Abstract
Invasive fungal disease (IFD) early diagnosis improves hematological patient survival. Non-culture-based methods may reduce diagnostic time to identify IFD. As complex data on the value of 1,3-β-d-glucan (BDG) from bronchoalveolar lavage fluid (BALF) compared to serum for the most frequent invasive pulmonary aspergillosis (IPA) diagnosis are scarce, particularly including evaluation of potential factors adversely affecting BDG assay, we provided prospective single-center analysis evaluating 172 episodes of pulmonary infiltrates with BDG detection in BALF and serum samples collected in parallel among hematological patients from 2006 to 2015. Proven and probable IPA were documented in 13.4% of the episodes. Sensitivity (SEN), specificity (SPE), positive and negative predictive value (PPV; NPV), and diagnostic odds ratio (DOR) of the BDG assay using standard (80 pg/ml) cut-off for BALF were: 56.5%; 83.2%; 34.2%; 92.5%, and 6.5, respectively, and for serum were: 56.5%; 82.6%; 33.3%; 92.5%, and 6.2, respectively. The same BDG assay parameters employing a calculated optimal cut-off for BALF (39 pg/ml) were: 78.3%; 72.5%; 30.5%; 95.6%, and 9.5, respectively; and for serum (40 pg/ml) were: 73.9%; 69.1%; 27.0%; 94.5%, and 6.3, respectively. While identifying acceptable SEN, SPE, and DOR, yet low PPV of both BALF and serum BDG assay for IPA diagnosis, neither the combination of both materials nor the new optimal BDG cut-off led to significant test quality improvement. Absolute neutrophil count and aspirated BALF volume with a significant trend affected BDG assay performance. The BDG test did not outperform galactomannan assay.
Similar content being viewed by others
Introduction
Invasive fungal diseases (IFD) are life-threatening infections in patients with hematological malignancies1,2,3. Pulmonary infiltrates could represent an IFD warning sign, and differential diagnosis is crucial for the early start of preemptive antibiotic and antifungal therapy4,5,6,7.
Non-culture-based methods with their high sensitivity (SEN) and specificity (SPE) reduce diagnostic time to identify IFD. 1,3-β-d-glucan (BDG), a major cell wall component of most fungal species, is released into blood and tissues during IFD (except mucormycetes and Cryptococcus spp.). The Fungitell test is the only BDG antigenemia assay recommended4,5,8,9,10. Galactomannan (GM) is well established as a reliable BALF and serum marker in early detection of invasive aspergillosis4,5,9,11,12,13.
Several studies and meta-analyses concerning serum BDG assay performance in IFD diagnosis with variable outcomes have been provided8,14,15,16,17,18,19,20,21. Data regarding BDG from bronchoalveolar lavage fluid (BALF) for differential diagnosis of pulmonary infiltrates exists although limited and heterogeneous22,23,24,25,26. Heterogeneity is caused by various factors and lacks a complex analysis. Data is also insufficient regarding added value of a BDG combination from serum and BALF23,24,25,26.
We therefore recognized the need to evaluate and reconsider the role of BDG from serum and BALF in detection of early invasive pulmonary aspergillosis (IPA) the most common IFD. Reassessing BDG detection accuracy regarding a commonly employed 80 pg/ml cut-off, we implemented superior measures to propose a new optimal BDG cut-off value in serum and BALF samples for IPA diagnosis. Our secondary goal was to analyze defined clinical factor impact on test accuracy with a standard of 80 pg/ml cut-off. Furthermore, we sought to identify and qualify increased IPA detection accuracy when serum and BALF samples were obtained in parallel.
Methods
Study population
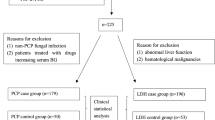
Our prospective cohort study involved consecutive non-selected hematological patients treated at our department from 2006 to 2015. We reviewed database clinical and laboratory records concerning epidemiology, diagnosis, and therapy of patients who underwent bronchoscopy with BALF and serum testing for both BDG and GM at exactly the same time to evaluate pulmonary infiltrates on chest high resolution computed tomography (HRCT). Patients with repeated sampling were included in the analysis if the BAL was performed on a clearly new presentation of pulmonary infiltrate evaluated by an expert radiologist. Episodes with apparent false-positive results for GM in the serum caused by the administration of GM-positive tested lots of piperacillin-tazobactam or Plasma-Lyte solution (Baxter Healthcare) were excluded from the analysis. The sample collection and research were approved by the Local Ethics Committee of the University Hospital Brno, Czech Republic, Number 01-170920/EK. All research was performed in accordance with relevant guidelines and regulations. Informed consent was obtained from all participants.
Fiberoptic bronchoscopy and sample investigation
The fiberoptic bronchoscopy site was guided by the HRCT pathological finding. Eight to 10 sequential, 20-ml aliquots of sterile saline solution were infused into the lower respiratory tract, and each aliquot was immediately aspirated. The first bronchial sample aliquot's return was processed separately from subsequent aliquot returns, which were pooled together and homogenized (BALF). BALF was subjected to cytology assessment, direct examination, bacterial, fungal, mycobacterial culture; galactomannan and BDG detection; polymerase chain reaction (PCR) testing for Aspergillus fumigatus, mucormycetes, Pneumocystis jirovecii, and viral pathogens. Serum samples obtained simultaneously with bronchoscopic material were examined for GM and BDG detection, bacterial and fungal culture.
1,3-β-d-glucan detection
For BDG detection, a commercial kit (Fungitell, Associates of Cape Cod, Inc., Cape Cod, MA, USA) was used according to manufacturer’s instructions for sera samples and equally for BALF samples. BALF specimens were centrifuged at 1000 rpm for 10 min, and supernatant was used for BDG detection. Thereafter, the BALF samples were equally treated as sera samples. Samples were frozen at − 20 °C until BDG level determination. A positive test result was defined as a sample with cut-off level ≥ 80 pg/ml for both, serum and BALF. A detailed methodology of BDG assessment in serum and BALF has been previously described27.
Case definition and important clinical parameters
Each case was classified as proven, probable, possible, or no IFD according to EORTC/MSG (European Organization for Research and Treatment of Cancer/Mycoses Study Group) criteria4, however Fungitell test results were not included as one of the microbiological criteria. Cases classified as proven and probable IFD were considered true-positives; no IFD as true-negatives. Eighty-three episodes with possible IFD, 8 episodes with invasive mucormycosis, one episode caused by Alternaria sp., and 18 Pneumocystis pneumonia episodes were not included in our final analysis.
We not only assessed BDG detection accuracy in BALF and serum with 80 pg/ml cut-off, moreover we sought to set up new accurate IFD detection cut-off levels based on sensitivity (SEN) and specificity (SPE) optimal combination. Parameters possibly affecting BDG assay were analyzed from BALF: Antifungals administration and its duration prior to sampling, concomitant Candida spp. positive culture from BALF or oral cavity, positive bacterial culture from BALF, absolute neutrophil value, and aspirated BALF volume; and from serum: Antifungals administration and its duration prior to sampling, concomitant bacteremia, and absolute neutrophil value. Furthermore, we compared BDG to GM test accuracy in both, BALF and serum, for IFD diagnosis.
Statistical analysis
Continuous variables were compared using the Mann–Whitney U test and the Spearman correlation coefficient (RS). Categorical parameters relation was evaluated using Pearson’s Chi-squared and Kendall’s tau tests. For all analyses, α = 0.05 was used as a level of statistical significance. Sensitivity (SEN), specificity (SPE), negative predictive value (NPV), positive predictive value (PPV), diagnostic odds ratio (DOR), accuracy, were calculated for both serum and BALF BDG assay, and combination of both materials. A new BDG-max variable was evaluated based on the higher BDG value of the pair samples, BALF, and serum. A receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to estimate BDG assay discriminatory capability performed in samples of BALF and serum for IFD detection. The influence of all monitored variables on IFD prediction was evaluated using a multidimensional logistic regression model. For statistical analysis, software R version 3.5.2 was used.
Results
In total, 172 unique episodes (from 152 adult patients) were analyzed with characteristics described in Table 1. Proven, probable, and no IFDs were documented in 8 (5%), 15 (9%), and 149 (86%) episodes, respectively. Invasive aspergillosis was present in all eight proven and fifteen probable cases.
BDG assay evaluated by a standard cut-off of 80 pg/ml
BDG values median in BALF and serum was 16.0 pg/ml (min-0; max-1594) and 22.0 pg/ml (min-0; max-1138), respectively. GM values median in BALF and serum was 0.13 IP (index of positivity) (min-0.04; max-1.98) and 0.13 IP (min-0.03; max-0.52), respectively. The correlation between BDG and GM levels in BALF and serum is indicated in Fig. 1.
Correlation between continuous parameters possibly influencing the BDG assay quality in IFD diagnosing. In this figure shows Spearman correlation matrix representing the relation of continuous parameters evaluated by the Spearman coefficient. The diagonal from the upper left corner to the lower right corner contains frequency histograms of each variable. In the histogram “IFD diagnosis”, the episode numbers are divided according to the probability of IFD: 0—no IFD; 2—probable IFD; 3—proven IFD. On the bottom of the diagonal, there are the bivariate scatter dot plots with fitted lines displayed of the variable pairs. On the top of the diagonal, the pairwise correlations of variables (Spearman rank correlation coefficients; S) are with the significance level represented by stars: *p < 0.05; **p < 0.01; ***p < 0.001. BDG, 1,3-β-d-glucan; BALF, bronchoalveolar lavage fluid; IFD, invasive fungal disease; GM, galactomannan; BAL, bronchoalveolar lavage.
Median BDG levels in proven and probable episodes versus no IFD were in BALF 111 versus 13 pg/ml and in serum 113 versus 20 pg/ml. The correlation between BALF or serum BDG levels and IFD probabilities (RS = 0.255; and RS = 0.255, respectively) appears in Fig. 1.
Based on ROC analysis, BDG assay performance for BALF and serum indicated SEN (56.5%; and 56.5%), PPV (34.2%; and 33.3%), AUC (0.671; and 0.677), SPE (83.2%; and 82.6%), NPV (92.5%; and 92.5%), and IFD prediction accuracy 0.8 with a standard cut-off of 80 pg/ml (Table 2). BDG-max assay affirmed SEN (65.2%), SPE (73.2%), and 0.6 diagnostic accuracy (Table 2).
BDG assay evaluated by a new suggested optimal cut-off
Based on ROC analysis, our challenging task was to determine optimal BDG cut-off value in both serum and BALF. We identified new BDG cut-offs for both BALF (39 pg/ml) and serum (40 pg/ml). The new suggested BALF and serum value reached SEN (78.3% and 73.9%), SPE (72.5% and 69.1%), and DOR (9.5 and 6.3) (Table 2).
Clinical factors possibly influencing BDG performance accuracy
We did not verify any significant relationship between probability of final IFD diagnosis and concomitant Candida spp. positive culture of oral cavity or BALF at time of sampling (ρ = 5.7, and 2.0; p > 0.05; respectively) (Fig. 2).
Correlation between categorical parameters evaluated by using the Pearson’s Chi-squared tests (upper right) and Kendall’s tau tests (lower left). In this figure represents the relation between categorical parameters. The diagonal from the upper left corner to the lower right corner contains frequency histograms of each variable. In the histogram “IFD diagnosis”, episode numbers are divided according to the probability of IFD: 0—no IFD; 2—probable IFD; 3—proven IFD. Concerning “Antifungals”: 0—no antifungals prior to sampling; 1—any antifungals prior to sampling; “Antifungals (type)”: 0—no antifungals; 1—fluconazole; 2—antifungals with broader spectrum; other histograms: 0—absent; 1—present. The Pearson’s Chi-squared tests (on the right top of the diagonal) measure the strength of a linear association between categorical variables presented by Pearson correlation coefficient (ρ) valued between + 1 and − 1, where 1 is a total positive linear correlation, 0 is no linear correlation, and − 1 is total negative linear correlation. The Kendall’s tau tests (on the bottom left of the diagonal) represent the ordinal dependence between two measured quantities based on the τ coefficient. Each significance level is represented by stars: *p < 0.05; **p < 0.01; ***p < 0.001. BDG, 1,3-β-d-glucan; BALF, bronchoalveolar lavage fluid; IFD, invasive fungal disease; GM, galactomannan; BAL, bronchoalveolar lavage.
Similarly, the positivity of BALF bacterial culture and bacteremia did not suggest any significant correlation with IFD probability (ρ = 2.8, and 1.5, respectively; p > 0.05). (Fig. 2).
Significant correlation between lower absolute neutrophil value at the time of sampling and the higher degree of IFD probability was substantiated in our analysis (RS = −0.223; p < 0.01) (Fig. 1).
More effective agents with a broader antifungal spectrum were more frequently administered at the time of BALF among episodes finally defined as proven or probable IFD with statistical significance (p < 0.01) (Fig. 2). Prolonged antifungals administration prior to sampling correlated with higher serum BDG levels (RS = 0.136) (Fig. 1).
Eventually, episodes with higher aspirated BALF volume > 80 ml had a lower median of BALF-BDG value compared to episodes with lower aspirated BALF volume ≤ 80 ml (10 pg/ml vs. 21 pg/ml). Moreover, lower BALF BDG levels corresponded to higher aspirated BALF volume in the Spearman correlation test, yet results were not statistically significant (RS = −0.059; p > 0.05) (Fig. 1).
Logistic regression model evaluating clinical factors potentially influencing BDG and GM test accuracy
The model evaluating only BDG revealed an absolute number of neutrophils as a factor influencing BDG predictive value for IPA (Table 3). Higher absolute neutrophil count decreased BDG assay performance predictive value with statistical trend (p = 0.099). Additionally, we affirmed a trend in correlation between higher aspirated BALF volume and reduced opportunity to correctly predict IPA using BALF BDG (p = 0.085). No other factors significantly affected BDG assay prediction for IPA.
Finally, our multivariate analysis, most consistent with real clinical practice, evaluated the quality of both BDG and GM diagnostic tests together during IPA diagnosis. Despite both tests being statistically significant, the GM test confirmed a 203-fold higher predictive value for serum and a 19-fold higher value for BALF compared to the BDG test (Table 4). Furthermore, there was correlation between higher BALF volume and reduced IPA predictive probability using the BDG test (p = 0.069) (Table 4).
Discussion
Our study substantiates efficacy of concomitantly obtained BALF and serum BDG samples from a large set of unselected consecutive hematological patients with pulmonary infiltrates for IFD diagnosis. BDG and GM values levels significantly correlated with each other in both serum and BALF (Fig. 1). We documented a substantially higher median of both BALF and serum BDG levels in episodes of proven and probable IFDs compared to no IFDs (p < 0.001). Moreover, the correlation between BALF or serum BDG levels and IFD probability was evidenced with statistical significance (p < 0.001; and p < 0.05; respectively) (Fig. 1). BDG displayed similar DOR in BALF compared to serum (6.5 vs. 6.2) at the same levels of SEN and SPE (57% and 83%). The BDG-max assay affirmed higher SEN (65%) but lower SPE (73%) and DOR (5.1).
BDG test quality is affected by a number of different factors. First, IFD definition may vary among studies. A vast majority of studies (including our investigation) did not include possible IFD in their analysis23,24,25. The quality of the BALF and serum BDG test did not significantly differ in the cohort with versus without 83 possible IFD in our analysis (DOR—6.9 vs. 6.5; and 4.2 vs. 6.2).
Additionally, degree and type of immunosuppression may facilitate BDG test accuracy discrepancies among studies. Our results are consistent with published data of Rose et al. and Theel et al., who reported BALF and serum BDG sensitivity in the range of 50–53%, and 40–55%, with a similar spectrum of hematological patients, respectively23,25. Most published studies used the same Fungitell cut-off level (≥ 80 pg/ml) for both serum and BALF as our study. He et al. in a large meta-analysis, reported serum BDG diagnostic accuracy and set the optimal cut-off level of 60 pg/ml as optimum for distinguishing patients with and without IFD16. Similar to He's study, we confirmed a lower optimal cut-off compared to the standard cut-off in both serum and BALF with better SEN and DOR, yet slightly lower SPE (Table 2).
Candida spp. colonization or infection
Concomitant Candida spp. colonization or non-invasive respiratory infections may influence BALF BDG specimen false positivity and assay accuracy. Therefore, BALF BDG test SPE differences between published studies (39–68%) and our data (83%) could be caused by the variable frequency of Candida spp. positive cultures from BALF in episodes with no IFD compared to our study (32–43% vs. 6%)23,24,25. Furthermore, our study recognized a lower proportion of Candida spp. positive cultures from BALF in Fungitell positive episodes with no IFD compared to Theel's study (16–38%)25. Nonetheless, based on a multivariate analysis and in accordance with Rose's study23, we did not substantiate concomitant positive Candida spp. culture from BALF or oral cavity as a factor significantly affecting BALF BDG assay performance (Table 3).
Bacterial infection
In concordance with published data, we confirmed BDG test lower predictive value in episodes with positive bacterial culture from BALF (Enterococcus sp., Pseudomonas aeruginosa, Klebsiella sp., Streptococcus sp.) or concomitant bacteremia (Enterococcus sp., Klebsiella sp.), although without statistical significance using the multivariate analysis (Table 3)28,29,30. As with our data, Rose's BALF BDG assay performance was not significantly affected by concurrent pulmonary bacterial infection23.
Neutropenia
Published studies analyzing a BALF or serum BDG test in non-neutropenic patients or in cohorts with a lower proportion of neutropenic patients determined a lower SPE (26–65%) compared to our study17,18,19,23,24,25,26. Our analysis affirmed a higher neutrophil count during sampling as a factor adversely affecting BDG test performance within an IFD prediction with a statistical trend in significance (Table 3). To our knowledge, such a comprehensive analysis evaluating BDG test accuracy within an IFD diagnosis according to degree of neutropenia has not yet been conducted.
Antifungal therapy
Antifungal therapy was significantly associated with false-negative BDG results in both BALF and serum in Rose's analysis and with serum in Ostrosky-Zeichner's study20,23. In contrast to published studies, we did not determine any significant relationship between antifungal administration or antifungal treatment duration and BALF or serum BDG assay performance in the multivariate analysis (Table 3). However, we are aware of our high proportion of episodes treated with antifungals at the time of cohort sampling reflecting clinical practice. For this reason, stricter criteria are set for BDG diagnostic test performance with IFD diagnosis.
Sampling method and BAL standardization
Sampling method and timing could be another source of heterogeneity and affect fungal antigens detection accuracy in both BALF and serum. Theel et al. analyzed serum samples collected within 72 h of the BAL, which was at variance with our study sampling BALF and serum at precisely the same time25. Other investigations failed to note sampling time23,24. As predicted, specificity increased up to 100% by using two serum BDG test sequential positivities31,32.
BAL procedure standardization is a significant factor. Instilled solution during bronchoscopy ranging from 100 to 200 ml represents one of the crucial factors contributing to the final amount of aspirated BALF volume and consequently to BDG concentration and assay reactivity21,25. Notably, specific published data concerning BDG assay is lacking. Comparable to Racil's study evaluating the BALF GM test, our analysis affirmed better BALF BDG performance in patients with lower aspirated BALF volume (Tables 3, 4)33.
In our cohort, both BDG and GM antigens were simultaneously investigated from serum and BALF. In concordance with our results, a galactomannan test substantiated superiority to the BDG tested in both BALF and serum when diagnosing invasive pulmonary aspergillosis in literature26,34.
Our study highlights the advantage of BALF and serum BDG concomitant obtained samples including one of the largest published sets of non-selected consecutive hematological patients with pulmonary infiltrates relating to IFD diagnosis. Furthermore, our analysis precisely assesses predictable factors affecting BDG test accuracy.
Nonetheless, potential study limitations reflecting real clinical practice depict a low number of proven/probable IFDs, a high proportion of antifungal therapy at the time of sampling, and BALF specimen serum cut-off implementation without updated established criteria. Moreover, patients were only tested once with serum Fungitell assay. Although our study is a single-center analysis, it is a homogeneous, reproducible, and verifiable cohort.
Conclusions
In conclusion, we confirmed acceptable SEN, SPE, DOR, and a low PPV of both BALF and serum BDG assay for pulmonary IPA diagnosis. Furthermore, we recorded high NPV for both BALFs and sera, predisposing a basic BDG assay utility to exclude IFDs indicating serum value with patient IFD screenings. BDG test sensitivity and DOR were not substantially increased by BALF compared to sole serum testing, and their combination did not improve test quality. Dedicated efforts to determine optimal BDG limit value did not facilitate significant test quality improvement.
We confirmed that: (1) absolute neutrophil count at the time of sampling and (2) aspirated BALF volume both considerably affect assay performance.
Consequently, although BALF and serum GM and BDG values correlated with each other, BDG continued to reveal reduced test quality compared to GM in IPA diagnosis. Our study fully supports the recent Infectious Diseases Society of America (IDSA) and EORTC/MSG recommendations to avoid using BDG for defining IFD and to restrict use to specific clinical settings in conjunction with other clinical findings.
References
Herbrecht, R., Bories, P., Moulin, J.-C., Ledoux, M.-P. & Letscher-Bru, V. Risk stratification for invasive aspergillosis in immunocompromised patients. Ann. N. Y. Acad. Sci. 1272, 23–30 (2012).
Pagano, L. et al. Risk assessment and prognostic factors for mould-related diseases in immunocompromised patients. J. Antimicrob. Chemother. 66(Suppl 1), i5-14 (2011).
Racil, Z. et al. Invasive aspergillosis in patients with hematological malignancies in the Czech and Slovak republics: fungal infection database (FIND) analysis, 2005–2009. Int. J. Infect. Dis. 17, e101-109 (2013).
De Pauw, B. et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46, 1813–1821 (2008).
Donnelly, J. P. et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciz1008 (2019).
Neofytos, D. et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 48, 265–273 (2009).
Freifeld, A. G. et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 52, 427–431 (2011).
White, S. K., Walker, B. S., Hanson, K. E. & Schmidt, R. L. Diagnostic accuracy of β-d-glucan (fungitell) testing among patients with hematologic malignancies or solid organ tumors: a systematic review and meta-analysis. Am. J. Clin. Pathol. 151, 275–285 (2019).
Patterson, T. F. et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 63, e1–e60 (2016).
Pappas, P. G. et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 62, e1-50 (2016).
D’Haese, J. et al. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. J. Clin. Microbiol. 50, 1258–1263 (2012).
Leeflang, M. M. G. et al. Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD007394.pub2 (2015).
Mennink-Kersten, M. A. S. H., Donnelly, J. P. & Verweij, P. E. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4, 349–357 (2004).
Hou, T.-Y. et al. The screening performance of serum 1,3-β-d-glucan in patients with invasive fungal diseases: a meta-analysis of prospective cohort studies. PLoS ONE 10, e0131602 (2015).
Lamoth, F. et al. β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the third European conference on infections in leukemia (ECIL-3). Clin. Infect. Dis. 54, 633–643 (2012).
He, S. et al. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-β-d-glucan for invasive fungal infection: focus on cutoff levels. J. Microbiol. Immunol. Infect. 48, 351–361 (2015).
Azoulay, E. et al. (1, 3)-β-d-glucan assay for diagnosing invasive fungal infections in critically ill patients with hematological malignancies. Oncotarget 7, 21484–21495 (2016).
Levesque, E. et al. Detection of (1,3)-β-d-glucan for the diagnosis of invasive fungal infection in liver transplant recipients. Int. J. Mol. Sci. 18, 862 (2017).
Prattes, J. et al. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am. J. Respir. Crit. Care Med. 190, 922–929 (2014).
Ostrosky-Zeichner, L. et al. Multicenter clinical evaluation of the (1 → 3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41, 654–659 (2005).
Acosta, J. et al. A prospective comparison of galactomannan in bronchoalveolar lavage fluid for the diagnosis of pulmonary invasive aspergillosis in medical patients under intensive care: comparison with the diagnostic performance of galactomannan and of (1 → 3)-β-d-glucan chromogenic assay in serum samples. Clin. Microbiol. Infect. 17, 1053–1060 (2011).
Shi, X.-Y. et al. Diagnostic value of (1 → 3)-β-d-glucan in bronchoalveolar lavage fluid for invasive fungal disease: a meta-analysis. Respir. Med. 117, 48–53 (2016).
Rose, S. R. et al. The utility of bronchoalveolar lavage beta-d-glucan testing for the diagnosis of invasive fungal infections. J. Infect. 69, 278–283 (2014).
Mutschlechner, W. et al. Bronchoalveolar lavage fluid (1,3)β-d-glucan for the diagnosis of invasive fungal infections in solid organ transplantation: a prospective multicenter study. Transplantation 99, e140-144 (2015).
Theel, E. S. et al. Detection of (1, 3)-β-d-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia 175, 33–41 (2013).
Boch, T. et al. Detection of invasive pulmonary aspergillosis in critically ill patients by combined use of conventional culture, galactomannan, 1-3-beta-d-glucan and Aspergillus specific nested polymerase chain reaction in a prospective pilot study. J. Crit. Care 47, 198–203 (2018).
Weinbergerova, B., Kocmanova, I., Racil, Z. & Mayer, J. Serological approaches. Methods Mol. Biol. 1508, 209–221 (2017).
Pickering, J. W., Sant, H. W., Bowles, C. A. P., Roberts, W. L. & Woods, G. L. Evaluation of a (1 → 3)-beta-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 43, 5957–5962 (2005).
Mennink-Kersten, M. A. S. H., Ruegebrink, D. & Verweij, P. E. Pseudomonas aeruginosa as a cause of 1,3-beta-d-glucan assay reactivity. Clin. Infect. Dis. 46, 1930–1931 (2008).
Albert, O. et al. Reactivity of (1 → 3)-β-d-glucan assay in bacterial bloodstream infections. Eur. J. Clin. Microbiol. Infect. Dis. 30, 1453–1460 (2011).
Ellis, M. et al. Assessment of the clinical utility of serial beta-d-glucan concentrations in patients with persistent neutropenic fever. J. Med. Microbiol. 57, 287–295 (2008).
Racil, Z. et al. Difficulties in using 1,3-{beta}-d-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies-high frequency of false-positive results and their analysis. J. Med. Microbiol. 59, 1016–1022 (2010).
Racil, Z. et al. Galactomannan detection in bronchoalveolar lavage fluid for the diagnosis of invasive aspergillosis in patients with hematological diseases-the role of factors affecting assay performance. Int. J. Infect. Dis. 15, e874-881 (2011).
Hoenigl, M. et al. Performance of galactomannan, beta-d-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J. Clin. Microbiol. 52, 2039–2045 (2014).
Acknowledgements
This work was supported by The CzEch Leukemia Study Group—for Life (CELL), the Health Ministry [FNBr, 65269705], and the 2020 Czech Ministry of Education, Youth, and Sports [MUNI/A/1395/2019].
Author information
Authors and Affiliations
Contributions
B.W.: contributed to the study conception and design; performed material preparation, data collection and analysis; wrote the manuscript. T.K., I.K., M.L., Z.P., Z.K., J.M.: contributed to the study conception and design; performed material preparation, data collection and analysis; commented on previous versions of the manuscript; read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weinbergerova, B., Kabut, T., Kocmanova, I. et al. Bronchoalveolar lavage fluid and serum 1,3-β-d-glucan testing for invasive pulmonary aspergillosis diagnosis in hematological patients: the role of factors affecting assay performance. Sci Rep 10, 17963 (2020). https://doi.org/10.1038/s41598-020-75132-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75132-3
This article is cited by
-
Evaluation of the Dynamiker® Fungus (1–3)-β-d-Glucan Assay for the Diagnosis of Invasive Aspergillosis in High-Risk Patients with Hematologic Malignancies
Infectious Diseases and Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.