0.531; p = 0.003).
Discussion
In this secondary analysis of a randomized trial with patients undergoing OAGB surgery, a high rate of depressive symptoms at T0/baseline was observed. There was an improvement in BDI total score and the subdivision scores at 6 and also at 12 months, except for the cognitive-affective subdivision at 12 months. BMI loss showed no association with depressive symptomatology at 6 and 12 months. Further, patients undergoing OAGB in both depressive symptomatology groups showed the same amount of weight at baseline. Moreover, sUA levels decreased significantly following bariatric surgery. Patients with moderate to severe depressive symptomatology presented with lower sUA levels than patients with no or minimal to mild depressive symptomatology. ROC analysis revealed that sUA levels of 5.0 or 4.5 mg/dl (T6 or T12) had a prognostic accuracy for depression severity in patients undergoing OAGB. Decrease in sUA correlated with changes in weight, BMI and waist circumference.
There is evidence, that bariatric surgery is a powerful tool for understanding the complex cascade of events associated with the regulation of body weight, improvement of health conditions, and mental health32,33,34,35. A prospective, well-controlled study, the Swedish Obese Subjects (SOS) study, involving 4047 patients with obesity assessed the change in psychological aspects postoperatively. This study demonstrated that depressive symptomatology decreased after weight reduction, but the decrease only remained stable among those who lost ≥ 25% of their initial weight36. Additionally, numerous studies, also using the BDI score, showed elevated depressive symptomatology in patients undergoing bariatric surgery25,37,38,39,40,41,42,43,44. The results from the present study are also comparable to the results of a study by Mitchell et al.25, where the authors reported on a significant decline in reported depression scores among patients within the first 6 months, after which the scores remained steady at the 1 year follow up. Interestingly, after the 1 year mark there was an observed modest, yet significant increase in the depression scores at 2 and 3 year follow up25. It is hypothesized that this deterioration in mood improvement may be linked to unrealistic expectations of the surgical outcomes and the related disappointment45, weight regain and/or reoccurrence of comorbiditie46,47, various nutritional deficiencies, which theoretically could present with depressive features48 or relative malabsorption of antidepressants49,50.
The results from the present study are consistent with previous literature28,29, where patients undergoing bariatric surgery are more likely to endorse more somatic and vegetative symptoms, rather than mental aspect of depression. As with the BDI it is possible to distinguish between cognitive-affective and somatic-vegetative items by using subscales. With these subscales it might be possible to evaluate whether the symptoms are depressive ones or concomitant symptoms of obesity, which may be important for a clinician to see if a high BDI total score is being primarily driven by concomitant symptoms of obesity29.
Moreover, some authors have found that patients with depressive symptomatology presented poor weight or BMI loss after surgery38,51,52,53. One explanation was that the former affects one’s ability to adapt to post-surgical behavioural changes53. However, the results from the present study showed that the improvement in BDI score was independent of the magnitude of BMI loss. These are in line with a study from Ayloo et al.37, which observed that weight loss had no independent influence on BDI improvement. The results indicated that the change in mood following bariatric surgery is related to the multiple factor interaction including initial weight loss. Here, psychosocial factors possibly have a more substantial role in the change in mood than was previously known37.
To our knowledge, this is the first analysis that compares levels of sUA and depressive symptomatology in patients undergoing OAGB. sUA levels demonstrated a moderate to good prognostic accuracy as a biomarker for patients with depressive symptomatology and sUA levels were higher with moderate to severe depressive symptomatology.
Another possible mechanism may be related to the effects of depression in decreasing antioxidant defences and increasing oxidative stress as reported54,55. The few studies that, to date, investigated the association between plasma uric acid levels and depression report conflicting results. These were mostly case–control studies that found increased56,57, decreased58,59, and no difference60 in levels of uric acid. Recently published meta-analyses has shown that participants with major depressive disorder have levels of the antioxidant uric acid lower than healthy controls12,61,62. The results could have broader implications given previous hypotheses regarding oxidative stress status63 and purinergic role64 in major depressive disorder.
Another possible explanation of the association between depression and uric acid could be the dietary habits of patients with obesity (e.g., increased sugar-sweetened soft drinks intake, fructose consumption, and increased purines intake)65, as lifestyle factors, including alcohol use, decreased physical activity and smoking. All these factors are associated with oxidative stress and are found to be more prevalent in people with depression. Increased oxidative stress could lead to depletion of antioxidants, including uric acid. A longitudinal cohort study included these factors and determined little effect on the association in major depressive and anxiety disorders66.
Moreover, a pilot study investigated the association between sUA concentrations and loss of body weight following laparoscopic sleeve gastrectomy or laparoscopic Roux-en-Y-gastric bypass in severely adolescents with morbid obesity67 and demonstrated a strong association of sUA change with changes in body weight. The authors hypothesized that sUA concentration may be influenced by factors that bariatric surgery procedures have in common, namely change in weight, adiposity, and dietary patterns.
This secondary analysis has certain limitations. First, the sample size is rather small, although it was based on the sample size calculation taking into account differences of serum 25(OH)D concentrations at 6 months between the intervention and control group. Indeed, we were able to demonstrate significant differences, which validate the sample size calculation. This study included a high percentage of women (80%), which, however, is very common in candidates for bariatric surgery, and might thus not be generalized to a male population. Another limitation might be that the parent study is a randomized trial of vitamin D supplementation. Evidence shows an association between vitamin D and uric acid levels68 and between vitamin D and depression69,70,71. However in this secondary analysis there was no association between vitamin D and uric acid levels and between vitamin D and depression, therefore vitamin D is not a confounding factor on this associations.
The strengths of the study include a detailed pre- and postoperative data of patients undergoing OAGB, which, as a rather new bariatric procedure, has not been evaluated in that regard. Furthermore, this study showed that sUA levels are associated with depressive symptomatology in patients undergoing OAGB.
Conclusion
In conclusion, this study reports in patients undergoing the relatively new OAGB an improvement in depressive symptomatology postoperatively after 6 and 12 months, which was not associated with BMI loss. Furthermore, sUA levels also decreased significantly postoperatively. Patients with moderate to severe depressive symptomatology presented lower sUA levels than patients with none or minimal to mild depressive symptomatology. This decrease correlated with changes in weight, BMI and waist circumference. sUA levels below 5 mg/dl had a prognostic accuracy for depression severity in patients undergoing OAGB, which might be useful in clinical practice.
References
Carter, P. L. The evolution of bariatric surgery. Am. J. Surg. 209(5), 779–782 (2015).
Chang, S. H. et al. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 149(3), 275–287 (2014).
Kessler, R. C. et al. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 289(23), 3095–3105 (2003).
Kelly, T., Yang, W., Chen, C. S., Reynolds, K. & He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. (Lond.). 32(9), 1431–1437 (2008).
Roberts, R. E., Kaplan, G. A., Shema, S. J. & Strawbridge, W. J. Are the obese at greater risk for depression?. Am. J. Epidemiol. 152(2), 163–170 (2000).
Marcus, M. D., Kalarchian, M. A. & Courcoulas, A. P. Psychiatric evaluation and follow-up of bariatric surgery patients. Am. J. Psychiatry. 166(3), 285–291 (2009).
Muller, A., Mitchell, J. E., Sondag, C. & de Zwaan, M. Psychiatric aspects of bariatric surgery. Curr. Psychiatry Rep. 15(10), 397 (2013).
Luppino, F. S. et al. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 67(3), 220–229 (2010).
Kalarchian, M. A. et al. Psychiatric disorders and weight change in a prospective study of bariatric surgery patients: A 3-year follow-up. Psychosom. Med. 78(3), 373–381 (2016).
Kalarchian, M. A. et al. Psychiatric disorders among bariatric surgery candidates: Relationship to obesity and functional health status. Am. J. Psychiatry. 164(2), 328–334 (2007) (quiz 74).
Parmar, C. D. & Mahawar, K. K. One anastomosis (mini) gastric bypass is now an established bariatric procedure: A systematic review of 12,807 patients. Obes. Surg. 28(9), 2956–2967 (2018).
Bartoli, F. et al. Antioxidant uric acid in treated and untreated subjects with major depressive disorder: A meta-analysis and meta-regression. Eur. Arch. Psychiatry Clin. Neurosci. 268(2), 119–127 (2018).
Thonney, B., Pataky, Z., Badel, S., Bobbioni-Harsch, E. & Golay, A. The relationship between weight loss and psychosocial functioning among bariatric surgery patients. Am. J. Surg. 199(2), 183–188 (2010).
Cheffer, A. et al. Purinergic system in psychiatric diseases. Mol. Psychiatry. 23(1), 94–106 (2018).
Kesebir, S., Tatlidil Yaylaci, E., Suner, O. & Gultekin, B. K. Uric acid levels may be a biological marker for the differentiation of unipolar and bipolar disorder: The role of affective temperament. J. Affect. Disord. 165, 131–134 (2014).
Bos, M. J., Koudstaal, P. J., Hofman, A., Witteman, J. C. & Breteler, M. M. Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam study. Stroke 37(6), 1503–1507 (2006).
Luger, M. et al. Vitamin D3 loading is superior to conventional supplementation after weight loss surgery in vitamin D-deficient morbidly obese patients: A double-blind randomized placebo-controlled trial. Obes. Surg. 27(5), 1196–1207 (2017).
Luger, M. et al. The link between obesity and vitamin D in bariatric patients with omega-loop gastric bypass surgery—A vitamin D supplementation trial to compare the efficacy of postoperative cholecalciferol loading (LOAD): Study protocol for a randomized controlled trial. Trials. 16(1), 328 (2015).
Rutledge, R. The mini-gastric bypass: Experience with the first 1,274 cases. Obes. Surg. 11(3), 276–280 (2001) (Epub 2001/07/04. eng).
Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Trials. 11, 32 (2010).
Dale, O. & Salo, M. The Helsinki Declaration, research guidelines and regulations: present and future editorial aspects. Acta Anaesthesiol. Scand. 40(7), 771–772 (1996).
International Society for the Advancement of Kinanthropometry (ISAK). International Standards for Anthropometric Assessment. 2001. https://www.ceap.br/material/MAT17032011184632.pdf.
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J. & Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry. 4, 561–571 (1961) (Epub 1961/06/01. eng).
Beck, A. T., Steer, R. A. & Garbin, M. G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 8(1), 77–100 (1998).
Mitchell, J. E. et al. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity (Silver Spring). 22(8), 1799–1806 (2014) (Epub 2014/03/19).
Gruszka, W. et al. The occurrence of depressive symptoms in obese subjects starting treatment and not seeking treatment for obesity. Eat. Weight Disord. EWD. 25(2), 283–289 (2020).
Thombs, B. D. et al. Somatic symptom overlap in Beck Depression Inventory-II scores following myocardial infarction. Br. J. Psychiatry. 197(1), 61–66 (2010).
Munoz, D. J. et al. Considerations for the use of the Beck Depression Inventory in the assessment of weight-loss surgery seeking patients. Obes. Surg. 17(8), 1097–1101 (2007).
Hayden, M. J., Dixon, J. B., Dixon, M. E. & O’Brien, P. E. Confirmatory factor analysis of the Beck Depression Inventory in obese individuals seeking surgery. Obes. Surg. 20(4), 432–439 (2010).
Jorde, R., Sneve, M., Figenschau, Y., Svartberg, J. & Waterloo, K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: Randomized double blind trial. J. Intern. Med. 264(6), 599–609 (2008).
Zhang, S. et al. Empirical comparison of four baseline covariate adjustment methods in analysis of continuous outcomes in randomized controlled trials. Clin. Epidemiol. 6, 227–235 (2014).
Jumbe, S., Hamlet, C. & Meyrick, J. Psychological aspects of bariatric surgery as a treatment for obesity. Curr. Obes. Rep. 6(1), 71–78 (2017).
Herpertz, S. et al. Does obesity surgery improve psychosocial functioning? A systematic review. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 27(11), 1300–1314 (2003).
Buchwald, H. et al. Bariatric surgery: A systematic review and meta-analysis. JAMA 292(14), 1724–1737 (2004).
Bocchieri, L. E., Meana, M. & Fisher, B. L. A review of psychosocial outcomes of surgery for morbid obesity. J. Psychosom. Res. 52(3), 155–165 (2002).
Ryden, A. & Torgerson, J. S. The Swedish Obese Subjects Study—what has been accomplished to date?. Surg. Obes. Relat. Dis. 2(5), 549–560 (2006).
Ayloo, S., Thompson, K., Choudhury, N. & Sheriffdeen, R. Correlation between the Beck Depression Inventory and bariatric surgical procedures. Surg. Obes. Relat. Dis. 11(3), 637–642 (2015).
White, M. A. et al. Prognostic significance of depressive symptoms on weight loss and psychosocial outcomes following gastric bypass surgery: A prospective 24-month follow-up study. Obes. Surg. 25(10), 1909–1916 (2015).
Alabi, F. et al. Depression before and after bariatric surgery in low-income patients: The utility of the beck depression inventory. Obes. Surg. 28(11), 3492–3498 (2018).
Sarwer, D. B. et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg. Obes. Relat. Dis. 4(5), 640–646 (2008).
Dixon, J. B., Dixon, M. E. & O’Brien, P. E. Depression in association with severe obesity: Changes with weight loss. Arch. Intern. Med. 163(17), 2058–2065 (2003).
de Zwaan, M. et al. Characteristics of morbidly obese patients before gastric bypass surgery. Compr. Psychiatry. 44(5), 428–434 (2003).
Krukowski, R. A., Friedman, K. E. & Applegate, K. L. The utility of the Beck Depression Inventory in a bariatric surgery population. Obes. Surg. 20(4), 426–431 (2010).
Hayden, M. J., Dixon, J. B., Dixon, M. E., Shea, T. L. & O’Brien, P. E. Characterization of the improvement in depressive symptoms following bariatric surgery. Obes. Surg. 21(3), 328–335 (2011).
Wadden, T. A. et al. Psychosocial aspects of obesity and obesity surgery. Surg. Clin. N. Am. 81(5), 1001–1024 (2001).
Schernthaner, G., Brix, J. M., Kopp, H. P. & Schernthaner, G. H. Cure of type 2 diabetes by metabolic surgery? A critical analysis of the evidence in 2010. Diabetes Care 34(Suppl 2), S355–S360 (2011).
Jimenez, A. et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann. Surg. 256(6), 1023–1029 (2012).
Mechanick, J. I. et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring). 21(Suppl 1), S1-27 (2013).
Roerig, J. L. et al. A comparison of duloxetine plasma levels in postbariatric surgery patients versus matched nonsurgical control subjects. J. Clin. Psychopharmacol. 33(4), 479–484 (2013).
Roerig, J. L. et al. Preliminary comparison of sertraline levels in postbariatric surgery patients versus matched nonsurgical cohort. Surg. Obes. Relat. Dis. 8(1), 62–66 (2012).
Semanscin-Doerr, D. A., Windover, A., Ashton, K. & Heinberg, L. J. Mood disorders in laparoscopic sleeve gastrectomy patients: does it affect early weight loss?. Surg. Obes. Relat. Dis. 6(2), 191–196 (2010).
Kalarchian, M. A. et al. Relationship of psychiatric disorders to 6-month outcomes after gastric bypass. Surg. Obes. Relat. Dis. 4(4), 544–549 (2008).
Wimmelmann, C. L., Dela, F. & Mortensen, E. L. Psychological predictors of weight loss after bariatric surgery: A review of the recent research. Obes. Res. Clin. Pract. 8(4), e299-313 (2014).
Black, C. N., Bot, M., Scheffer, P. G., Cuijpers, P. & Penninx, B. W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 51, 164–175 (2015).
Palta, P., Samuel, L. J., Miller, E. R. 3rd. & Szanton, S. L. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom. Med. 76(1), 12–19 (2014).
Tao, R. & Li, H. High serum uric acid level in adolescent depressive patients. J. Affect. Disord. 15(174), 464–466 (2015).
Kesebir, S., Suner, O., Yaylaci, E. T., Bayrak, A. & Turan, C. Increased uric acid levels in bipolar disorder: Is it trait or state?. J. Biol. Regul. Homeost. Agents. 27(4), 981–988 (2013).
Wen, S. et al. Serum uric acid levels and the clinical characteristics of depression. Clin. Biochem. 45(1–2), 49–53 (2012).
Chaudhari, K. et al. Clinical correlation of alteration of endogenous antioxidant-uric acid level in major depressive disorder. Indian J. Clin. Biochem. IJCB. 25(1), 77–81 (2010).
Lyngdoh, T. et al. Associations of serum uric acid and SLC2A9 variant with depressive and anxiety disorders: A population-based study. PLoS ONE 8(10), e76336 (2013).
Jimenez-Fernandez, S. et al. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: Results from a meta-analysis. J. Clin. Psychiatry. 76(12), 1658–1667 (2015).
Liu, T. et al. A meta-analysis of oxidative stress markers in depression. PLoS ONE 10(10), e0138904 (2015).
Michel, T. M., Pulschen, D. & Thome, J. The role of oxidative stress in depressive disorders. Curr. Pharm. Des. 18(36), 5890–5899 (2012).
Sperlagh, B. et al. The role of purinergic signaling in depressive disorders. Neuropsychopharmacol. Hung. 14(4), 231–238 (2012).
Singh, J. A., Reddy, S. G. & Kundukulam, J. Risk factors for gout and prevention: A systematic review of the literature. Curr. Opin. Rheumatol. 23(2), 192–202 (2011).
Black, C. N., Bot, M., Scheffer, P. G., Snieder, H. & Penninx, B. Uric acid in major depressive and anxiety disorders. J. Affect. Disord. 1(225), 684–690 (2018).
Oberbach, A. et al. Bariatric surgery in severely obese adolescents improves major comorbidities including hyperuricemia. Metab. Clin. Exp. 63(2), 242–249 (2014).
Charoenngam, N., Ponvilawan, B. & Ungprasert, P. Vitamin D insufficiency and deficiency are associated with a higher level of serum uric acid: A systematic review and meta-analysis. Mod. Rheumatol. 4, 1–6 (2019).
Vellekkatt, F. & Menon, V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 65(2), 74–80 (2019).
Jamilian, H. et al. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Prog. Neuropsychopharmacol. Biol. Psychiatry 30(94), 109651 (2019).
Parker, G. B., Brotchie, H. & Graham, R. K. Vitamin D and depression. J. Affect. Disord. 15(208), 56–61 (2017).
Acknowledgements
We would like to thank Miranda Adelfang, Michaela Faustmann and Hacer Geyik for their support. We would also like to thank Alexandra Kaider for the statistical input and, last but not least, the bariatric patients who participated in this study.
Funding
The work of this paper was partly supported by the Austrian Biobanking and BioMolecular resources Research Infrastructure (BBMRI.at) funded by the Austrian Federal Ministry of Science, Research and Economy (BMWFW GZ 10.470/0016-II/3/2013), by the non-profit organization “Special Institute for Preventive Cardiology And Nutrition—SIPCAN” (Salzburg) for personnel costs and Fresenius Kabi supplied, cost-free, the Oleovit and placebo oil but all had no role in the design and conduct of the study, the collection, analysis, and interpretation of data, in the preparation of the manuscript, or in the review or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
The project idea came from M.W., R.K., B.L., and R.M. M.W., R.K., R.M., B.L., K.S., G.P., and F.H. designed the research. M.W., R.K., and C.K. conducted the research. M.W., E.W. and I.G. analyzed the data, performed statistical analyses, and drafted the manuscript with appreciable input from R.K., B.L., C.K., K.S., R.M., G.P., and F.H. E.W. and M.W. had prime responsibility for the final manuscript content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
GP reports educational grants from Medtronic, other for Operating Courses for Medtronic, outside the submitted work. All other authors declare that they have no conflict of interest. The authors have no commercial associations that might be a conflict of interest in relation to this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Winzer, E., Ludvik, B., Grabovac, I. et al. Course of depressive symptomatology and its association with serum uric acid in one-anastomosis gastric bypass patients. Sci Rep 10, 18405 (2020). https://doi.org/10.1038/s41598-020-75407-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75407-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.