Abstract
Cannabis produces a class of isoprenylated resorcinyl polyketides known as cannabinoids, a subset of which are medically important and exclusive to this plant. The cannabinoid alkyl group is a critical structural feature that governs therapeutic activity. Genetic enhancement of the alkyl side-chain could lead to the development of novel chemical phenotypes (chemotypes) for pharmaceutical end-use. However, the genetic determinants underlying in planta variation of cannabinoid alkyl side-chain length remain uncharacterised. Using a diversity panel derived from the Ecofibre Cannabis germplasm collection, an extreme-phenotype genome-wide association study (XP-GWAS) was used to enrich for alkyl cannabinoid polymorphic regions. Resequencing of chemotypically extreme pools revealed a known cannabinoid synthesis pathway locus as well as a series of chemotype-associated genomic regions. One of these regions contained a candidate gene encoding a β-keto acyl carrier protein (ACP) reductase (BKR) putatively associated with polyketide fatty acid starter unit synthesis and alkyl side-chain length. Association analysis revealed twenty-two polymorphic variants spanning the length of this gene, including two nonsynonymous substitutions. The success of this first reported application of XP-GWAS for an obligate outcrossing and highly heterozygote plant genus suggests that this approach may have generic application for other plant species.
Similar content being viewed by others
Introduction
Cannabis is a multi-use predominantly dioecious and highly heterozygote plant genus1 within the angiosperm family Cannabaceae2. Medicinal use of Cannabis is thought to have spanned several millennia3, although incorporation of plant-derived drug products into modern western medicine has been hampered for several decades due to the narcotic status of this plant4,5.
Therapeutic activity of Cannabis is associated with a class of secondary metabolites commonly identified as cannabinoids6,7. Mature plants are prolific producers of these compounds, which are predominantly synthesised in capitate stalked trichomes and subsequently accumulate as exudates within their storage cavities8. The United States Food and Drug Administration (FDA) recently approved the first Cannabis-based medicine9, a purified plant extract containing the cannabinoid cannabidiol (CBD). This compound represents one of more than 100 naturally occurring plant cannabinoids10,11,12. In addition to CBD and the intoxicant delta-(9)-tetrahydrocannabinol (THC) other ‘minor’ cannabinoids produced by Cannabis show promise as therapeutic molecules13,14. However, these usually occur in planta at much lower concentrations15.
Cannabinoids are isoprenylated resorcinyl polyketides16, which are fused from intermediates derived from polyketide and isoprenoid biosynthetic pathways17,18. They are synthesised in plants with a carboxylated resorcinyl core, although this is readily decarboxylated non-enzymatically19. The dicyclic CBDA-type and tricyclic THCA-type cannabinoids are formed from the cyclisation of the isoprenoid residue by cannabidiolic acid synthase (CBDAS) and delta-(9)-tetrahydrocannabinolic acid synthase (THCAS)20,21. The activity of these synthases determines the structure of cannabinoid ligands, and so their ability to modulate the endocannabinoid system and associated physiological effects22. The alkyl side-chain is a further critical structural feature that influences potential therapeutic activity23,24, with side-chain length ranging in planta from between one to seven carbons25,26,27,28,29. However, cannabinoids with a five-carbon alkyl side-chain typically predominate in contemporary domesticated plants30,31,32.
Although there is potential for restructuring metabolic networks so that novel recombinant chemical phenotypes (chemotypes) can be developed for biopharmaceutical end-use33, the genetic and biosynthetic regulation of the alkyl group is largely unknown. Biosynthesis of cannabinoids within engineered yeast strains has demonstrated the promiscuity of cannabinoid pathway enzymes and their ability to produce alkyl cannabinoid homologs with varying chain lengths and configurations34. However, the biosynthetic origin of polyketide fatty acid (FA) starter units that determine alkyl homology is not fully understood35,36. Moreover, the genetic components underlying alkyl cannabinoid chemotypes have not been characterised37,38. This limits application of modern genetic improvement strategies, including marker-assisted selection (MAS) and genome engineering39.
The past decade has seen a rapid expansion in the use of high-throughput sequencing approaches to elucidate secondary metabolism in plants40,41,42. These have included using whole-genome sequencing such as used in Ocimum sanctum (holy basil)43 or RNA-Seq as used for Brassica juncea (Indian mustard)44. More targeted sequencing-based strategies in Nicotiana tabacum (tobacco)45 and Curcuma longa (turmeric)46, have contributed to improving the accuracy with which chemotype can be genetically characterised and predicted. However, the effectiveness of some of these methodologies can be limited in non-model orphan plant species47, where the inbred research lines required for linkage-based analysis such mapping-by-sequencing (MBS)48 may not be available49. This is especially problematic in Cannabis where the ability to acquire and exchange ex situ genetic resources for research continues to be constrained by regulatory requirements50.
Association mapping is an alternative high-throughput sequencing approach that circumvents the requirement for a dedicated crossing experiment. This approach relies on historical recombination for detection of genetic intervals associated with a trait51,52, and has been demonstrated through genome-wide association analysis (GWAS) to provide greater resolution than would be achievable from similar sized family mapping populations53. However, GWAS can be prohibitively expensive when applied to large sets of individuals, an issue amplified in heterozygote organisms due to the requirement for a high sequencing depth per sample to determine genotype accurately54. Extreme-phenotype GWAS (XP-GWAS) has been developed as a novel solution to this problem and involves bulked segregant analysis (BSA) of phenotypes within a diversity panel, with plants being selected at the extremes of the trait distribution52. XP-GWAS has resolved QTL for kernel row number in Zea mays (maize)52 and has also been successfully applied to inbred lines of Beta vulgaris (sugar beet)51 and its crop wild relatives55 to identify trait-associated variants. More recently, this analysis has identified variants associated with caffeine and trigonelline content in the self-fertilising species Coffea arabica (Arabian coffee)56. However, few studies have evaluated the utility of XP-GWAS in obligate outcrossing and highly heterozygote plants such as Cannabis.
Here we demonstrate the potential for XP-GWAS to provide high-resolution gene level mapping using a globally representative Cannabis diversity panel. This was achieved using whole-genome resequencing of pools comprised of chemotypically extreme individuals, where pools were divergent for cyclic (dicyclic vs tricyclic) and alkyl (C3 vs C5 chain-length) cannabinoid composition. Mapping of reads to two Cannabis reference genomes and comparison of allele frequencies between bulked DNA pools allowed detection of a known cannabinoid synthase locus and de novo discovery of polymorphic regions harbouring putative candidate genes linked to alkyl side-chain length.
Results
Alkyl-cannabinoid profiling and demarcation of chemotypic pools
We established a chemotype diversity panel from germplasm derived from the Ecofibre Global Germplasm Collection (EFGGC). Each accession in the panel is a provisional population, due to the heterozygous obligate outcrossing nature of Cannabis. Seven hundred and eleven juvenile Cannabis sativa L. plants derived from 72 accessions were chemotyped. The relative proportions of C3-/C5-alkyl cannabinoids and di-/tri-cyclic cannabinoids were calculated as a percentage of total cannabinoid content from fresh plant material, with fresh weight concentrations determined from liquid chromatography–mass spectrometry (LC–MS) analysis (Fig. 1). The alkyl cannabinoid values of the diversity panel were skewed towards the wild type pentyl cannabinoid chemotype, while cyclic cannabinoid values exhibited a tripartite chemotypic distribution comprised of low, intermediate and high dicyclic values (Fig. 1).
Chemotypic distribution of cyclic and alkyl cannabinoid composition in 711 Cannabis sativa L. plants. (a) Scatter plot showing distribution of bi-axial cannabinoid chemotypes. (b) Histogram and kernel density estimates of C3-alkyl cannabinoid chemotypes. (c) Histogram and kernel density estimates of dicyclic cannabinoid chemotypes. Plants within the diversity panel were selected from the Ecofibre Global Genetic Resource Collection. Cannabinoid composition was determined from liquid chromatography-mass spectrometry (LC–MS). Dicyclic and C3-alkyl cannabinoid fractions within the total cannabinoid fraction were calculated from fresh weight (w/w) cannabinoid content. Alkyl cannabinoid values are skewed towards low C3-alkyl, while dicyclic values exhibit a tripartite chemotypic distribution. Blue diamond (CBDA) and yellow diamond (THCVA) data points represent individual plants selected for an extreme-phenotype genome-wide association study.
The frequency of individuals in the diversity panel that exhibited chemotypically extreme values was sufficient to enable the pooling of plants divergent for both alkyl and cyclic chemotypes (Fig. 1, Supplementary Table S1). Two pools were constructed, each with seventy individuals that either exhibited [low C3-alkyl + high dicyclic (e.g. CBDA)] or [high C3-alkyl + low dicyclic (e.g. THCVA)] values at the extreme ends of alkyl and cyclic cannabinoid chemotypic distributions (Fig. 1). Each pool had a selectivity of 9.8% (Supplementary Fig. S1). For the pentyl dicyclic CBDA pool, values ranged from 94.3–97.1%, with C3 alkyl values ranging from 0.5–0.9% (Fig. 1, Supplementary Fig. S1), while those of the propyl tricyclic THCVA pool ranged from 0.1–2.5% and 64.6–92.9%, respectively.
DNA sequencing and mapping
Bulked DNA from each chemotypically extreme pool was subject to whole genome sequencing with Illumina sequencing-by-synthesis technology, generating 250,996,133 paired end (PE) reads for the CBDA pool and 238,918,478 PE reads for the THCVA pool (Table 1). For each pool, reads were mapped to Cannabis var. Finola (FN) and Cannabis var. Purple Kush (PK) genomes, representative of dicyclic CBDA and tricyclic THCA pentyl alkyl cannabinoid chemotypic lineages, respectively. For the FN reference sequence, mean sequencing depths were 53.8 × for the CBDA pool and 52.7 × for the THCVA pool (Table 1). Since the PK reference sequence had a smaller haploid genome length (0.9 Gb for PK vs 1.0 Gb for FN), depth of coverage for PK mapped reads was higher for the CBDA (66.1 ×) and THCVA pools (63.2 ×) (Table 1). Breadth of coverage based on a mean sequencing depth ≥ 15 ×, averaged 73.5% (± 2.1) for the FN reference and 78.9% (± 0.4) for the PK reference (Table 1).
Variant calling resulted in 25,285,455 (FN) and 23,360,033 (PK) short variants (SNP and InDels) when bulked pools were mapped to the reference sequences. Following hard filtering and removal of multiallelic variations and variant sites lacking genotypes for both chemotypic pools, two sets of high confidence biallelic variants were obtained for FN and PK mapped reads (Table 2). Post-call filtering identified 9,133,504 SNPs and 2,593,786 InDels when bulked pools were mapped to the FN reference genome, while a total of 7,926,104 SNPs and 2,714,230 InDels were identified from post-call filtering of PK mapped reads (Table 2). Allele frequency estimates (AFe) at each variant site were then determined for CBDA and THCVA bulked pools. An AFe of 0 indicates 100% of reads support the reference allele, while an AFe of 1 indicates 100% of reads support the alternative allele. To allow variant comparison of pools, we calculated per site delta-AFe values as the absolute difference in AFe. A delta-AFe value ≥ 0.9 was used to indicate homozygote dissimilarities between pools. Low coverage contributes to higher error in determining allele frequencies due to stochastic effects while high coverage indicates the possibility of collapsed repetitive sequences. To reduce erroneous AFe values, only delta-AFe values supported by 0.75–2.5 × the average depth of coverage were included.
Chemotypic pools are able to detect the known CBDAS locus
Bulked DNA from individual plants with divergent cannabinoid values allowed genome-wide variant comparison for alkyl and cyclic cannabinoid chemotypes (Fig. 1). Prior knowledge of the location of the CBDAS locus in the CBDAS homozygote and chemotypically CBDA predominant FN genome served as a control for our XP-GWAS facilitated variant discovery in Cannabis. Genetic intervals represent resolvable intervals between two marker loci. Those displaying delta-AFe values of 1 were delineated algorithmically by plotting contiguous allele frequencies of the CBDA pool that matched the wild-type reference sequence (AFe ≤ 0.1), with deviant allele frequencies between the dicyclic CBDA pool and the mutant tricyclic THCVA pool introduced through chemotypic selection of pooled individuals. As such, fluctuating delta-AFe values within intervals were considered as variants exclusive to the tricyclic THCVA pool, which is comprised of individuals having only minor dicyclic cannabinoid levels (e.g. CBDA/CBDVA).
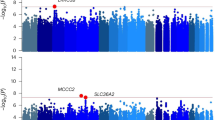
Consistent with the known genomic position of the CBDAS locus, two intervals were detected on chromosome six sensu [GenBank: ASM341772v2] (Fig. 2a). One of these was coincident with the 6924 bp region that incorporates the 1632-nucleotide open reading frame (ORF) of CBDAS (Fig. 2b, Supplementary Table S2), with the CBDAS locus allele variant corresponding to GenBank accession AB292682.1 (100% query cover, 99.4% identity). THCVA variants were supported by a mean depth of coverage of 156 × (compared with an average of 58 × for the CBDA pool) along the length of the CBDAS locus, with a subset of THCVA pool variants > 250 × coverage. Higher read coverage in the THCVA pool is consistent with multiple collapsed CBDAS gene copies being mapped to this locus. In total, 43 short variants (SNPs and InDels) with AFe values ≥ 0.9 were identified in the ORF of CBDAS (Supplementary Table S2). No intervals were detected which overlapped the coordinates of the THCAS locus when comparing chemotypic pools using the PK genome. However, a large gap (~ 350 kb) in delta-AFe values was detected in the middle of chromosome seven sensu [GenBank: ASM23057v2] (Fig. 3a). This region encompasses the coordinates of THCAS and this region was confirmed as non-homologous between pooled samples from raw alignment data (Fig. 3b). Reads from the THCVA pool mapped to the THCAS locus, while those from the CBDA pool failed to align to this region (Fig. 3b).
Detection of interval overlapping the CBDAS locus. (a) Plot of delta-AFe values encompassing two genomic regions (intervals) located within proximity to the chemotype determining CBDAS locus. AFe sorted by coordinate along chromosome six of the Cannabis sativa L. var. Finola (FN) genome. Delta-AFe indicates the absolute difference between AFe of CBDA and THCVA pools. Red dotted line indicates delta-AFe of 0.9. (b) Plot of AFe for the interval overlapping the 1632-nucleotide open reading frame of CBDAS. To reduce background noise, only delta-AFe supported by 0.75–2.5 × the average depth of coverage was plotted. Black arrow indicates orientation of gene (+/− strand). Pink line indicates genomic interval. Intervals displaying delta-AFe values of ≥ 0.9 were delineated using an algorithm which plots contiguous AFe values ≤ 0.1 in the CBDA pool (see “Methods”); AFe: allele frequency estimates; CBDAS: gene encoding cannabidiolic acid synthase.
Non-homologous genomic region encompassing the THCAS locus. (a) Delta AFe values form a gap that overlaps the coordinates of the THCAS locus. delta-AFe is the absolute difference between AFe of CBDA and THCVA pools. (b) Integrative Genomics Viewer (IGV) snapshot of the THCAS locus. Reads from the THCVA pool were mapped to the PK reference sequence but reads for this region were absent from the CBDA pool; delta-AFe plotted by coordinate along chromosome seven of the Cannabis sativa L. var. Purple Kush (PK) genome. Bold red line indicates position of the THCAS locus. Red dotted line indicates delta-AFe of 0.9. AFe allele frequency estimates, THCAS gene encoding delta(9)-tetrahydrocannabinolic acid synthase.
Intervals detect putative candidate alkyl cannabinoid genes
We next focused on interval detection for alkyl side-chain length. AFe values from the high pentyl alkyl CBDA pool and the high propyl alkyl THCVA pool were plotted for the remaining chromosomes and unplaced scaffolds for the genomes of FN and PK, both of which have a pentyl alkyl cannabinoid chemotype. To allow ad-hoc identification of genetic intervals relevant to alkyl cannabinoid chemotypes, AFe values were plotted from the CBDA pool which is chemotypically uniform for pentyl alkyl cannabinoids and therefore expected to match the reference sequence at sites determining alkyl chain length. Contiguous AFe values ≤ 0.1 from the CBDA pool revealed twenty-two intervals within the FN reference sequence (Fig. 4a), and nine intervals within the PK genome (Fig. 4b). Intervals ranged from 1–57 kb, with an average length of 10.3 kb (Supplementary Table S3, 4), and had a random distribution within both reference assemblies (Fig. 4a,b).
Delta-AFe between chemotypic pools and interval detection. (a) Delta-AFe plotted by chromosome number and coordinate along the Cannabis sativa L. var. Finola (FN) genome. Delta-AFe is the absolute difference between allele frequency estimates of CBDA and THCVA DNA pools. (b) Delta-AFe plotted by chromosome number and coordinate along the Cannabis sativa L. var. Purple Kush (PK) genome. Intervals displaying delta-AFe values of ≥ 0.9 were delineated using an algorithm which plots contiguous AFe values ≤ 0.1 in the CBDA pool (see “Methods”). To reduce background noise, only delta-AFe supported by 0.75–2.5 × the average depth of coverage was plotted. Pink lines indicate location of intervals. Red dotted line indicates delta-AFe of 0.9; AFe allele frequency estimates.
At the time of analysis, a stable annotated reference sequence for Cannabis was unavailable. Genic regions within intervals were identified using FGENESH (v 2.6), with Basic Local Alignment Search Tool (BLAST)p alignments of predicted protein sequences used to determine potential gene function. Intervals were principally comprised of transposable element-like sequences (Supplementary Table S3, 4). However, PK intervals 106 (2.8 kb), 107 (12.1 kb) and 108 (12.4 kb) clustered on chromosome nine (Figs. 4b, 5a) and revealed three putative candidate genes (Fig. 5b). One of these encoded a protein with high identity to a β-keto acyl carrier protein (ACP) reductase (BKR; EC 1.1.1.100) (Supplementary Table S4). This protein has 81.7% identity to BKR homologs (80% query cover, E-value 4e−127) and incorporates a conserved fabG protein domain (NCBI accession PRK05557, E-value 1.44e−92). The two other putative candidate genes encoded a fkbH-like protein and a TLC domain-containing protein (NCBI accession smart00724, E-value 1.76e−13) (Fig. 5b, Supplementary Table S4). These intervals were not proximal to CBDAS and THCAS related sequences, and no copies of these genes were present on chromosome nine of the PK genome.
Genomic region encompassing putative candidate alkyl cannabinoid pathway genes. (a) Plot of delta-AFe within a region of chromosome nine on the Cannabis sativa L. var. Purple Kush (PK) genome. Red dotted line indicates delta-AFe of 0.9. Pink lines indicate location of intervals. Red line indicates location of putative candidate genes. (b) AFe of CBDA and THCVA pools under intervals. Pink lines indicate location of intervals. Red line indicates location of putative candidate genes. Black arrow indicates orientation of gene (+/− strand). (c) Positions of exons and gene features for the predicted BKR homolog gene. Gene features: CDSf, first exon including start codon; CDSi, internal exon; CDSl: last exon including stop codon; TSS, transcription start. Graphical output generated using FGENESH (v 2.6) (Softberry, http://www.softberry.com/berry.phtml)87. (d) Alignment of the mRNA sequence of the putative BKR gene homolog with the Purple Kush canSat3 draft representative transcriptome. High scoring alignments were found with segments of the PK transcript PK02233.1 (Supplementary Table S6). Graphic output generated using an in-house TimeLogic DeCypher system (Active Motif Inc., Carlsbad, CA) and TimeLogic Tera-BLASTN algorithm (v2.2.29) (Active Motif Inc., Carlsbad, CA) (https://www.timelogic.com).
Association analysis reveals putative alkyl cannabinoid-linked polymorphic variants
Analysis of polymorphisms within the three putative candidate genes revealed 25 variant sites with delta-AFe values ≥ 0.9, of which 22 spanned the length of the predicted BKR gene homolog located on PK interval 106 (Fig. 5b,c, Supplementary Table S5). Three of the BKR associated SNPs were in the 5′ untranslated region (UTR) and four were in exonic regions. Two of these were nonsynonymous and located within exon 6 (locus ID: Cs_BKR_06, Cs_BKR_07) (Supplementary Table S5, Fig. 5c). Nonsynonymous variants included a G/A SNP that would result in an Ala to Val substitution, as well as a T/C SNP that would result in a Ser to Asn substitution (Supplementary Table S5), with Val and Asn substitutions associated with the THCVA pool. Depth of coverage for the BKR homolog variant sites averaged 67 × for the CBDA pool and 74 × for the THCVA pool, with nonsynonymous SNPs supported by 62/61 reads for the CBDA pool and 65/64 reads for the THCVA pool (Supplementary Table S5). BKR nonsynonymous variants with delta-AFe values ≥ 0.9 did not overlap with the NAD(P) binding site or active site residues associated with the fabG conserved domain, and no other variant sites with AFe ≥ 0.9 were observed in exonic regions of the fkbH-like and TLC domain-encoding genes. However, mRNA sequences of putative candidate genes, including the BKR homolog (Fig. 5d), aligned to transcripts of the draft PK transcriptome (Supplementary Table S6).
Discussion
To allow for the visual identification of genic regions under selection, an established algorithm which allows comparison between two phenotypically divergent DNA pools was used51,55. This has been successful in identifying the known BvCYP76AD1 gene that encodes a cytochrome P450 enzyme responsible for betalain synthesis in Beta vulgaris (sugar beet)51. This algorithm has also been used to identify alleles of the Rz2 gene which encodes a nucleotide-binding site leucine-rich repeat (NBS-LRR) protein conferring resistance against rhizomania disease in Beta crop wild relatives55. The criterion the algorithm uses for interval detection is a series of AFe values close to zero in the pool with the phenotype matching the reference sequence. This is calculated to allow a limited number of non-supportive reads (see “Methods”). Seed variants are used as starting locations for intervals, with intercepts (variants) not considered if they are flanked on each side by an AFe ≤ 0.1.
The algorithm for automatic interval detection was successful in identifying the location of the known CBDAS locus present on the FN genome (Fig. 2a,b). While the algorithm allows ad hoc identification of intervals, it does not assign a level of confidence. Given the lack of biological replicates for associations, calculating probability values for intervals is not straightforward. This could be addressed by sequencing individuals within pools or by sequencing a physical random pool to check for spurious associations52. However, both approaches would negate to some extent the lower costs associated with sequencing a maximum of two phenotypically extreme DNA pools52,56. One approach that could be assessed is to apply a Chi-squared test to the read counts for the pools surrounding intervals, in order to reject the null hypothesis that the phenotype and AFe are not correlated. As reported elsewhere55, a subset of intervals were fragmented over genic regions. This may be an indication that the tolerance for variants requires adjustment and that the algorithm is not exhaustive in its ability to capture genic sites associated with phenotype.
The genomic structure underlying dicyclic (CBDA/CBDVA) and tricyclic (THCA/THCVA) cannabinoid composition is now known to possess a high level of complexity and may vary considerably among different lineages and recombinants of Cannabis37,38. While segregation patterns support the hypothesis that cannabinoid synthases are isoforms at a single genetic locus B57, their genomic organisation appears to diverge significantly from this model37,38,58. Recent versions of the PK (tricyclic THCA-type) and FN (dicyclic CBDA-type) genomes, which were used for the current analysis, show two discrete loci that are non-homologous between chemotypes37. Within the PK assembly there is a THCAS locus, and in the FN assembly a CBDAS locus, neither of which has a clear counterpart in the opposite genome.
Analysis of AFe between CBDA and THCVA pools provides further insight into the genomic structure of the locus B complex. In our study, reads from the CBDA pool mapped to the CBDAS locus (FN genome), while no reads from the CBDA pool mapped to the THCAS locus (PK genome) (Fig. 3b). This is congruent with previous observations, where CBDA-predominant plants lack cannabinoid synthases gene copies that either align to the THCAS locus or that have > 95% nucleotide identity to functional THCAS sequences37,58. However, analysis of reads from the tricyclic THCVA pool showed differences in synthase heterogeneity as compared with the tricyclic PK genome. Previously, when sequence reads of the THCA predominant PK genome were mapped to the FN assembly, none aligned to the CBDAS locus37. In our analysis of the THCVA pool, not only did reads map to the CBDAS locus, but the THCVA pool yielded a high depth of coverage for this locus.
The tricyclic propyl alkyl THCVA chemotype is rarely found among domesticated forms of Cannabis, including those associated with medicinal25, industrial hemp31,32,59 and recreational drug end-uses30. One explanation for synthase heterogeneity between the PK genome and THCVA pool is that selection for THCA in PK has led to a loss in CBDAS gene copy number. High coverage of CBDAS by reads from the THCVA pool suggests that the current assemblies underestimate the total number of CBDAS gene copies that are present within the broader gene pool37,38. Low levels of the dicyclic cannabinoids (e.g. CBDA/CBDVA) in the tricyclic propyl alkyl THCVA pool would suggest that the CBDAS sequences from this pool are null alleles as evident by the presence of a 4 bp deletion previously associated with loss of function (Supplementary Table S2).
It is predicted that the sequencing of alternative bi-axial chemotypic pools THCA vs CBDVA would have provided additional insight into cannabinoid synthase heterogeneity and that this would have further validated the XP-GWAS approach through the identification of the CBDAS locus using independent bulked pools. However, this analysis was not possible due to the low representation of CBDVA chemotypes in the sample population. While not directly relevant to the chemotype selection of bulked pools, no intervals were detected which corresponded to THCAS-like CBCAS loci. These genes have 96% identical to THCAS at the nucleotide level37, although the position of these loci on unmapped scaffolds may have influenced mapping quality and subsequent variant analysis.
Although the reference genome sequences used are comprised of ten chromosome pseudomolecules, they remain fragmented with 2352 (FN) and 6295 (PK) unlocalised scaffolds37 and are predicted to contain unresolved THCA/CBDA loci38. While these and other near-complete haploid reference sequences are a substantial improvement on the previous draft genome58, development of a fully phased diploid assembly for Cannabis will accelerate understanding of haplotype structure and improve allele-specific analyses of complex loci such as those governing cannabinoid synthase activity. Given that evidence exists for linage-specific changes in cannabinoid synthase gene content38, development of ‘pan genomes’ should also aid in resolving the genetic molecular basis for cannabinoid composition among diverse subtaxa while also improving the analysis of pooled samples60.
13C labelling patterns in Cannabis have indicated that the alkyl group of cannabinoids originate from a fatty acid (FA) precursor61. This is also supported by experiments with engineered yeast strains fed various FA precursors, which result in altered production of cannabinoid alkyl homologs34. Polyketide FA starter units could arise via several metabolic routes. One hypothesis involves the breakdown of FAs by desaturases, lipoxygenases (LOX) and hydroperoxide lyases36,58. Alternative paths involve the breakdown of branched-chain amino acids, as occurs in prenylated polyketide synthesis in the closely related Cannabaceae species Humulus lupulus (hops)62, as well as the de novo FA synthesis pathway in Cannabis35. The latter two examples both involve BKR activity for the development of polyketide starter units35,63.
In type II FA synthesis, which occurs in plants and many bacteria, FAs are constructed from the condensation of two-carbon units by a series of discrete mono-functional enzymes: β-ketoacyl-ACP synthase (KAS), BKR, β-hydroxyacyl-ACP dehydrase (HAAD) and enoyl-ACP reductase (ENR)64. BKR catalyses the first reductive step in FA elongation using malonyl-CoA-derived building blocks65. Analogous with its function in de novo FA synthesis, this enzyme performs a similar reductive step in polyketide synthesis66. It shares significant sequence similarity with the second FA synthesis reductase ENR, as well as with other members of the short-chain dehydrogenases/reductases (SDR) family66,67,68. This is also found with the protein sequence of the predicted BKR homolog, with BLASTp analysis yielding a specific hit to the SDR family domain adh_short (GenBank accession pfam00106, E-value 6.56e−70).
The BKR homolog identified on PK interval 106 may have a putative role in the synthesis of polyketide FA starter units. However, it is difficult to speculate on the mechanism by which this gene contributes to alkyl side-chain length without biochemical examination of its encoded protein. This is due to the similarity of BKR with other oxidoreductases of the SDR superfamily69, as well as limited structural data on the transient complexes BKR forms with other FA synthesis proteins70. As with KAS71 and ENR72 type II FA synthesis enzymes, BKR (fabG) isoforms have demonstrated the ability to shift FA composition67. In Capsicum, the CaKR1 gene has been found to encode a BKR which facilitates FA elongation of the capsaicinoid intermediate 8-methyl-6-nonenoic acid63, with CaKR1 gene silencing influencing capsaicinoid anabolism. The role of BKR in cannabinoid biosynthesis is also supported by quantitative PCR (qPCR) analysis, where BKR-associated unigenes were found to be expressed at high levels in trichome vs leaf tissue35. In addition, the BKR homolog identified on PK interval 106 aligned to the draft PK transcriptome, with 91% query cover and ≥ 99% identity with segments of the PK02233.1 transcript (Supplementary Table S6, Fig. 5d).
Other predicted candidate genes found clustered within 25 kb of the BKR homolog also have interesting putative functions relating to polyketide and FA synthesis. Proteins containing the fkbH domain are associated with the formation of glyceryl-ACP, where they function as part of a larger protein cluster involved in the synthesis of methoxymalonyl-ACP polyketide extender units73,74. TLC (TRAM/LAG1/CLN8) lipid-sensing domain-containing proteins are associated with several functions including acyl-CoA-dependent ceramide synthesis75 and lipid transfer76. PK and other high-yielding cannabinoid strains can produce small quantities of cannabinoids with varying alkyl-chain lengths including C3-alkyl homologs26,29. Contemporary industrial hemp cultivars such as FN, which are thought to have been derived from a narrow subset of the genepool and are more uniform in chemotype30,50, are principally associated with CBDA production21,59. This may explain why analysis of pooled chemotypes against the FN genome were not meaningful in the detection of the putative alkyl cannabinoid gene clusters.
While it is not obvious how intervals harbouring transposable element-like sequences are relevant to alkyl cannabinoid composition (Fig. 4a,b, Supplementary Table S3, 4), they may have utility in determining phylogeny and genetic diversity between chemotypic lineages or act as markers to tag and track traits of interest77,78. This information could be used to inform ex situ germplasm conservation and to develop representative core collections79.
We successfully demonstrated the application of a modified version of the GWAS approach with sequencing of bulked pools derived from a diversity panel of Cannabis51,52, an obligate outcrossing and highly heterozygote plant genus1,80. The approach was validated through the identification of a known complex locus involved in the synthesis of the dicyclic cannabinoid CBDA37, and has led to the de novo identification of putative candidate cannabinoid pathway genes. Resequencing of pools divergent for both CBDA and THCVA allowed genetic characterisation of two distinct chemotypes, resulting in the detection of trait-associated variants within a candidate gene putative linked to alkyl cannabinoid synthesis. This study made use of rare allelic variation present within a subset of the Cannabis genepool and highlights the importance of ex situ germplasm conservation and the systematic phenotyping of these resources for genetic improvement50,81.
The analysis presented here offers insight into the biosynthesis of alkyl cannabinoids and provides a platform for further genetic characterisation of alkyl cannabinoid metabolism. The putative variants located within intervals and their flanking sequences provide useful templates for the development of PCR-based markers or as probes for targeted high-throughput DNA sequencing. This approach could be applied to analysing progeny of a segregating population or used to screen germplasm with intent to develop marker haplotypes for alkyl-cannabinoid composition. The outcome of this analysis also provides putative targets for gene editing and other interventions, including opportunities to validate trait-associated genetic regions and associated candidate genes using reverse genetic approaches.
Methods
Genetic materials and cultivation
All research activities, including the procurement and cultivation of the prohibited plant Cannabis, were executed in accordance with the NSW Drug Misuse and Trafficking Act 1985 and under authorisations granted to Professor Graham King by the New South Wales Ministry of Health, Pharmaceutical Regulatory Unit, Legal and Regulatory Services Branch, Australia. Cannabis sativa L. seed pack accessions were sourced from the Ecofibre Global Germplasm Collection. Seventy-two accessions were used to develop a chemotypic diversity panel comprising of 711 individual plants. Dr. Omid Ansari (omid@ecofibre.com) is the contact person for enquiries on germplasm used in this study and the associated Ecofibre ex situ Cannabis genetic resource collection.
Seeds were sown directly into 7.5 cm (diameter) × 10 cm (height) 400 mL round pots at a depth of 1.5 cm. Soil media consisted of one part perlite, one part peat moss, and one part vermiculite as well as dolomite at a concentration of 110 g/100 L. Plants were grown under environmentally uniform conditions within Ecofibre’s purpose-built growth chambers, and were subject to 11 h of high pressure sodium (HPS)/metal halide (MH) light (luminous flux = 72,000 lumens) per day. Temperature was kept constant between 26 and 28 °C. Plants were watered daily. Upon full extension of the first leaflet pair plants were supplemented with CANNA Aqua Vega solution.
LC–MS chemotyping
Cannabinoids were extracted from the sub-apical raceme of juvenile plants (code 1008)82 at opposing phyllotaxis (2 × 250 mg) and analysed using liquid chromatography-mass spectrometry (LC–MS) as previously described31. Dicyclic, tricyclic, C3-alkyl and C5-alkyl cannabinoid fractions within the total cannabinoid fraction were calculated from the fresh weight (w/w) content of cannabidivarinic acid (CBDVA), tetrahydrocannabivarinic acid (THCVA), cannabidiolic acid (CBDA), delta(9)-tetrahydrocannabinolic acid (THCA), cannabidivarin (CBDV), delta(9)-tetrahydrocannabivarin (THCV), cannabidiol (CBD) and delta(9)-tetrahydrocannabinol (THC).
DNA isolation, library preparation and sequencing
DNA was isolated from each plant prior to bulking. DNA extraction and purification involved using a Qiagen DNeasy Plant Mini Kit in accordance with the manufacturer’s instructions, with tissue disruption performed using a Qiagen TissueLyser. A Qubit Fluorometer and Qubit dsDNA BR Assay Kit was used for DNA quantification. DNA concentration (w/v) in Qiagen AE buffer (10 mM Tris–Cl 0.5 mM EDTA; pH 9.0) was normalised, pooled and concentrated to 129 μg/μL using an Eppendorf Concentrator #5301 with diaphragm vacuum pump. Molecular weight (≥ 20 kb) and RNA contamination were assessed by gel electrophoresis.
Library preparation and sequencing of pooled DNA samples was undertaken at the Australian Genome Research Facility Ltd (AGRF, Melbourne, Australia; https://agrf.org.au). DNA libraries were prepared using an Illumina TruSeq DNA PCR-Free Library Prep Kit, with DNA fragmentation, end repair, size selection, A-tailing and adapter ligation conducted according to the manufacturer's guidelines. Whole-genome sequencing of DNA pools was performed on an Illumina HiSeq2500 platform in high-output mode. Samples were run over two flow cells and sequenced according to the 2 × 125 bp (550 bp insert) paired end (PE) scheme using HiSeq HT chemistry. Image analysis was performed by HiSeq Control Software (HCS, v2.2.68) and Real Time Analysis (RTA, v1.18.66.3). Data was generated using the bcl2fastq 2.20.0.422 pipeline.
Data pre-processing and mapping
All data pre-processing and short variant discovery steps were performed using the Genome Analysis Toolkit (GATK, v4.0.3.0)83. Sequence reads of pooled samples were separately mapped to modified versions of Cannabis sativa L. var. Finola (FN) [GenBank: ASM341772v2] and var. Purple Kush (PK) [GenBank: ASM23057v2] reference assemblies37. Modified reference sequences were developed using the tool ScaffoldStitcher (Python) (https://bitbucket.org/dholab/scaffoldstitcher/src). ScaffoldStitcher concatenated unplaced scaffolds using spacers of 550 Ns.
Analysis-ready Binary Alignment Map (BAM) files were developed with consideration of the Broads GATK Best Practices workflows (https://gatk.broadinstitute.org). FastqToSam was used to generate unmapped BAM (uBAM) files. Adapter sequences were tagged using MarkIlluminaAdapters. uBAMs were reverted to FASTQ and purged of metadata using SamToFastq. Reads were then mapped to modified FN and PK reference assemblies using Burrows-Wheeler Aligner's maximal exact matches (BWA-MEM, v0.7.17) algorithm84. Duplicates were tagged using MarkDuplicates. Pooled sample BAM files were sorted by coordinate with SortSam and validity using ValidateSamFile. Sequencing and alignment statistics were determined from CollectAlignmentSummaryMetrics. Depth and breadth of coverage were assessed using AWK, mpileup and depth commands (Sequence Alignment/Map (SAM)tools, v 1.7)85.
Short variant discovery
Base Quality Score Recalibration (BQSR) was performed as outlined by GATK guidelines. Recalibration tables were written with BaseRecalibrator and ApplyBQSR was used to recalibrate BAM files. BQSR was bootstrapped and convergence of successive recalibration outputs assessed using AnalyzeCovariates.
Single nucleotide polymorphism (SNPs) and small insertion-deletion (InDels) were identified using the Broads GATK HaplotypeCaller. HaplotypeCaller runs were performed in GVCF mode, with chemotypic pool gVCF files consolidated using CombineGVCFs. Variants were joint called using GenotypeGVCFs. Variants were then hard filtered with SelectVariants and VariantFiltration tools in accordance with GATK generic filtering parameters. Variants were restricted to biallelic allelicity. Variants supported by a depth of coverage (DP) < 15 or a genotype quality (GQ) value < 20 were not considered. Sites with no-call genotypes that were deficient in one of two chemotypic pool genotypes were excluded. Bi-sample SNP and InDel VCFs were then merged using MergeVcfs to accommodate downstream analysis.
Allele frequency estimation
Allele frequency estimates (AFe) were calculated at each site per DNA pool using the relative number of reads supportive of either the alternative or reference allele. An AFe of 0 indicates 100% of reads support the reference allele, while an AFe of 1 indicates 100% of reads support the alternative allele. To demarcate AFe values associated with either chemotypic pool, per site delta-AFe values were calculated as the absolute difference of AFe values between pools. Delta-AFe values ≥ 0.9 indicated homozygote differences between pools. AFe and delta-AFe values were calculated for biallelic variants and plotted by coordinate along FN and PK genome assemblies. To reduce false positive variants, only delta-AFe values supported by 0.75–2.5 x the average depth of coverage was plotted51.
Interval detection and visualization
Genomic regions exhibiting AFe value ≥ 0.9 were delineated using an established algorithm for automatic interval detection51,55. Intervals were demarcated by a series of AFe values ≤ 0.1 in the CBDA pool. Interruptions by an intercept (variant) were ignored if they were comprised of a maximum of 1 variant with an AFe ≥ 0.1, flanked on both sides by a variant with an AFe of ≤ 0.1. The starting point of intervals was determined from seed variants exhibiting AFe minima values (≤ 0.1). AFe minima was calculated to permit a small number of non-supportive reads using X = (P – 100 × E/C), with bulked pool phenotypic difference P = 0.9, combined coverage of the pools C and an estimate sequencing error from the Illumina HiSeq platform E = 0.01. Variants overlapping intervals were then filtered for AFe ≥ 0.9. The Python script VCF2AFAnalysis.py (https://github.com/davidries84/vcf2AFAnalysis) was used to generate intervals and to plot AFe and delta-AFe values51. Visualisation of raw alignment data and regions of interest was performed using Integrative Genomics Viewer (IGV, v2.6.0) software86.
Gene predication and annotation
Multifasta files containing interval sequences were indexed using faidx (SAMtools, v 1.7). Putative genes and associated protein sequences were predicted using FGENESH (v2.6) (https://www.softberry.com/)87. FGENESH was performed using Cannabis-specific gene finding parameters (date accessed 04/12/2019). For functional annotation of predicted genes, Basic Local Alignment Search Tool (BLAST)p analysis of amino acid sequences were performed against the non-redundant protein sequence database (National Center for Biotechnology Information (NCBI) nr protein, accessed 12/04/2019 containing 198,058,131 sequences)88. The highest scoring characterised protein was extracted, with consideration for identity, coverage, then E-value. Nucleotide sequences [mRNA] of putative candidate genes identified on PK intervals on chromosome nine were queried using an in-house TimeLogic DeCypher system (Active Motif Inc., Carlsbad, CA) and TimeLogic Tera-BLASTN algorithm (v2.2.29) (Active Motif Inc., Carlsbad, CA) (https://www.timelogic.com) against the canSat3 representative transcriptome (Cannabis Genome Browser, https://genome.ccbr.utoronto.ca/downloads.html), with an E-value cut-off limit of 1e−10 applied.
Data availability
The Illumina sequencing data for chemotypic extreme pools from this study have been submitted to the NCBI BioProject database under accession number PRJNA669610 (https://www.ncbi.nlm.nih.gov/bioproject). For enquiries relating to the Ecofibre germplasm collection and commercial arrangements contact Omid Ansari: omid@ecofibre.com.
References
Soler, S. et al. Genetic structure of Cannabissativa var. indica cultivars based on genomic SSR (gSSR) markers: implications for breeding and germplasm management. Ind. Crops Prod. 104, 171–178. https://doi.org/10.1016/j.indcrop.2017.04.043 (2017).
Small, E. & Cronquist, A. A practical and natural taxonomy for Cannabis. Taxon 25, 405–435. https://doi.org/10.2307/1220524 (1976).
Pisanti, S. & Bifulco, M. Medical Cannabis: a plurimillennial history of an evergreen. J. Cell. Physiol. 234, 8342–8351 (2019).
Nutt, D. Illegal drugs laws: clearing a 50-year-old obstacle to research. PLoS Biol. 13, e1002047. https://doi.org/10.1371/journal.pbio.1002047 (2015).
Pain, S. A potted history. Nature 525, S10–S11. https://doi.org/10.1038/525S10a (2015).
Lichtman, A. H. et al. Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J. Pain Symptom Manag. 55, 179-188.e171. https://doi.org/10.1016/j.jpainsymman.2017.09.001 (2018).
Thiele, E. A. et al. Cannabidiol in patients with seizures associated with Lennox–Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 391, 1085–1096. https://doi.org/10.1016/S0140-6736(18)30136-3 (2018).
Rodziewicz, P., Loroch, S., Marczak, Ł, Sickmann, A. & Kayser, O. Cannabinoid synthases and osmoprotective metabolites accumulate in the exudates of Cannabis sativa L. glandular trichomes. Plant Sci. 284, 108–116. https://doi.org/10.1016/j.plantsci.2019.04.008 (2019).
Chandra, S., Lata, H., ElSohly, M. A., Walker, L. A. & Potter, D. Cannabis cultivation: methodological issues for obtaining medical-grade product. Epilepsy Behav. 70, 302–312. https://doi.org/10.1016/j.yebeh.2016.11.029 (2017).
ElSohly, M. A. & Slade, D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 78, 539–548. https://doi.org/10.1016/j.lfs.2005.09.011 (2005).
Hanuš, L. O. et al. Enantiomeric cannabidiol derivatives: synthesis and binding to cannabinoid receptors. Org. Biomol. Chem. 3, 1116–1123. https://doi.org/10.1039/B416943C (2005).
Radwan, M. M. et al. Isolation and pharmacological evaluation of minor cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 78, 1271–1276. https://doi.org/10.1021/acs.jnatprod.5b00065 (2015).
Huizenga, M. N., Sepulveda-Rodriguez, A. & Forcelli, P. A. Preclinical safety and efficacy of cannabidivarin for early life seizures. Neuropharmacology 148, 189–198. https://doi.org/10.1016/j.neuropharm.2019.01.002 (2019).
Jadoon, K. A. et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 39, 1777–1786. https://doi.org/10.2337/dc16-0650 (2016).
Hazekamp, A., Tejkalová, K. & Papadimitriou, S. Cannabis: from cultivar to chemovar II—a metabolomics approach to Cannabis classification. Cannabis Cannabinoid Res. 1, 202–215. https://doi.org/10.1089/can.2016.0017 (2016).
Hanuš, L. O., Meyer, S. M., Muñoz, E., Taglialatela-Scafati, O. & Appendino, G. Phytocannabinoids: a unified critical inventory. Nat. Prod. Rep. 33, 1357–1392. https://doi.org/10.1039/c6np00074f (2016).
Fellermeier, M. & Zenk, M. H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett. 427, 283–285. https://doi.org/10.1016/S0014-5793(98)00450-5 (1998).
Gagne, S. J. et al. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc. Natl. Acad. Sci. U.S.A. 109, 12811–12816. https://doi.org/10.1073/pnas.1200330109 (2012).
Dussy, F. E., Hamberg, C., Luginbühl, M., Schwerzmann, T. & Briellmann, T. A. Isolation of Δ9-THCA-A from hemp and analytical aspects concerning the determination of Δ9-THC in Cannabis products. Forensic Sci. Int. 149, 3–10. https://doi.org/10.1016/j.forsciint.2004.05.015 (2005).
Sirikantaramas, S. et al. The gene controlling marijuana psychoactivity molecular cloning and heterologous expression of Δ1-tetrahydrocannabinolic acid synthase from Cannabis sativa L. J. Biol. Chem. 279, 39767–39774. https://doi.org/10.1074/jbc.M403693200 (2004).
Taura, F. et al. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett. 581, 2929–2934. https://doi.org/10.1016/j.febslet.2007.05.043 (2007).
McPartland, J., Glass, M. & Pertwee, R. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br. J. Pharmacol. 152, 583–593 (2007).
Martin, B. et al. Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists. J. Pharmacol. Exp. Ther. 290, 1065–1079 (1999).
Thakur, G. A., Duclos, R. I. & Makriyannis, A. Natural cannabinoids: templates for drug discovery. Life Sci. 78, 454–466. https://doi.org/10.1016/j.lfs.2005.09.014 (2005).
De Meijer, E. P. M. & Hammond, K. M. The inheritance of chemical phenotype in Cannabissativa L. (V): regulation of the propyl-/pentyl cannabinoid ratio, completion of a genetic model. Euphytica 210, 291–307. https://doi.org/10.1007/s10681-016-1721-3 (2016).
Shoyama, Y., Hirano, H., Makino, H., Umekita, N. & Nishioka, I. Cannabis. X. The isolation and structures of four new propyl cannabinoid acids, tetrahydrocannabivarinic acid, cannabidivarinic acid, cannabichromevarinic acid and cannabigerovarinic acid, from Thai Cannabis, “Meao variant”. Chem. Pharm. Bull. 25, 2306–2311 (1977).
Smith, R. Identification of butyl cannabinoids in marijuana. J. Forensic Sci. 42, 610–618. https://doi.org/10.1520/JFS14173J (1997).
Vree, T., Breimer, D., Ginneken, C. & Rossum, J. Identification in hashish of tetrahydrocannabinol, cannabidiol and cannabinol analogues with a methyl side-chain. J. Pharm. Pharmacol. 24, 7–12. https://doi.org/10.1111/j.2042-7158.1972.tb08857.x (1972).
Citti, C. et al. A novel phytocannabinoid isolated from Cannabissativa L. with an in vivo cannabimimetic activity higher than Δ 9-tetrahydrocannabinol: Δ 9-tetrahydrocannabiphorol. Sci. Rep. 9, 20335. https://doi.org/10.1038/s41598-019-56785-1 (2019).
Hillig, K. W. & Mahlberg, P. G. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am. J. Bot. 91, 966–975. https://doi.org/10.3732/ajb.91.6.966 (2004).
Welling, M. T., Liu, L., Raymond, C. A., Ansari, O. & King, G. J. Developmental plasticity of the major alkyl cannabinoid chemotypes in a diverse Cannabis genetic resource collection. Front. Plant Sci. 9, 1510. https://doi.org/10.3389/fpls.2018.01510 (2018).
Welling, M., Liu, L., Shapter, T., Raymond, C. & King, G. Characterisation of cannabinoid composition in a diverse Cannabissativa L. germplasm collection. Euphytica 208, 463–475. https://doi.org/10.1007/s10681-015-1585-y (2016).
Kai, G. et al. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab. Eng. 13, 319–327. https://doi.org/10.1016/j.ymben.2011.02.003 (2011).
Luo, X. et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 567, 123–126. https://doi.org/10.1038/s41586-019-0978-9 (2019).
Marks, M. D. et al. Identification of candidate genes affecting Δ9-tetrahydrocannabinol biosynthesis in Cannabis sativa. J. Exp. Bot. 60, 3715–3726. https://doi.org/10.1093/jxb/erp210 (2009).
Stout, J. M., Boubakir, Z., Ambrose, S. J., Purves, R. W. & Page, J. E. The hexanoyl-CoA precursor for cannabinoid biosynthesis is formed by an acyl-activating enzyme in Cannabis sativa trichomes. Plant J. 71, 353–365. https://doi.org/10.1111/j.1365-313X.2012.04949.x (2012).
Laverty, K. U. et al. A physical and genetic map of Cannabis sativa identifies extensive rearrangements at the THC/CBD acid synthase loci. Genome Res. 29, 146–156. https://doi.org/10.1101/gr.242594.118 (2019).
Grassa, C. J. et al. A complete Cannabis chromosome assembly and adaptive admixture for elevated cannabidiol (CBD) content. BioRxiv https://doi.org/10.1101/458083 (2018).
Pacifico, D. et al. Genetics and marker-assisted selection of the chemotype in Cannabis sativa L. Mol. Breed. 17, 257–268. https://doi.org/10.1007/s11032-005-5681-x (2006).
Kim, J. & Buell, C. R. A revolution in plant metabolism: genome-enabled pathway discovery. Plant Physiol. 169, 1532–1539. https://doi.org/10.1104/pp.15.00976 (2015).
Tripathi, S., Jadaun, J. S., Chandra, M. & Sangwan, N. S. Medicinal plant transcriptomes: the new gateways for accelerated understanding of plant secondary metabolism. Plant. Genet. Resour. 14, 256–269. https://doi.org/10.1017/S1479262116000162 (2016).
Zhao, N., Wang, G., Norris, A., Chen, X. & Chen, F. Studying plant secondary metabolism in the age of genomics. Crit. Rev. Plant Sci. 32, 369–382. https://doi.org/10.1080/07352689.2013.789648 (2013).
Rastogi, S. et al. Unravelling the genome of holy basil: an “incomparable” “elixir of life” of traditional Indian medicine. BMC Genomics 16, 413. https://doi.org/10.1186/s12864-015-1640-z (2015).
Liu, X. et al. De novo transcriptome of Brassicajuncea seed coat and identification of genes for the biosynthesis of flavonoids. PLoS ONE 8, e71110. https://doi.org/10.1371/journal.pone.0071110 (2013).
Reddy, T. V. & Saiprasad, G. V. S. Identification of SNPs in nicotine biosynthesis related genes by targeted re-sequencing of TILLING population and germplasm with varying nicotine levels in tobacco. Euphytica 203, 659–671. https://doi.org/10.1007/s10681-014-1300-4 (2015).
Li, D. et al. Targeted integration of RNA-seq and metabolite data to elucidate curcuminoid biosynthesis in four curcuma species. Plant Cell Physiol. 56, 843–851. https://doi.org/10.1093/pcp/pcv008 (2015).
Hirsch, C. N. & Buell, R. C. Tapping the promise of genomics in species with complex, nonmodel genomes. Annu Rev Plant Biol 64, 89–110 (2013).
Hartwig, B., James, G. V., Konrad, K., Schneeberger, K. & Turck, F. Fast isogenic mapping-by-sequencing of ethyl methanesulfonate-induced mutant bulks. Plant Physiol. 160, 591–600. https://doi.org/10.1104/pp.112.200311 (2012).
Varshney, R. K., Glaszmann, J.-C., Leung, H. & Ribaut, J.-M. More genomic resources for less-studied crops. Trends Biotechnol. 28, 452–460. https://doi.org/10.1016/j.tibtech.2010.06.007 (2010).
Welling, M. T. et al. A belated green revolution for Cannabis: virtual genetic resources to fast-track cultivar development. Front. Plant Sci. 7, 1113. https://doi.org/10.3389/fpls.2016.01113 (2016).
Ries, D., Holtgräwe, D., Viehöver, P. & Weisshaar, B. Rapid gene identification in sugar beet using deep sequencing of DNA from phenotypic pools selected from breeding panels. BMC Genomics 17, 236. https://doi.org/10.1186/s12864-016-2566-9 (2016).
Yang, J. et al. Extreme-phenotype genome-wide association study (XP-GWAS): a method for identifying trait-associated variants by sequencing pools of individuals selected from a diversity panel. Plant J. 84, 587–596. https://doi.org/10.1111/tpj.13029 (2015).
Myles, S. et al. Association mapping: critical considerations shift from genotyping to experimental design. Plant Cell 21, 2194–2202. https://doi.org/10.1105/tpc.109.068437 (2009).
Song, K., Li, L. & Zhang, G. Coverage recommendation for genotyping analysis of highly heterologous species using next-generation sequencing technology. Sci. Rep. 6, 35736. https://doi.org/10.1038/srep35736 (2016).
Capistrano-Gossmann, G. G. et al. Crop wild relative populations of Beta vulgaris allow direct mapping of agronomically important genes. Nat. Commun. 8, 15708. https://doi.org/10.1038/ncomms15708 (2017).
Tran, H. T. et al. SNP in the Coffea arabica genome associated with coffee quality. Tree Genet. Genomes 14, 72. https://doi.org/10.1007/s11295-018-1282-9 (2018).
De Meijer, E. P. M. et al. The inheritance of chemical phenotype in Cannabis sativa L. Genetics 163, 335–346. https://doi.org/10.1007/s10681-005-1164-8 (2003).
Van Bakel, H. et al. The draft genome and transcriptome of Cannabis sativa. Genome Biol. 12, R102. https://doi.org/10.1186/gb-2011-12-10-r102 (2011).
Staginnus, C., Zörntlein, S. & de Meijer, E. A PCR marker linked to a THCA synthase polymorphism is a reliable tool to discriminate potentially THC-rich plants of Cannabis sativa L. J. Forensic Sci. 59, 919–926. https://doi.org/10.1111/1556-4029.12448 (2014).
Hübner, S. et al. Sunflower pan-genome analysis shows that hybridization altered gene content and disease resistance. Nat. Plants 5, 54. https://doi.org/10.1038/s41477-018-0329-0 (2019).
Fellermeier, M., Eisenreich, W., Bacher, A. & Zenk, M. H. Biosynthesis of cannabinoids: incorporation experiments with 13C-labeled glucoses. Eur. J. Biochem. 268, 1596–1604. https://doi.org/10.1046/j.1432-1327.2001.02030.x (2001).
Xu, H. et al. Characterization of the formation of branched short-chain fatty acid: CoAs for bitter acid biosynthesis in hop glandular trichomes. Mol. Plant 6, 1301–1317. https://doi.org/10.1093/mp/sst004 (2013).
Koeda, S. et al. Mutation in the putative ketoacyl-ACP reductase CaKR1 induces loss of pungency in Capsicum. Theor. Appl. Genet. 132, 65–80. https://doi.org/10.1007/s00122-018-3195-2 (2019).
Marchive, C., Nikovics, K., To, A., Lepiniec, L. & Baud, S. Transcriptional regulation of fatty acid production in higher plants: molecular bases and biotechnological outcomes. Eur. J. Lipid Sci. Technol. 116, 1332–1343. https://doi.org/10.1002/ejlt.201400027 (2014).
White, S. W., Zheng, J., Zhang, Y.-M. & Rock, C. O. The structural biology of type II fatty acid biosynthesis. Annu. Rev. Biochem. 74, 791–831 (2005).
Fisher, M. et al. The X-ray structure of Brassica napus β-keto acyl carrier protein reductase and its implications for substrate binding and catalysis. Structure 8, 339–347. https://doi.org/10.1016/S0969-2126(00)00115-5 (2000).
Feng, S.-X. et al. Ralstonia solanacearum fatty acid composition is determined by interaction of two 3-ketoacyl-acyl carrier protein reductases encoded on separate replicons. BMC Microbiol. 15, 223. https://doi.org/10.1186/s12866-015-0554-x (2015).
Rafferty, J. B. et al. Common themes in redox chemistry emerge from the X-ray structure of oilseed rape (Brassica napus) enoyl acyl carrier protein reductase. Structure 3, 927–938. https://doi.org/10.1016/S0969-2126(01)00227-1 (1995).
Wang, H. & Cronan, J. E. Only one of the two annotated Lactococcuslactis fabG genes encodes a functional β-ketoacyl-acyl carrier protein reductase. Biochemistry 43, 11782–11789. https://doi.org/10.1021/bi0487600 (2004).
Beld, J., Lee, D. J. & Burkart, M. D. Fatty acid biosynthesis revisited: structure elucidation and metabolic engineering. Mol. Biosyst. 11, 38–59. https://doi.org/10.1039/C4MB00443D (2015).
Leonard, J. M., Knapp, S. J. & Slabaugh, M. B. A Cuphea β-ketoacyl-ACP synthase shifts the synthesis of fatty acids towards shorter chains in Arabidopsis seeds expressing Cuphea FatB thioesterases. Plant J. 13, 621–628. https://doi.org/10.1046/j.1365-313X.1998.00066.x (1998).
González-Thuillier, I., Venegas-Calerón, M., Garcés, R., von Wettstein-Knowles, P. & Martínez-Force, E. Sunflower (Helianthus annuus) fatty acid synthase complex: enoyl-[acyl carrier protein]-reductase genes. Planta 241, 43–56. https://doi.org/10.1007/s00425-014-2162-7 (2015).
Hanh, N. P. K., Hwang, J. Y. & Nam, D. H. Biosynthesis of methoxymalonyl-acyl carrier protein (ACP) as an extender unit for bafilomycin polyketide in Streptomyces griseus DSM 2608. Biotechnol. Bioprocess Eng. 23, 693–703. https://doi.org/10.1007/s12257-018-0427-x (2018).
Wu, K., Chung, L., Revill, W. P., Katz, L. & Reeves, C. D. The FK520 gene cluster of Streptomyceshygroscopicus var. ascomyceticus (ATCC 14891) contains genes for biosynthesis of unusual polyketide extender units. Gene 251, 81–90. https://doi.org/10.1016/S0378-1119(00)00171-2 (2000).
Schorling, S., Vallée, B., Barz, W. P., Riezman, H. & Oesterhelt, D. Lag1p and Lac1p are essential for the acyl-CoA–dependent ceramide synthase reaction in Saccharomyces cerevisae. Mol. Biol. Cell 12, 3417–3427. https://doi.org/10.1091/mbc.12.11.3417 (2001).
Ito, T. et al. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. U.S.A. 98, 4569–4574. https://doi.org/10.1073/pnas.061034498 (2001).
Pearce, S., Knox, M., Ellis, T., Flavell, A. & Kumar, A. Pea Ty1-copia group retrotransposons: transpositional activity and use as markers to study genetic diversity in Pisum. Mol. Gen. Genet. 263, 898–907. https://doi.org/10.1007/s004380000257 (2000).
Tatout, C., Warwick, S., Lenoir, A. & Deragon, J.-M. SINE insertions as clade markers for wild crucifer species. Mol. Biol. Evol. 16, 1614–1614 (1999).
Thachuk, C. et al. Core Hunter: an algorithm for sampling genetic resources based on multiple genetic measures. BMC Bioinform. 10, 243. https://doi.org/10.1186/1471-2105-10-243 (2009).
Faeti, V., Mandolino, G. & Ranalli, P. Genetic diversity of Cannabis sativa germplasm based on RAPD markers. Plant Breed. 115, 367–370. https://doi.org/10.1111/j.1439-0523.1996.tb00935.x (1996).
Welling, M. T. et al. Complex patterns of cannabinoid alkyl side-chain inheritance in Cannabis. Sci. Rep. 9, 11421. https://doi.org/10.1038/s41598-019-47812-2 (2019).
Mediavilla, V., Jonquera, M., Schmid-Slembrouck, I. & Soldati, A. Decimal code for growth stages of hemp (Cannabissativa L.). J. Ind. Hemp 5, 68–74 (1998).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. https://doi.org/10.1101/gr.107524.110 (2010).
Li, H. Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics 30, 2843–2851. https://doi.org/10.1093/bioinformatics/btu356 (2014).
Li, H. et al. The Sequence Alignment/Map format and SAM tools. Bioinformatics 25, 2078–2079. https://doi.org/10.1093/bioinformatics/btp352 (2009).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26. https://doi.org/10.1038/nbt.1754 (2011).
Solovyev, V., Kosarev, P., Seledsov, I. & Vorobyev, D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 7, S10. https://doi.org/10.1186/gb-2006-7-s1-s10 (2006).
Zhang, Z., Schwartz, S., Wagner, L. & Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7, 203–214. https://doi.org/10.1089/10665270050081478 (2000).
Acknowledgements
The authors would like to acknowledge Ecofibre for generously providing Southern Cross University access to the Ecofibre Global Germplasm Collection, as well as for providing insightful information necessary for the selection of accessions. The authors are also grateful for the material and financial support provided by Ecofibre. Finally, the authors would like to acknowledge Dr. Abdul Baten for providing support with the bioinformatic analysis of bulked DNA samples.
Author information
Authors and Affiliations
Contributions
M.W. prepared the manuscript, designed and performed experiments, including the development of the diversity panel as well as chemical and bioinformatics analysis. L.L. contributed to the development of analytical procedures and performed detailed review and revision of the manuscript. T.K. contributed to the analysis of chemotype-associated variants as well as review and revision of the manuscript. R.M. contributed to the bioinformatics analysis of intervals, review and revision of the manuscript. O.A. provided background information and performed review and revision of the manuscript. G.K. provided substantial contributions to conception and design of the research project and performed detailed review and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Southern Cross University receives funding from the commercial entity Ecofibre Pty Ltd. Ananda Hemp Ltd are a subsidiary company of Ecofibre. Matthew Welling received a stipend from Ecofibre. Omid Ansari is an employee of Ecofibre. Lei Liu, Tobias Kretzschmar, Ramil Mauleon and Graham J. King declare no potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Welling, M.T., Liu, L., Kretzschmar, T. et al. An extreme-phenotype genome‐wide association study identifies candidate cannabinoid pathway genes in Cannabis. Sci Rep 10, 18643 (2020). https://doi.org/10.1038/s41598-020-75271-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75271-7
This article is cited by
-
Fine mapping of ClLOX, a QTL for powdery mildew resistance in watermelon (Citrullus lanatus L.)
Theoretical and Applied Genetics (2024)
-
Using a global diversity panel of Cannabis sativa L. to develop a near InfraRed-based chemometric application for cannabinoid quantification
Scientific Reports (2023)
-
Evaluation of nine statistics to identify QTLs in bulk segregant analysis using next generation sequencing approaches
BMC Genomics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.