Abstract
The earth has been undergoing climate change, especially in recent years, driven by increasing concentration of atmospheric carbon dioxide (CO2) and rising earth-surface temperature, which could reduce N allocation to Bt toxin for transgenic Bt crops (Bt crops), but the N fertilization is considered to be an effective method to enhance the C–N balance in Bt crops in the case of elevated CO2 in future. DNA methylation not only in promoterregion but also in codingregion of transgene plays a critical role in transgene expression regulation and silencing of transgenic crops. Recent research has emphasized the risks of increased transgene silencing of Bacillus thuringiensis (Bt) rice under elevated CO2. In this study, the effects of elevated CO2 (vs. ambient CO2) on exogenous Bt toxins and transgene expression in promoterregion and codingregion of Bt rice during tillering stage (cv. HH1 expressing fused Cry1Ab/Cry1Ac) were evaluated under three nitrogen (N) fertilizer rate (1/4, 1 and 2 N levels). The aboveground and belowground biomass, and foliar Bt protein content of Bt rice were all significantly increased with the augmentation of N-fertilizer. And elevated CO2 significantly increased belowground biomass, total soluble protein content, transgene methylation levels in promoterregion (P1), and in total of promoterregion(P1) and codingregion (P2 + P3) (i.e., P1 + P2 + P3) at 1 N level, and it also increased transgene methylation levels in codingregion (P2), and in total of promoterregion and codingregion (P1 + P2 + P3) at 2 N level. In addition, elevated CO2 decreased foliar Bt protein content at 1 N level. The transgene methylation levels in promoterregion and codingregion were negatively correlated with Bt-transgene expression level. The methylation level of cytosines located at CG sites was higher than those at CHG and CHH sites in P1, P2 and P3 fragments regardless of the CO2 or N-fertilizer level. The correlation of transgene mehtylation in promoterregion with transgene expression is even stronger than that in codingregion. These data indicate that N fertilization supply will increase the Bt toxin content in transgenic Bt rice, especially under elevated CO2.
Similar content being viewed by others
Introduction
Global atmospheric carbon dioxide (CO2) concentration has increased from 280 ppm in pre-industrial to 404 ppm currently1. It has been projected that it will grow up to 700 ppm at the end of this century2. Elevated CO2 can increase photosynthetic rate, biomass, and C:N ratio of plants3,4,5,6. Plants grown under elevated CO2 accumulate increased level of nonstructural carbohydrates and afford lower nutritional quality of plant tissues for herbivorous insect pests7. Broadly speaking, assimilation and allocation profiles of carbon and nitrogen in plant under elevated CO2 will change the primary and secondary metabolites of plants, thereby affecting the aboveground and belowground herbivorous insects8,9,10.
Rice (Oryza sativa L.) is a stable food for more than half of the world’s population11. Unfortunately, rice yields suffer huge losses by insect pests especially lepidopteran pests12. Researchers have developed transgenic rice varieties that produce insecticidal Cry toxins from Bacillus thuringiensis (Bt) in order to control target lepidopteran pests13,14. Among them, Huahui 1 (HH1), expressing the fused Cry1Ab/1Ac gene, has high resistance to the target lepidopteran pests of rice and has been issued bio-safety certificates in China15.
Because a biologically effective exogenous insect-resistant Bt toxin is expressed in transgenic rice, the stability of Bt toxin expression in plant tissues of Bt rice under elevated CO2 has been of great interests among researchers. Previous studies have investigated the effects of elevated CO2 on performance of Bt crops and stability of the transgenic traits16,17,18,19. Some studies have suggested that the exogenous gene expression in Bt plants transfers certain nutrients from the normal physiological pathways which may change the C-N balance, especially in the case of changed abiotic conditions16,17,18,20. The application of N fertilization can alleviate such nutrient diversion21. Coviella et al. found that elevated CO2 reduced N allocation to Bt toxin, but the reduction was largely diverted by the augmentation of nitrogen22. Hence, the N fertilization is considered to be an effective method to enhance the C–N balance in Bt plants in the case of elevated CO2 in the future16,22.
DNA methylation represents a stable epigenetic mechanism in regulating gene expression23,24,25,26. Numerous studies have proven that DNA methylation plays a critical role on many aspects of plant growth, including flower development, responses to environment stress, transgene expression regulation and silencing27,28,29,30,31. Transgene silencing mainly includes two forms, that is, transcriptional gene silencing (TGS), in which DNA methylation occurs in promoter-region, and posttranscriptional gene silencing (PTGS), in which DNA methylation occurs in coding sequences32,33,34. Li et al. reported that promoter-region methylation repressed gene expression and coding-region methylation usually positively associated with gene expression35. During seedling stage of Bt rice, the foliar coding-region methylation keeped at low level and showed a moderate regulation of Bt gene expression under elevated CO2 and N augmentation situation19. However, how did promoter-region methylation regulate the Bt-transgene expression of Bt rice under elevated CO2 was still unclear. Tillering stage is a key period for the construction of rice population. The number of tillers and the quality of growth determine the formation of final yield. So, the higher foliar exogenous-toxin protein content of Bt rice grown under elevated CO2 is important to control target lepidopteran pests and thus get higher yields. Investigating how transgene methylation in promoterregion and codingregion regulate the exogenous transgene expression under elevated CO2 is vital to ensure higher Bt-transgene expression level for Bt rice.
In this study, the effects of elevated CO2 on Bt-transgene expression in promoterregion and codingregion of Bt rice during tillering stage were investigate under different N-fertilizer levels. The aims of this study were to: (1) explore whether N-fertilizer application under elevated CO2 condition can alleviate or eliminate the nitrogen limitation in Bt rice, (2) investigate how the transgene methylation levels in promoterregion and codingregion regulates Bt-transgene expression under elevated CO2 condition.
Results
Belowground and aboveground biomass of Bt rice
CO2, N-fertilizer levels and their interaction were significantly affected both the belowground and aboveground biomass of Bt rice (P < 0.05 or 0.001; Table 1). Both the belowground and aboveground biomass significantly increased with increased N-fertilizer augmentation, respectively (P < 0.05; Fig. 1). Compared with ambient CO2, elevated CO2 significantly increased the aboveground biomass of Bt rice grown at 2 N-fertilizer level (+ 25.74%), and belowground biomass of Bt rice grown at 1 N and 2 N-fertilizer levels (+ 27.71% and + 21.19%; P < 0.05, Fig. 1).
Aboveground (A) and belowground (B) biomass of Bt rice with fused Cry1Ab/Ac during tillering stage, grown under ambient and elevated CO2 with different N-fertilizer levels. (Values are mean ± SE. Values denoted by different lowercase and uppercase letters indicate significant differences between the ambient CO2 and elevated CO2 for same N-fertilizer rates, and between the different N-fertilizer rates for same CO2 level by LSD test at P < 0.05. The same in Figs. 2, 3, 4, 5, 6, 7).
Pearson’s analysis on the correlations between the methylation levels in the promoterregion (P1), and codingregion (P2, P3, P2 + P3) of Bt-transgene (P1 + P2 + P3) and the Cry1Ab/1Ac expression level in the leaves of Bt rice leaves during tillering stage, grown under ambient and elevated CO2 with different N-fertilizer level. (P1, CpG island 1 (promoterregion); P2, CpG island 2 (codingregion); P3, CpG island 3 (codingregion); P2 + P3, CpG island 2 + CpG island 3 (codingregion); P1 + P2 + P3, CpG island 1 + CpG island 2 + CpG island 3 (Bt-transgene)).
Foliar contents of total soluble protein and Bt protein of Bt rice
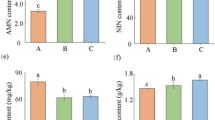
N-fertilizer level significantly affected the foliar content of total soluble protein of Bt rice (P < 0.001; Table 1). Under ambient CO2, the foliar content of total soluble proteins of Bt rice grown at 1/4 N level were significantly lower (− 16.14%) than that at 2 N level (P < 0.05; Fig. 2A). Under elevated CO2, the foliar content of total soluble proteins of Bt rice grown at reduced N-fertilizer level (1/4 N) were significantly lower than that at 1 N and 2 N levels (− 17.27% and − 15.70%; P < 0.05, Fig. 2A). Compared with ambient CO2, elevated CO2 significantly increased the foliar content of total soluble proteins of Bt rice grown at 1 N level (+ 10.75%; P < 0.05, Fig. 2A).
N-fertilizer level (P < 0.001) and its interaction with CO2 level (P < 0.05) significantly influenced the foliar Bt protein content of Bt rice (Table 1). Under ambient CO2, the foliar Bt protein content of Bt rice significantly increased with the N fertilizer augmentation (P < 0.05; Fig. 2B). Under elevated CO2, the foliar Bt protein content of Bt rice grown at 2 N level was significantly higher than that at 1/4 and 1 N levels (+ 88.21% and + 61.47%; P < 0.05; Fig. 2B). Compared with ambient CO2, elevated CO2 significantly decreased the foliar Bt protein content of Bt rice grown at 1 N level (− 16.04%; P < 0.05; Fig. 2B).
Bt transgene expression in the leaves of Bt rice
N-fertilizer level (P < 0.05) and its interaction with CO2 level (P < 0.001) significantly affected the Bt transgene expression in the leaves of Bt rice (Table 1). Under ambient CO2, the Bt-transgene expression level in the leaves of Bt rice grown at 1/4 N and 2 N level was significantly down-regulated when compared with that at 1 N level (− 38.16% and − 19.04%; P < 0.05; Fig. 3). Compared with ambient CO2, elevated CO2 just significantly up-regulated the Bt-transgene expression level in the leaves of Bt rice grown at 1/4 N level (+ 48.03%; P < 0.05; Fig. 3).
Methylation status in the promoterregion and codingregion of Bt-transgene in the leaves of Bt rice
Promoterregion (P1) of Bt-transgene
CO2, N-fertilizer levels and their interaction significantly affected the methylation levels in the promoterregion (P1) of Bt-transgene in the leaves of Bt rice (P < 0.05; Table1). N-fertilizer level differently affected the methylation in the P1 fragment of Bt-transgene in the leaves of Bt rice. The methylation percentages in the P1 fragment of Bt-transgene in the leaves of Bt rice grown at 1/4 N level (+ 135.89%) and 2 N level (+ 157.23%) were markedly higher than that at 1 N level under ambient CO2, respectively (P < 0.05; Fig. 4), while it was contrary tendency under elevated CO2. Significant decreases in the methylation percentages were found in the P1 fragment of Bt-transgene in the leaves of Bt rice grown at 1/4 N level (− 62.52%) and 2 N level (− 33.75%) in contrast to that at 1 N level under elevated CO2 (P < 0.05; Fig. 4). In addition, compared with ambient CO2, elevated CO2 obviously decreased the methylation percentages in the P1 fragment of Bt-transgene in the leaves of Bt rice grown at reduced N-fertilizer level (1/4 N) (− 24.21%; P > 0.05), and markedly enhanced the methylation percentages in the P1 fragment of Bt-transgene in the leaves of Bt rice grown at recommended normal (1 N: + 376.96%; P < 0.05) and increased N-fertilizer level (2 N: + 22.84%; P > 0.05, Fig. 4).
CO2, N-fertilizer levels and CO2 × N-fertilizer interactions significantly affected the methylation levels of cytosines located at CG and CHH sites in the P1 fragment of Bt-transgene in the leaves of Bt rice (P < 0.05; Table 2). The methylation levels of cytosines located at CHG site in the P1 fragment of Bt-transgene in the leaves of Bt rice was just significantly affected by CO2 and CO2 × N interactions (P < 0.05; Table 2). Under ambient CO2, the methylation level of cytosines located at CG and CHH sites in the P1 fragment of Bt-transgene in the leaves of Bt rice grown at 1/4 N level (+ 122.95% and + 140.32%; P < 0.05) and 2 N level (+ 112.82% and + 249.95%; P < 0.05) were markedly higher than that at 1 N level. In contrast, the methylation level of cytosines located at CG, CHG and CHH sites in the P1 fragment of Bt-transgene in the leaves of Bt rice grown at 1/4 N level (− 57.77%, − 58.41 and − 72.66%; P < 0.05) were significantly lower than that at 1 N level under elevated CO2 (Fig. 5). Moreover, compared with ambient CO2, elevated CO2 markedly enhanced the methylation percentages of cytosines located at CG, CHG and CHH sites in the P1 fragment of Bt-transgene in the leaves of Bt rice grown at 1 N level (+ 313.79%, + 397.40% and + 511.32%; P < 0.05), and CG sites in the P1 fragment of Bt-transgene in the leaves of Bt rice grown at increased N-fertilizer level (2 N: + 35.67%; P < 0.05) (Fig. 5). The methylation level of cytosines located at CG sites was higher than those at CHG and CHH in the P1 fragment of Bt-transgene in the leaves of Bt rice regardless of the CO2 or N-fertilizer level (Fig. 5).
Codingregion (P2, P3, P2 + P3) of Bt-transgene
The interaction between CO2 and N-fertilizer levels significantly affected the methylation levels in the codingregion (P2 + P3) of Bt-transgene in the leaves of Bt rice (P < 0.05; Table 1). Compared with ambient CO2, elevated CO2 significantly enhanced the methylation percentages in the P2 + P3 fragments of Bt-transgene in the leaves of Bt rice grown at increased N-fertilizer level (2 N: + 47.24%; P < 0.05) (Fig. 6). CO2 × N-fertilizer interaction significantly affected the methylation levels of cytosines located at CG site in the P2 + P3 fragments of Bt-transgenein the leaves of Bt rice (P < 0.05; Table 2). Compared with ambient CO2, elevated CO2 significantly enhanced the methylation percentages of cytosines located at CG sites in the P2 + P3 fragments of Bt-transgene in the leaves of Bt ricegrown at 2 N level (+ 67.52%; P < 0.05; Fig. 5). The methylation level of cytosines located at CG sites was higher than those at CHG and CHH sites in the P2 + P3 fragments of Bt-transgene in the leaves of Bt rice regardless of the CO2 or N-fertilizer level (Fig. 5).
CO2, Nitrogen-fertilizer levels and their interaction did not significantly affect methylation levels in the codingregion (P2) and codingregion (P3) of Bt-transgene in the leaves of Bt rice (P > 0.05; Table 1). In the codingregion (P2), the methylation percentage at 2 N level under elevated CO2 (16.28%) was significantly higher than that under ambient CO2 (9.72%) (P < 0.05, Fig. 6). There were no CHG and CHH sites as potential targets in the P2 fragment of Bt-transgene (Fig. 5). In the codingregion (P3), the methylated level was very low, not exceeding 5.74% (Fig. 6). The methylation level in the P3 fragment was lower than that in the P2 fragment of Bt-transgene in the leaves of Bt rice (Fig. 6). N-fertilizer level significantly influenced the methylation level of cytosines located at CG site in the P3 fragment of Bt-transgene in the leaves of Bt rice (P < 0.05; Table 2). Under ambient CO2, methylation level of cytosines located at CG sites in the P3 fragment of Bt-transgene in the leaves of Bt rice grown under increased N-fertilizer level (2 N) was significantly lower that at 1 N level (− 70.47%, P < 0.05; Fig. 5).
Bt-transgene (P1 + P2 + P3)
CO2 and its interaction with N-fertilizer significantly affected the methylation levels in the Bt-transgene (P1 + P2 + P3) in the leaves of Bt rice (P < 0.001; Table 1). The methylation percentages in the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice grown at 1/4 N level were significantly lower than that at 1 N and 2 N level under elevated CO2 respectively (− 37.10% and − 15.80%; P < 0.05, Fig. 7). In addition, compared with ambient CO2, elevated CO2 markedly enhanced the methylation percentages in the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice grown at recommended normal (1 N: + 87.17%; P < 0.05) and increased N-fertilizer level (2 N: + 36.17%; P > 0.05) (Fig. 7).
CO2, N-fertilizer levels and their interactions significantly affected the methylation levels of cytosines located at CHH sites in the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice (P < 0.05; Table 2). The methylation levels of cytosines located at CG in the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice was significantly affected by CO2 and its interaction with N-fertilizer (P < 0.05; Table 2), while the methylation levels of cytosines located at CHG sites in the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice was just significantly affected by CO2 level. The methylation level of cytosines located at CG sites was higher than those at CHG and CHH sites in the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice regardless of the CO2 or N-fertilizer level (Fig. 5). Under ambient CO2, the methylation level of cytosines located at CHH sites in the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice grown at 2 N level were markedly higher than that at 1 N level (+ 128.29%; P < 0.05, Fig. 5). Under elevated CO2, the methylation level of cytosines located at CG and CHH sites in the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice grown at 1/4 N level were significantly lower than that at 1 N level respectively (− 33.79% and − 61.01%; P < 0.05, Fig. 5). In addition, compared with ambient CO2, elevated CO2 markedly enhanced the methylation percentages of cytosines located at CG, CHG and CHH sites in the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice grown at 1 N level (+ 62.03%, + 284.85% and + 229.98%; P < 0.05) and at CG sites in the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice grown at increased N-fertilizer level (2 N: + 53.77%; P < 0.05) (Fig. 5).
The correlation between the transgene methylation in promoterregion and codingregion, and the Bt-transgene expression level
The Pearson’s analysis showed that the methylation level in the promoterregion (P1) of Bt-transgene was negatively correlated with the Cry1Ab/1Ac expression level in the leaves of Bt rice (Fig. 8). The methylation level in the codingregion (P2 + P3) was slightly negatively correlated with the Cry1Ab/1Ac expression level in the leaves of Bt rice (Fig. 8). The methylation level in the Bt-transgene (P1 + P2 + P3) was negatively correlated with the Cry1Ab/1Ac expression level in leaves of Bt rice during tillering stage (Fig. 8).
Discussion
Previous studies showed that elevated CO2 can stimulate plant growth and increase photosynthetic rate, photosynthate production, biomass and C: N ratios36. Hao et al. reported that the biomass of leaf, stem, pod, and total aboveground biomass of soybean increased with elevated CO237. Our results indicated that elevated CO2 and increased N-fertilizer both increased the biomass of Bt rice. Also, it appeared that elevated CO2 showed a positive effect on the aboveground biomass of Bt rice grown under higher N-fertilizer (i.e., 2 N level) and belowground biomass of Bt rice grown under 1 N and 2 N-fertilizer. The biomass of Bt rice was significantly increased with increased augmentation of N fertilizer. It is expected that the increased nitrogen uptake by the plant would enhance the rate of photosynthesis, resulting in increased biomass accumulation via increased CO2 diffusion conductance and Rubisco content in Bt rice leaves38,39,40. Hence, elevated CO2 and augmentation of N supply simultaneously increased the rice biomass, likely manifesting synergistically additive effects on biomass accumulation.
In recent years, the potential impacts of future CO2 levels on Bt crops have attracted increasing attention. Our results show that foliar Bt protein content of Bt rice grown at elevated CO2 were significantly lower than that under ambient CO2 at 1 N level. It may be related to the decreased N allocation to Bt protein caused by elevated CO216. Similarly, Coviella et al. found that elevated CO2 decreased Bt protein content in Bt cotton22. In this study, the foliar Bt toxin content of Bt rice at 2 N level was significantly higher than those at 1 N and 1/4 N level, indicating that the doubling of nitrogen augmentation (i.e., 2 N) resulted in the enhanced foliar Bt protein content level in the leaves of Bt rice. Bruns and Abel reported that the Bt protein production of two transgenic Bt-transgenic maize lines increased with the augmentation of N fertilizer application41. Yang et al. found that the contents of Cry2A and Cry1C in Bt rice both increased in the tillering and milking stages with the higher N concentrations applied on rice42. Wang et al. documented that the Cry1Ab/1Ac content of Bt-SY63 at higher N fertilizer was significantly higher than that without N fertilizer treatment43. Moreover, the foliar content of total soluble protein at 1/4 N level was significantly lower than that at 1 N and 2 N level, respectively. The Bt protein content in plant tissues has been shown to significantly correlate with soluble protein and overall nitrogen content41,44. Hence, it is plausible to increase the Bt protein content in Bt crops by taking appropriate nitrogen management measures.
Epigenetic changes in DNA methylation can affect transgene expression for transgenic crops. DNA methylation occurs in codingregion has a more complex association with gene expression, whereas DNA methylation in promoterregion plays a vital role in transgene silencing35. For example, the resistance marker expression of transformed tobacco cultivars was rapidly lost and transgene expression were down-regulated, and hypermethylation within the 35S and NOS-promoters of these cultivars were found45. Additionally, environmental factors, such as drought and extreme temperature can potentially influence the methylation status46,47,48. In rice, 70% of the drought-induced methylation changing sites were reversed to their original status after water recovery49. In this study, our results showed that elevated CO2 significantly enhanced the methylation percentages in the promoterregion (P1), and the P1 + P2 + P3 fragments of Bt-transgene in the leaves of Bt rice during tillering stage grown at 1 N level. In the codingregion, the methylation level in the P2 fragment of Bt-transgene, the fagment near the top strand of Bt-transgene, was higher than that in the P3 fragment, the fragment amplified from the bottom strand of Bt-transgene. Though the methylation level was low in P3 fragment of Bt-transgene, it was negatively correlated with the Cry1Ab/1Ac expressi in the leaves of Bt rice during tillering stage. In general, the methylation status in codingregion in Bt-transgene was slightly negatively correlated with the Cry1Ab/1Ac expression level in the leaves of Bt rice during tillering tage. Jiang et al. found that the PTGS methylation in the codingregion of Bt-transgene in the leaves of Bt rice during seeding stage remained at a relatively low level, lower than 5%19. The methylation level in the codingregion of Bt-transgene shows a weak regulation to the transgene expression. Thus, the methylation level in codingregionof Bt-transgene in the leaves of Bt rice has a weak regulation to the transgene expression both in tillering and seeding stage. The methylation levels in the promoterregion likely affected transgene expression more than that in the codingregion of Bt-transgene in the leaves of Bt rice. In addition, the Pearson’s analysis also showed that the methylation level in the P1 + P2 + P3 fragments of Bt-transgene was negatively correlated with the Cry1Ab/1Ac expression in the leaves of Bt rice. Thus, the methylation level in the P1 + P2 + P3 fragments of Bt-transgene was showed moderate regulation to the transgene expression in the leaves of Bt rice during tillering stage.
Stable transgene expression and heritability are key factors for the development and application of transgenic crops. Environmental factors, such as soil salinity, water accessibility and temperature all play crucial roles in Bt transgene expression50,51. Trtikova et al. found that the Cry1Ab expression in MON 810 maize under hot/dry stress was significantly lower than that under optimal conditions52. Other studies with Bt crops have also indicated that environment might influence the levels of transgene expression differently53. Our results indicated that the Bt transgene expression was significantly up-regulated by elevated CO2 under 1/4 N level, and Bt transgene expression level in the leaves of Bt rice grown at 1/4N and 2N level was significantly down-regulated when compared with that at 1N level under ambient CO2. Considering the methylation level in promoterregion and codingregion of Bt-transgene was negatively correlated with the Cry1Ab/1Ac expression level in the leaves of Bt rice during tillerage stage, so we speculate that the different transgene expression level among different CO2 and N treatments was caused by methylation in promoterregion and codingregion of Bt-transgene and post-transcriptional regulation in the leaves of Bt rice during tillering stage.
In conclusion, the methylation level in the promoterregion and codingregion of Bt-transgene were negatively correlated with the Bt transgene expression level in the leaves of Bt rice during tillering stage. The methylation levels in the promoterregion likely affected transgene expression more than that in the codingregion of Bt-transgene in the leaves of Bt rice during tillering stage. Elevated CO2 showed positively effect on the transgene methylation level and negatively effect on the foliar Bt toxin content of Bt rice grown under 1 N level. The increased N-fertilizer level showed positively effect on the foliar Bt toxin content of Bt rice during tillering stage. Under elevated CO2 situation in the future, moderate application of N-fertilizer can increase the foliar Bt toxin content in Bt rice. Futhermore, additional studies should be performed to evaluate the efficacy of the transgenic proteins against the target organisms under elevated CO2, and thus the biological meaning behind it.
Materials and methods
Plant materials
The Bt rice cultivar HH1 (Huahui 1) was used in the study. The rice seeds were provided by Prof. Yongjun Lin from Huazhong Agricultural University (Wuhan, China). HH1 was developed by using MH63 as the recipient to harbor the fusion gene Cry1Ab/Ac from transgenic event TT51-1 (GenBank Accession Number: EU880444.1). Expression of the Cry1Ab/Ac gene is driven by the rice actin 1 promoter and the nopaline synthase (NOS) gene terminator (seen in Fig. 9).
Plant growth conditions
This experiment was performed in electronically controlled growth incubator (GDN-400D-4/CO2; Ningbo Southeast Instrument CO., LTD, Ningbo, China) connected with a gas-tank system for maintaining the desired atmospheric CO2 concentration. The conditions in the chambers were maintained at 28 °C (day) and 25 °C (night) under a 16: 8 h light/dark photoperiod. The light intensity was 20,000 lx. Two CO2 concentrations levels were applied continuously, i.e., elevated CO2 (800 ppm, predicted CO2 concentration in 2100), and ambient CO2 (about 400 ppm). With each CO2 level, the N-fertilizer was set at three levels, 1/4, 1 and 2 N; the 1 N was 1.25 mM NH4NO3. Therefore, the experiment was consisted of 2 CO2 concentrations × 3 N-fertilizer levels (total 6 treatment combinations) deployed in six electronically controlled growth incubators as three replications for CO2 main factors.
The rice seeds of Bt rice (cv. HH1) were soaked in water for one day, and germinated on a board covered with wet cotton gauze for one day. Then, these seeds were sown into plastic foam covering (0.6 cm thick) on plastic cups (9 cm diameter, 7 cm height) and placed in the electronically controlled growth incubators of ambient and elevated CO2. In the cup, there were two holes in the plastic foam and one rice seeds into each hole (total two seeds per cup). Thirty cups were placed in each electronically controlled growth chambers with 10 cups per N-fertilizer level. The cups were filled with modified culture solutions54; the solution was replaced with fresh solution every day. The composition of modified culture solutions was as follows (per liter): NH4NO3, 1.25 mM; KH2PO4, 0.3 mM; K2SO4, 1 mM; CaCl2·2H2O, 1 mM; MgSO4·7H2O, 1 mM; Na2SiO3·9H2O, 0.5 mM. (2) Micronutrient solution: MnCl2·4H2O, 9 μM; Na2MoO4·2H2O, 0.39 μM; H3BO3, 20 μM; ZnSO4·7H2O, 0.77 μM; CuSO4·5H2O, 0.32 μM; FeSO4·7H2O + Na2-EDTAN54. The plastic cups (plants) were re-randomized every two days within the chamber to minimize the positional effect. At tillering stage, the rice plants were collected, labelled, and stored at − 80 °C for various measurements.
Measurement of plant biomass
After sixty-five days for Bt rice grown under ambient and elevated CO2 with different N-fertilizer levels (i.e., tillering stage), ten Bt rice plants for each N-fertilizer level were randomly selected from each growth incubator (i.e., 30 rice plants for each fertility-fertilizer level per CO2 level). The biomass of belowground (root) and aboveground (stem and leaves) plant tissues were individually weighted with an electronic balance (Mettler Toledo AL 104; readability = 0.1 mg, repeatability < ± 0.1 mg).
Measurement of foliar contents of total soluble protein and Bt protein
After the measurement of plant biomass, the foliar contents of total soluble protein and Bt protein in the sampled rice plants were measured using the diagnostic kit, A045-2 (Nanjing Jiancheng Bioengineering Institute) and ELISA kits from EnviroLogix (Portland, ME; catalog number AP003), respectively. Three leaves from each sampled plant were taken as a sample unit and weighed. Five samples were measured for each treatment. The samples were individually placed into 2 ml microreaction tubes and homogenized in a Tissue Lyser II (Qiagen) by shaking for 3 min at 30 Hz with two steel balls in each tube. For the determination of foliar total soluble protein content, 0.9% saline was used as an extraction buffer in a proportion of 1:9 (m/v). Then, the measurement was performed by following the kit instructions. Optical density (OD) values were measured using a UV–Vis spectrophotometer (UV-1800PC, Mapada, Shanghai, China) at 595 nm wavelength. For the determination of foliar Bt protein content, samples were mixed with extraction buffer PBST (provided with the kit) in a proportion of 1: 10 to 1: 100 (m/v) and then measured the foliar Bt protein content in the leaves of Bt rice during tillering stage according to the kit instructions. The OD values were measured using a UV–Vis spectrophotometer at 450 nm wavelength.
Bioassay of the transcript expression levels of Bt-transgene
RNA extraction and reverse transcription
One leaf per rice plant was excised from 3 plants (total 3 leaves per replication) of each treatment combination of CO2 and N-fertilizer levels for quantification of transcript expression levels of Bt-transgene in the leaves of Bt rice during tillering stage. Three samples were measured for each treatment. Total RNA was extracted from leaf tissues using TRIzol reagent following the supplier’s protocol (Invitrogen). RNA concentration and integrity were evaluated using the NanoDrop spectrophotometer (Thermo Scientific). First strand cDNA templates were synthesized using Prime Script RT reagent kit (TaKaRa, Japan).
Real-time PCR analysis
Quantitative real-time PCR (qRT-PCR) experiment was carried out using SYBR Premix Ex Taq (TaKaRa, Japan) following the kit instructions. Expression of the target gene (i.e., Bt-transgene) was normalized relative to the expression of the housekeeping genes actin1 and ubiquitin. Quantification of the transcript level of Bt-transgene in the leaves of Bt rice during tillering stage was based on the method of Livak and Schmittgen55. Primers used for qRT-PCR are listed in Table 3.
Methylation analysis of Bt-transgene
Genomic DNA were extracted and purified from 30 mg treated leaves of Bt rice from each treatment combination of CO2 and N-fertilizer levels during tillering stage using DNAsecure Plant Kit (TIANGEN, Beijing, China) following the product instructions. DNA concentration was quantified in the NanoDrop spectrophotometer. Then, 100 ng of isolated DNA was submitted to bisulfite treatment to convert non-methylated cytosines into uracil. The conversion was performed using the DNA Bisulfite Conversion Kit (TIANGEN, Beijing, China). Three types of cytosines -CG, CHG and CHH were analyzed in two regions of transgene: a fragment of the Actin 1 promoter (P1, CpG island 1) and two fragments of Cry1Ab/1Ac coding region (P2, CpG island 2 and P3,CpG island 3) (Table 4). The bisulfite sequencing primers were designed using Methyl Primer Express Software (Applied Biosystems) (Table 5).
The target sequences of Bt-transgene were amplified from the Bisulfite-treated genomic DNA by PCR with Methylation-specific Kit (TIANGEN, Beijing, China). The PCR conditions consisted of denaturation at 95 °C for 5 min, followed by 35 cycles at 94 °C for 20 s, 60 °C for 30 s, 72 °C for 20 s, and annealing at 72 °C for 5 min. The PCR products were purified using AxyPrep DNA Gel Extraction Kit (Axygen, Union City, USA), cloned into pEASY-T3 Cloning Vector and transformed into Trans 1-T1 Phage Resistant Chemically Competent Cell (TransGen, Beijing, China). Positive clones were screened with PCR using M13R and M13F primers. Sequencing were done for at least ten independent positive clones from each PCR product was carried out.
Data analysis
All statistical analyses were conducted using SPSS (version 22.0; SPSS Inc., Chicago IL, USA; https://www.ibm.com/products/spss-statistics). DNA methylation levels (%) in CG, CHG and CHH cytosine types were assessed using the kismeth web tool. Two-way analysis of variances (ANOVAs) were performed to examine the effects of CO2 (Ambient vs. Elevated) and N-fertilizer (1/4, 1 and 2 N), and their interactions on plant biomass, foliar contents of total soluble protein and Bt protein, the gene expression levels of Cry1Ab/Ac, and the methylation level in the promoterregion (P1) and codingregion (P2, P3, P2 + P3) of Bt-transgene (P1 + P2 + P3) in the leaves of Bt rice during tillering stage. If there were significant effects of CO2 level, N-fertilizer level or their interaction, the least significant difference (LSD) test was used to separate the treatment means at P < 0.05. The Pearson’s test was performed by R software (version R i386 3.4.2; https://www.r-project.org/) to analyze correlations among methylation level in promoter region and coding region of Bt-transgene with the transgene expression level in the leaves of Bt rice during tillering stage, grown under ambient and elevated CO2 with different N-fertilizer levels.
References
Long, S. P. & Ort, D. R. More than taking the heat: Crops and global change. Curr. Opin. Plant Biol. 13, 241–248 (2010).
IPCC. Impacts, Adaptation and Vulnerability. Working Group II Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 1132 (Cambridge University Press, Cambridge, 2014).
Ainsworth, E. A. & Rogers, A. The response of photosynthesis and stomatal conductance to rising CO2: Mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270 (2007).
Jackson, R. B., Cook, C. W., Pippen, J. S. & Palmer, S. M. Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology 90, 3352–3366 (2009).
Liu, Y., Dang, Z., Parajulee, M. N. & Chen, F. Interactive effects of CO2 and temperature on plant chemistry of transgenic bt rice and population dynamics of a non-target planthopper, nilaparvata lugens (stal) under different levels of soil nitrogen. Toxins 11, 261. https://doi.org/10.3390/toxins11050261 (2019).
Zavala, J. A., Nabity, P. D. & DeLucia, E. H. An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 58, 79–97 (2013).
Hartley, S. E., Jones, C. G., Couper, G. C. & Jones, T. H. Biosynthesis of plant phenolic compounds in elevated atmospheric CO2. Glob. Change Biol. 6, 497–506 (2000).
Bidart-Bouzat, M. G., Mithen, R. & Berenbaum, M. R. Elevated CO2 influences herbivory-induced defense responses of Arabidopsis thaliana. Oecologia 145, 415–424 (2005).
Sun, Y., Cao, H., Yin, J., Kang, L. & Ge, F. Elevated CO2 changes the interactions between nematode and tomato genotypes differing in the JA pathway. Plant Cell Environ. 33, 729–739 (2010).
Xu, H. P., Xie, H. C., Wu, S. Y., Wang, Z. Y. & He, K. L. Effects of elevated CO2 and increased N fertilization on plant secondary metabolites and chewing insect fitness. Front. Plant Sci. 10, 739. https://doi.org/10.3389/fpls.2019.00739 (2019).
Li, Y., Hallerman, E. M., Liu, Q., Wu, K. & Peng, Y. The development and status of Bt rice in China. Plant Biotechnol. J. 14, 839–848 (2016).
Chen, F., Wu, G., Ge, F. & Parajulee, M. N. Relationships between exogenous-toxin quantity and increased biomass of transgenic Bt crops under elevated carbon dioxide. Ecotoxicol. Environ. Saf. 74, 1074–1080 (2011).
Li, Y., Peng, Y., Hallerman, E. M. & Wu, K. Biosafety management and commercial use of genetically modified crops in China. Plant Cell Rep. 33, 565–573 (2014).
Lu, B. R. Challenges of transgenic crop commercialization in China. Nat. Plants 2, 16077. https://doi.org/10.1038/nplants.2016.77 (2016).
Wang, Y. N. et al. Comparison of three transgenic Bt rice lines for insecticidal protein expression and resistance against a target pest, Chilo suppressalis (Lepidoptera: Crambidae). Insect Sci. 23, 78–87 (2016).
Coviella, C. E., Stipanovic, R. D. & Trumble, J. T. Plant allocation to defensive compounds: interactions between elevated CO2 and nitrogen in transgenic cotton plants. J. Exp. Bot. 53, 323–331 (2002).
Chen, F. J., Wu, G., Ge, F., Parajulee, M. N. & Shrestha, R. B. Effects of elevated CO2 and transgenic Bt cotton on plant chemistry, performance, and feeding of an insect herbivore, the cotton bollworm. Entomol. Exp. Appl. 115, 341–350 (2005).
Chen, M., Shelton, A. & Ye, G. Y. Insect-Resistant genetically modified rice in China: From research to commercialization. Annu. Rev. Entomol. 56, 81–101 (2011).
Jiang, S. et al. Impacts of elevated CO2 on exogenous Bacillus thuringiensis toxins and transgene expression in transgenic rice under different levels of nitrogen. Sci. Rep. 7, 14716. https://doi.org/10.1038/s41598-017-15321-9 (2017).
Himanen, S. J. et al. Interactions of elevated carbon dioxide and temperature with aphid feeding on transgenic oilseed rape: Are Bacillus thuringiensis (Bt) plants more susceptible to nontarget herbivores in future climate?. Glob. Change Biol. 14, 1437–1454 (2008).
Tsutsumi, K., Konno, M., Miyazawa, S. I. & Miyao, M. Sites of action of elevated CO2 on leaf development in rice: Discrimination between the effects of elevated CO2 and nitrogen deficiency. Plant Cell Physiol. 55, 258–268 (2014).
Coviella, C. E. & Trumble, J. T. Effect of elevated atmospheric carbon dioxide on the use of foliar application of Bacillus thuringiensis. Biocontrol 45, 325–336 (2000).
Hu, L. F. et al. Rice MADS3 regulates ROS homeostasis during late anther development. Plant cell 23, 515–533 (2011).
Jullien, P. E., Susaki, D., Yelagandula, R., Higashiyama, T. & Berger, F. DNA methylation dynamics during sexual reproduction in Arabidopsis thaliana. Curr. Biol. 22, 1825–1830 (2012).
Ma, Y. et al. Disrupted genome methylation in response to high temperature has distinct affects on microspore abortion and anther indehiscence. Plant Cell 30, 1387–1403 (2018).
Matzke, M. A. & Mosher, R. A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 15, 394–408 (2014).
Zhong, S. et al. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat. Biotechnol. 31, 154–159 (2013).
Yong-Villalobos, L. et al. Methylome analysis reveals an important role for epigenetic changes in the regulation of the Arabidopsis response to phosphate starvation. Proc. Natl. Acad. Sci. U.S.A. 112, E7293–E7302 (2015).
Mette, M. F. et al. Transcriptional silencing and promoter methylation triggered by double-stranded RNA. Embo J. 19, 5194–5201 (2000).
Matzke, M. et al. Genetic analysis of RNA-mediated transcriptional gene silencing. Biochim. Biophys. Acta 1677, 129–141 (2004).
Matzke, M., Kanno, T., Huettel, B., Daxinger, L. & Matzke, A. J. M. Targets of RNA-directed DNA methylation. Curr. Opin. Plant Biol. 10, 512–519 (2007).
Dalakouras, A., Dadami, E., Zwiebel, M., Krczal, G. & Wassenegger, M. Transgenerational maintenance of transgene body CG but not CHG and CHH methylation. Epigenetics 7, 1071–1078 (2012).
Lister, R. et al. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133, 523–536 (2008).
Vermeersch, L. et al. Transitive RNA silencing signals induce cytosine methylation of a transgenic but not an endogenous target. Plant J. 74, 867–879 (2013).
Li, M. Y. et al. NaCl-induced changes of ion fluxes in roots of transgenic Bacillus thuringiensis (Bt) cotton (Gossypium hirsutum L.). J. Integr. Agric. 12, 436–444 (2013).
Drake, B. G., Gonzalez-Meler, M. A. & Long, S. P. More efficient plants: A consequence of rising atmospheric CO2?. Annu. Rev. Plant Biol. 48, 609–639 (1997).
Hao, X. Y. et al. Effects of free air CO2 enrichment (FACE) on growth and yield of summer soybean. Acta Ecol. Sin. 29, 4595–4603 (2009) (in Chinese).
Yamori, W., Nagai, T. & Makino, A. The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C-3 crop species. Plant Cell Environ. 34, 764–777 (2011).
Reich, P. B., Hobbie, S. E. & Lee, T. D. Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nat. Geosci. 7, 920–924 (2014).
Ruiz, C., Pla, M., Company, N., Riudavets, J. & Nadal, A. High CO2 concentration as an inductor agent to drive production of recombinant phytotoxic antimicrobial peptides in plant biofactories. Plant Mol. Biol. 90, 329–343 (2016).
Bruns, H. A. & Abel, C. A. Nitrogen fertility effects on Bt delta-endotoxin and nitrogen concentrations of maize during-early growth. Agron. J. 95, 207–211 (2003).
Yang, Y. et al. Impacts of nitrogen fertilizer on major insect pests and their predators in transgenic Bt rice lines T2A–1 and T1C–19. Entomol. Exp. Appl. 106, 281–291 (2016).
Wang, F. et al. Effects of N treatments on the yield advantage of Bt-SY63 over SY63 (Oryza sativa) and the concentration of Bt protein. Field Crop. Res. 129, 39–45 (2012).
Dong, H. Z. & Li, W. J. Variability of endotoxin expression in Bt transgenic cotton. J. Agron. Crop Sci. 193, 21–29 (2007).
Weinhold, A., Kallenbach, M. & Baldwin, I. T. Progressive 35S promoter methylation increases rapidly during vegetative development in transgenic Nicotiana attenuata plants. Bmc Plant Biol. 13, 99. https://doi.org/10.1186/1471-2229-13-99 (2013).
Fan, H. H. et al. DNA methylation alterations of upland cotton (Gossypium hirsutum) in response to cold stress. Acta Physiol. Plant. 35, 2445–2453 (2013).
Xia, H. et al. Differentially methylated epiloci generated from numerous genotypes of contrasting tolerances are associated with osmotic-tolerance in rice seedlings. Front. Plant Sci. 8, 12. https://doi.org/10.3389/fpls.2017.00011 (2017).
Chen, B., Saltveit, M. E. & Beckles, D. M. Chilling-stress modifies DNA methylation level in cucumber (Cucumis sativus L.) seedling radicle to regulate elongation rate. Sci. Hortic. 252, 14–19 (2019).
Wang, W. et al. Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.). J. Exp. Bot. 62, 1951–1960 (2011).
Stam, M., Mol, J. N. M. & Kooter, J. M. The silence of genes in transgenic plants. Ann. Bot. 79, 3–12 (1997).
Vilperte, V., Agapito-Tenfen, S. Z., Wikmark, O. G. & Nodari, R. O. Levels of DNA methylation and transcript accumulation in leaves of transgenic maize varieties. Environ. Sci. Eur. 28, 29. https://doi.org/10.1186/s12302-016-0097-2 (2016).
Trtikova, M., Wikmark, O. G., Zemp, N., Widmer, A. & Hilbeck, A. Transgene expression and bt protein content in transgenic Bt maize (MON810) under optimal and stressful environmental conditions. PLoS ONE 10, e0123011. https://doi.org/10.1371/journal.pone.0123011 (2015).
Xia, L. Q. & Guo, S. D. The expression of Bt toxin gene under different thermal treatments. Sci. Agric. Sin. 37, 1733–1737 (2004) (in Chinese).
Kumar, A., Silim, S. N., Okamoto, M., Siddiqi, M. Y. & Glass, A. D. M. Differential expression of three members of the AMT1 gene family encoding putative high-affinity NH4+ transporters in roots of Oryza sativa subspecies indica. Plant Cell Environ. 26, 907–914 (2003).
Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25, 402–408 (2001).
Acknowledgements
This research was funded by the National Nature Science Foundations of China (NSFC) (31871963), the Special Program for New Transgenic Variety Breeding of the Ministry of Science and Technology, China (2016ZX08012005), the National Key Research and Development Program of China (2017 YFD0200400), the Fundamental Research Funds for the Central Universities (KYZ201818), the Qing-Lan Project of Jiangsu Province of China, Postgraduate Research &Practice Innovation Program of Jiangsu Province (KYCX19_0542) and Doctor Foundation of Qingdao Agricultural University (1119035).
Author information
Authors and Affiliations
Contributions
Y.M.L. and F.J.C. designed the study; Y.M.L., Y.H.W., G.C., and C.X.L. performed the experiments; Y.M.L. wrote the manuscript; Y.M.L., S.L.J, M.N.P. and F.J.C reviewed and polished the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Wang, Y., Chen, G. et al. Elevated CO2 alters transgene methylation not only in promoterregion but also in codingregion of Bt rice under different N-fertilizer levels. Sci Rep 10, 18138 (2020). https://doi.org/10.1038/s41598-020-75121-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75121-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.