Abstract
Pre-transplant prognostic scores help to optimize donor/recipient allocation and to minimize organ discard rates. Since most of these scores come from the US, direct application in non-US populations is not advisable. The Survival Benefit Estimator (SBE), built upon the Estimated Post-Transplant Survival (EPTS) and the Kidney Donor Profile Index (KDPI), has not been externally validated. We aimed to examine SBE in a cohort of Spanish kidney transplant recipients. We designed a retrospective cohort-based study of deceased-donor kidney transplants carried out in two different Spanish hospitals. Unadjusted and adjusted Cox models were applied for patient survival. Predictive models were compared using Harrell’s C statistics. SBE, EPTS and KDPI were independently associated with patient survival (p ≤ 0.01 in all models). Model discrimination measured with Harrell’s C statistics ranged from 0.57 (KDPI) to 0.69 (SBE) and 0.71 (EPTS). After adjustment, SBE presented similar calibration and discrimination power to that of EPTS. SBE tended to underestimate actual survival, mainly among high EPTS recipients/high KDPI donors. SBE performed acceptably well at discriminating post-transplant survival in a cohort of Spanish deceased-donor kidney transplant recipients, although its use as the main allocation guide, especially for high KDPI donors or high EPTS recipients requires further testing.
Similar content being viewed by others
Introduction
The survival benefit of kidney transplantation (KT) compared with remaining on dialysis and the rising prevalence of chronic kidney disease result in a continuous increase in the allograft demand/supply ratio.
As a consequence of this, changes in the acceptance criteria of donors into transplant programs have been implemented in order to increase the donor pool1,2,3,4,5. However, the improvement in survival associated with transplantation in old recipients (≥ 65 years) who receive marginal kidneys remains unclear6,7.
In 2014, the US Organ Procurement and Transplantation Network (OPTN) started a new kidney allocation system (KAS) based in donor (Kidney Donor Profile Index—KDPI) and recipient (Estimated Post Transplant Survival—EPTS) scores. Their aims were to improve donor/recipient matching and to decrease organ discard rates8. KDPI is a percentile measure calculated with ten donor variables which is currently applied in all deceased US donors to estimate the risk of post-transplant graft failure9,10. EPTS is also a percentile measure that includes four recipient variables and provides information about potential recipient survival after receiving a KT. Briefly, higher EPTS or KDPI scores indicate lower quality organs and worse expected survival of recipients. The OPTN KAS allocates kidneys with lower KDPI scores to candidates with lower EPTS scores to obtain the highest benefit11. Recently, a new scoring tool (Survival Benefit Estimator, SBE) has been developed to estimate post-transplant survival benefit based on KDPI and EPTS. SBE calculates the probability of 5-year post-transplant survival after receiving a kidney allograft from a specific donor. Results suggest that receiving a KT may be associated with lower mortality risk when compared to remaining on the waitlist across any possible KDPI and EPTS combination12.
Nevertheless, application of these scores in non-US patients might not be advisable due to the intrinsic differences existing between transplant programs in the US and other countries such as Spain4,13,14. In 2017, 45.7% of Spanish donors were ≥ 60 years, while only 4.9% of US donors were 65 years or older15. There were also significant differences regarding donor cause of death, diabetes or hypertension prevalence15,16. However, recent studies have shown the potential benefit that the application of these scores might have in non-US populations. KDPI has shown to improve the expanded criteria donor classification in order to provide an estimation of graft survival in a cohort of Spanish KT recipients17 while the introduction of the Kidney Donor Risk Index in the decision-making process to accept or decline deceased donor kidneys in a Eurotransplant center helped to increase its transplantation rate by 26%17,18,19,20.
The main objective of this study was to assess the prognostic performance of SBE in a two-center Spanish kidney transplant cohort. Secondary objectives of the study were (1) to evaluate the effectiveness of EPTS and KDPI when predicting post-transplant survival, (2) to describe the distribution of donors and recipients in our sample according to KDPI and EPTS scores and (3) to compare the characteristics of the study subjects with the US dataset.
Patients and methods
Study design and data collection
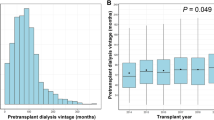
We designed a retrospective cohort study with adult (age ≥ 18 years) deceased-donor KT recipients performed from January 2000 to July 2015 in two different hospitals in Spain. KT from donors younger than 18 years old, living donors, multiple-organ transplants and recipients who were lost to follow-up during the first 5 years after transplantation were excluded from the analysis. Anthropometric, clinical, and analytical variables were collected from each site’s local transplant database. Time on dialysis was calculated considering all dialysis periods for patients that received more than one graft.
This project was conducted in accordance with the ethical standards of the 2000 Declaration of Helsinki as well as the 2008 Declaration of Istambul. No organs were procured from prisoners. The Institutional Review Boards and Ethics committees of both centers approved the study (PI 18-1051, Comité de Ética de la Investigación con Medicamentos, Área de Salud Valladolid Este). Informed consent was waived by the same authority that approved the study (Comité de Ética de la Investigación con Medicamentos, Área de Salud Valladolid Este) due to the observational and retrospective nature of the study. The study is reported in accordance with guidelines set forth in the 2007 STROBE statement21.
Survival estimation and actual survival
Predicted 5-year patient survival was calculated using the prognostic score developed by Bae et al.12, available at https://www.transplantmodels.com/kdpi-epts/. Briefly, SBE estimates the survival benefit (i.e. the absolute reduction in mortality risk expressed in percentage points) for a distinct recipient of receiving a kidney from a specific donor using a combination of EPTS and KDPI scores.
Actual post-transplant patient survival was defined as the time from KT to death, censoring by the end of the study. All patients were followed-up long enough to establish 60-month survival, regardless the allograft status (functioning or not).
The following donor variables were used to calculate KDPI: Age, race, height, weight, hypertension, diabetes, serum creatinine, hepatitis C seropositivity and cause of death, using the methods depicted in the OPTN site. KDPI scores range from 0 to 100% and indicate the potential longevity and quality of a kidney graft compared to a reference population9. EPTS was calculated using recipient variables including age, diabetes, time on dialysis and previous solid organ transplant, following the methods described in the OPTN site. EPTS scores vary from 0 to 100%. Patients with lower EPTS scores should experience more years of graft function from high-longevity kidneys compared to subjects with higher EPTS scores11.
To examine the association of SBE score with patient survival, we stratified our sample by SBE quintiles. Adjusted models were built using SBE, EPTS or KDPI and other recipient variables of clinical relevance (sex, hypertension, ischemic heart disease, congestive heart failure, peripheral vascular disease, stroke and hepatitis C status). Those variables already considered in the calculation of KDPI or EPTS scores were not included in this last analysis.
Statistical analysis
Data are expressed as mean and standard deviation (SD) or median and range for non-normal distributions. Qualitative variables are described as frequencies and percentages. T tests and analyses of variance were used to assess continuous variables. Chi-square test was used to compare categorical data between groups.
To address missing values, we first applied Little’s missing completely at random test to assess the assumption of missing completely at random for multivariate quantitative data22, which was non-significant (P > 0.05). Multiple imputation by fully conditional specification was applied to the dataset. A total run length of 1000 iterations was used, creating 10 imputed data sets to take into account uncertainty associated with missing data. All variables needed to calculate EPTS and KDPI score were included in the procedure (donor age, race, height, weight, hypertension, diabetes, serum creatinine, hepatitis C seropositivity and cause of death; recipient age, diabetes, time on dialysis and previous solid organ transplant). Final results were averaged across the created sets.
To compare the original SBE cohort with our study cohort, mean and standard deviation were estimated on the basis of the reported median and range according to the method developed by Hozo SP et al.23. Quintile distribution according to SBE score (Q1: 0–75; Q2: 75.1–83.5; Q3: 83.6–88.8; Q4: 88.9–93; Q5: 93.1–100) was used to obtain groups of comparable size. Overall survival probabilities for each group were estimated using the Kaplan–Meier method and compared by the log-rank test. Hazard ratios (HRs) with 95% confidence interval (CI) were calculated using unadjusted and adjusted Cox proportional hazards regression modelling. To ensure confidence interval coverage and type I error rate as defined by Vitinghoff et al.24 the study sample included at least ten events per predictor variable. Model discrimination was estimated using Harrell’s C statistic25. Harrell’s C estimates the proportion of correct predictions, serving as a concordance index. The results of Harrell’s C index varied from 0.5 (no discrimination) to 1 (perfect discrimination). The Benjamini–Hochberg procedure was used to control for the false discovery rate26. We examined the Akaike information criterion (AIC), a technique based on in-sample fit to estimate the likelihood of a model to predict future values. The statistical significance level was set at p < 0.05. Statistical analysis was performed using IBM SPSS v.22 (IBM SPSS, Chicago, IL, USA), GraphPad Prism v.7 (GraphPad Software, San Diego, CA, USA) and Microsoft Excel (Microsoft, Redmond, WA, USA).
Results
Baseline characteristics
A total of 1200 KT were performed in two Spanish hospitals between January 2000 and July 2015. The final analysis included 935 KT patients. Flow-chart of patients is represented in Fig. 1.
Regarding baseline characteristics, a comparative between Bae´s cohort and our study cohort is summarized in Table 1.
Spanish recipients and donors were older and predominantly Caucasians (92.2%). Spanish recipients showed lower prevalence of diabetes, less time on dialysis before KT and lower rates of re-transplantation. The proportion of pre-sensitized patients was higher in Bae´s cohort, while KDPI scores were higher and EPTS scores were lower in the Spanish cohort.
The study sample was divided into five groups according to SBE value distribution. Table 2 summarizes recipient, donor and transplant related data.
Patients with worse estimated post-transplant survival were older, had longer dialysis vintage and suffered more frequently from diabetes or cardiovascular disease. These patients received kidneys from older donors with a higher prevalence of hypertension and diabetes. There was a higher percentage of KT from cardiac-death donors among recipients with better estimated post-transplant survival.
Distribution of KDPI and EPTS scores in the Spanish cohort
In the Spanish cohort 36.6% of donors presented with KDPI > 80%, while a low number of recipients (10.3%) had high EPTS scores (EPTS > 80). The majority of recipients with EPTS > 85 (62.9%) received a kidney from a KDPI > 85 donor. The distribution of KDPI and EPTS scores in our cohort is shown in Fig. 2.
Comparison of 5-year predicted vs actual survival
SBE was used to estimate 5-year patient survival at the time of transplantation. The study sample was divided into quintiles using the results of the SBE score. Figure 3 compares the average estimated survival rate with the actual survival in each SBE quintile.
Actual survival rates in our cohort were higher than predicted ones in the first and second highest and lowest SBE quintiles.
In unadjusted analysis, higher SBE score was a significant protective factor for recipient death [HR: 0.95 (0.93–0.96)], while higher KDPI and EPTS scores were significant risk factors for recipient death [KDPI, HR: 1.01 (1–1.02); EPTS, HR: 1.03 (1.02–1.03)]. All three scores were independently associated with post-transplant survival after adjustment (Table 3).
Albeit patients with the worst SBE 5-year patient survival estimation (1st quintile) had a 23 median percentage point higher EPTS score and a 14 median percentage point higher KDPI score compared to the 2nd quintile group, actual survival between these two groups did not differ significantly (log Rank: 1.665; P = 0.197). This difference was not significant after adjustment for multiplicity.
Discrimination and calibration of SBE, EPTS and KDPI models are summarized in Table 3 and were tested with Harrell’s C statistic for Cox models. Discrimination was poor when using the KDPI model, with a Harrell’s C of 0.57. Using the SBE and EPTS scores improved discrimination when compared to the KDPI model, with a Harrell’s C of 0.69 and 0.71, respectively. Finally, adjusting also for clinically significant variables made a further improvement to a maximum c-statistic of 0.72 and 0.74 for the SBE and EPTS adjusted models, respectively.
Discussion
Clinical tools aimed to optimize selection of donor-recipient pairs and estimate the potential survival benefit that a specific organ could involve for a specific recipient have been recently developed.
The present study evaluated the predictive performance of SBE in terms of 5-year patient survival after kidney transplant, even if the patient lost the graft during that time. In our sample, SBE, EPTS and KDPI score are associated with 5-year patient survival and they remained significant even after multi-adjusted survival analysis by recipient comorbidities (Table 3).
In our study SBE proved to be an independent post-transplant survival predictor both in unadjusted and adjusted analysis. SBE showed acceptable discriminatory performance, with a C-statistic of 0.69 that was close to that of EPTS (0.71) and higher than that of KDPI (0.57). Surprisingly, the calculated C-statistic for the SBE score in our sample was higher than that obtained in its development sample (0.69 vs 0.64)12. However, in this European population SBE tended to underestimate actual survival mainly in the subsets of patients with either highest or lowest SBE scores, therefore reflecting limited utility of this tool in these specific populations. Subjects within the worst and second-worst quintiles according to SBE estimated 5-year survival did not demonstrate significant differences in actual survival (Fig. 3). This is not an isolated finding; in a sample of KT performed in a Eurotransplant center Schulte et al. described a mortality rate of 16 percentage points higher in recipients with an EPTS score between 61–80, compared to those with an EPTS score > 8027. The absence of differences in survival between worst and second-worst quintiles in our sample could be due to higher prevalence of cardiovascular disease (i.e. congestive heart failure or peripheral vascular disease) in the second-worst quintile compared with the worst (Table 2), which is not considered when calculating the EPTS or SBE scores. Conceivably, donor and recipient risk factors may affect post-transplant survival differently in non-US population, with older age possibly being the predominant risk trait in our Spanish cohort. Additionally, the sample size of these two groups is underpowered to detect a difference of 5.2 percentage point in the mortality rates of the worst and second-worst quintiles.
EPTS score showed moderate discrimination in the original US data, with a C-statistic of 0.6928. Clayton et al. evaluated this score in 4983 kidney transplant recipients from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry. The authors analyzed three different Cox models (EPTS only; EPTS plus donor age, hypertension status and HLA-DR mismatch; EPTS plus log-Kidney Donor Risk Index) and reported a Harrell’s C-statistic of 0.67, 0.68 and 0.69 for each model respectively18. We found similar discrimination power with a C-statistic of 0.71 in unadjusted analysis that improved to 0.74 when relevant clinical recipient variables were added to the model. EPTS was also tested as a prognostic tool after deceased-donor KT in Mexican patients, with an area under the receiver operating characteristic curve of 0.6429.
KDPI showed the lowest survival predictive power among the tools. This was an expected finding, as KDPI and Kidney Donor Risk Index were essentially designed as tools to estimate graft durability, but their potential application as predictors of post-transplant patient survival has been tested before. We found a 1.1% higher risk of mortality per percentage point of KDPI increase, and poor 5-year patient survival prediction capacity with a C-statistic of 0.57 in unadjusted analysis that improved to 0.65 when additional recipient variables were added to the analysis. Similar results were obtained by Peters-Sengers et al., reporting 5-year mortality C-statistic for Kidney Donor Risk Index (including deaths after graft loss) of 0.6830. Calvillo-Arbizu et al. also suggested that KDPI could constitute a potential indicator of patient survival, especially for recipients older than 60 years31. However, in another study with Spanish transplant patients no relationship was found between KDPI score and recipient death17.
The observed disparities between our results and American studies could be explained by several relevant demographic differences between the US and Spain. For instance, more than 90% of our donor and recipient sample was constituted by Caucasians whereas the cohort used in the development of SBE was much more ethnically diverse12. In addition, Spanish recipients were older but with less prevalence of diabetes, shorter dialysis vintage and lower sensitization degree. Cold ischemia time was also shorter in our sample. These differences were translated into a lower EPTS score compared to that of Bae’s study. On the other hand, our donors were older and more frequently diabetics and with hypertension, which translated in a higher KDPI compared to that registered in SBE development sample12.
Divergences in transplant era can also be a possible explanation of score performance discrepancies, as pointed by Rose et al.32. The Cox proportional hazards regression model used to develop Kidney Donor Risk Index was obtained from a sample of US kidney recipients from 1995–2005. Donor and recipient characteristics have changed significantly over the last 20 years, both in the US and in Europe33,34, as well as other factors such as dialysis-associated mortality or waitlist removal35. In addition, the increasing prevalence of chronic kidney disease, the growing demand for organ donations and the incorporation of expanded criteria donors as a viable organ source have modified even further the current transplant landscape in the last decades.
Differences also affect transplant policies and organ allocation. Between 2004 and 2015 more than 50% of the retrieved kidneys with KDPI > 85% were discarded in the US, even after the initiation of the KDPI era36,37. Although in Spain there are no official data regarding KDPI-associated discard rates as the use of KDPI is not routinely extended, Arias-Cabrales et al. reported that 35.5% of patients in their study received a kidney from a KDPI > 85 donor17, a percentage which was slightly lower (30.6%) in our current sample.
Results may also depend on other donor, recipient and procedure characteristics not accounted for during calculation of SBE, KDPI or EPTS scores, such as graft damage or abnormalities, excessive first warm ischemia time, additional recipient comorbidities or likelihood of transmission of diseases during transplantation. Additionally, divergences in health care systems between the US and Spain could explain some of the observed discrepancies. More than 70% of US kidney transplant patients report economic problems to access immunosuppressive medication38, with Medicare coverage for those drugs being lost after the first three years after transplantation39. In comparison, the transplant procedure, follow-up monitoring and all associated medication is completely covered in Spain and other European countries40, regardless of transplant duration.
The present analysis has some limitations. Extensive efforts were undertaken to adjust for potential confounding, but residual confounding due to unmeasured variables is still possible. Moreover, most of our sample consisted of Caucasians. Therefore, our results cannot be extrapolated to subjects of other ethnic groups. Likewise, most of the donors in this study were brain-dead so the studied scores may perform differently in other settings such as donation after cardiac death. Additionally, we only tested the predictive capabilities of the SBE score at the 5-year time point. As a consequence, our results should not be extrapolated to other time points. Finally, organ allocation strategies and policies may diverge considerably between countries and, to a lesser extent, between transplant centers, which may affect the predictive potential of scores such as SBE.
But the present study has several strengths too. To our knowledge, this is the first study that offers an external validation analysis of the SBE score in European population. SBE scoring considers both donor and recipient characteristics to build a post-transplant survival estimation. Although the discrimination power was similar to that of EPTS in our sample, due to recipient traits being the most relevant to determine recipient survival, the inclusion of donor associated variables provides a small but important improvement to survival prediction, thanks to their significant effect on future graft function. Our sample was built using data from two different transplant centers and included survival information regardless of actual graft function.
In sum, this is one of the first studies to provide external validation of the use of the SBE score as a standalone score in a non-US sample of kidney transplant recipients. Further analysis should be performed to adequately characterize its potential to produce accurate and individualized post-transplant survival predictions.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIC:
-
Akaike information criterion
- ANZDATA:
-
Australia and New Zealand Dialysis and Transplant Registry
- CI:
-
Confidence interval
- CIT:
-
Cold ischemia time
- DCD:
-
Donation after cardiac death
- Dis:
-
Disease
- DV:
-
Dialysis vintage
- EPTS:
-
Estimated post-transplant survival
- HR:
-
Hazard ratio
- HTN:
-
Hypertension
- IQR:
-
Interquartilic range
- KAS:
-
Kidney allocation system
- KDPI:
-
Kidney donor profile index
- KT:
-
Kidney transplantation
- OPTN:
-
Organ Procurement and Transplantation Network
- PRA:
-
Panel reactive antibodies
- Q:
-
Quintile
- SBE:
-
Survival benefit estimator
- SCr:
-
Serum creatinine
- SD:
-
Standard deviation
References
Garcia-Garcia, G., Harden, P. & Chapman, J. The global role of kidney transplantation for the world kidney day steering committee 2012. Int. J. Organ. Transplant Med. 3(1), 1–8 (2012).
Gridelli, B. & Remuzzi, G. Strategies for making more organs available for transplantation. N. Eng. J. Med. 343(6), 404–410 (2000).
Lloveras, J., Arcos, E., Comas, J., Crespo, M. & Pascual, J. A paired survival analysis comparing hemodialysis and kidney transplantation from deceased elderly donors older than 65 years. Transplantation 99(5), 991–996 (2015).
Ojo, A. O. et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J. Am. Soc. Nephrol. 12(3), 589–597 (2001).
Grams, M. E. et al. Trends in the prevalence of reduced GFR in the United States: A comparison of creatinine- and cystatin C-based estimates. Am. J. Kidney Dis. 62(2), 253–260 (2013).
Perez-Saez, M. J. et al. Survival benefit from kidney transplantation using kidneys from deceased donors aged >/=75 years: A time-dependent analysis. Am. J. Transplant. 16(9), 2724–2733 (2016).
Peters-Sengers, H. et al. Stretching the limits of renal transplantation in elderly recipients of grafts from elderly deceased donors. J. Am. Soc. Nephrol. 28(2), 621–631 (2017).
Stegall, M. D., Stock, P. G., Andreoni, K., Friedewald, J. J. & Leichtman, A. B. Why do we have the kidney allocation system we have today? A history of the 2014 kidney allocation system. Hum. Immunol. 78(1), 4–8 (2017).
Kidney Donor Profile Index Calculator. Organ Procurement and Transplantation Network. https://optn.transplant.hrsa.gov/resources/allocation-calculators/kdpi-calculator (2020).
Rao, P. S. et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation 88(2), 231–236 (2009).
Estimated Post Transplant Survival Calculator. Organ Procurement and Transplantation Network.https://optn.transplant.hrsa.gov/resources/allocation-calculators/epts-calculator (2020).
Bae, S. et al. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor-recipient combination. Am. J. Transplant. 19(2), 425–433 (2019).
Pascual, J. & Perez-Saez, M. J. Kidney donor profile index: Can it be extrapolated to our enviroment?. Nefrologia. 36(5), 465–468 (2016).
Ojo, A. O. et al. Comparison of the long-term outcomes of kidney transplantation: USA versus Spain. Nephrol. Dial. Transplant. 28(1), 213–220 (2013).
National Data. Organ Procurement and Transplantation Network. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ (2019).
Memoria Renal 2017. Organización Nacional de Trasplantes. https://www.ont.es/infesp/Paginas/Memorias.aspx(2018).
Arias-Cabrales, C. et al. Usefulness of the KDPI in Spain: A comparison with donor age and definition of standard/expanded criteria donor. Nefrologia. 38(5), 503–513 (2018).
Clayton, P. A., McDonald, S. P., Snyder, J. J., Salkowski, N. & Chadban, S. J. External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States. Am. J. Transplant. 14(8), 1922–1926 (2014).
Del Moral Martin, R. M. G. et al. Validation of KDRI/KDPI for the selection of expanded criteria kidney donors. Nefrologia.38(3), 297–303 (2018).
Philipse, E. et al. Does Kidney Donor Risk Index implementation lead to the transplantation of more and higher-quality donor kidneys? Nephrol. Dial. Transplant.32(11), 1934–1938.
Von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 61(4), 344–349 (2008).
Li, C. Little’s test of missing completely at random. Stata J. 13(4), 795–809 (2013).
Hozo, S. P., Djulbegovic, B. & Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 5, 13 (2005).
Vittinghoff, E. & McCulloch, C. E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 165(6), 710–718 (2007).
Harrell, F. E. Jr., Califf, R. M., Pryor, D. B., Lee, K. L. & Rosati, R. A. Evaluating the yield of medical tests. JAMA 247(18), 2543–2546 (1982).
Thissen, D., Steinberg, L. & Kuang, D. Quick and easy implementation for the Benjamini-Hochberg procedure for controlling the false positives in multiple comparisons. J. Educ. Behav. Stat. 27(1), 77–83 (2002).
Schulte, K. et al. Analysis of the Eurotransplant kidney allocation algorithm: How should we balance utility and equity?. Transplant Proc. 50(10), 3010–3016 (2018).
Proposal to substantially revise the national kidney allocation system. Organ Procurement and Transplantation Networkhttps://optn.transplant.hrsa.gov/PublicComment/pubcommentPropSub_311.pdf (2012).
Martínez-Mier, G. et al. Patient survival scale after kidney transplant from deceased donors in Veracruz, Mexico. Rev. Med. Inst. Mex Seguro Soc. 57(3), 149–155 (2019).
Peters-Sengers, H. et al. Validation of the prognostic kidney donor risk index scoring system of deceased donors for renal transplantation in the Netherlands. Transplantation 102(1), 162–170 (2018).
Calvillo-Arbizu, J. et al. Does the Kidney Donor Profile Index (KDPI) predict graft and patient survival in a Spanish population?. Nefrologia. 38(6), 587–595 (2018).
Rose, C., Sun, Y., Ferre, E., Gill, J., Landsberg, D. & Gill, J. An examination of the application of the Kidney Donor Risk Index in British Columbia. Can. J. Kidney Health Dis.5, 2054358118761052, https://doi.org/10.1177/2054358118761052 (2018).
Stewart, D. E. et al. Changes in deceased donor kidney transplantation one year after KAS implementation. Am. J. Transplant. 16(6), 1834–1847 (2016).
Coemans, M. et al. Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int. 94(5), 964–973 (2018).
Schold, J. D. et al. Dramatic secular changes in prognosis for kidney transplant candidates in the United States. Am. J. Transplant. 19(2), 414–424 (2019).
Bae, S. et al. Changes in discard rate after the introduction of the Kidney Donor Profile Index (KDPI). Am. J. Transplant. 16(7), 2202–2207 (2016).
Hart, A. et al. OPTN/SRTR 2015 annual data report: Kidney. Am. J. Transplant. 17(Suppl 1), 21–116 (2017).
Gill, J. S. & Tonelli, M. Penny wise, pound foolish? Coverage limits on immunosuppression after kidney transplantation. N. Eng. J. Med. 366(7), 586–589 (2012).
Gaston, R. S. Improving long-term outcomes in kidney transplantation: towards a new paradigm of post-transplant care in the United States. Trans. Am. Clin. Climatol. Assoc. 127, 350–361 (2016).
Lehner, L. J. et al. Assessment of the Kidney Donor Profile Index in a European cohort. Nephrol. Dial. Transplant. 33(8), 1465–1472 (2018).
Funding
CAC has support from a Rio Hortega contract, ISCIII-11453. This study had funds from FIS-FEDER PI16/0617 and Redinren RD16/0009/001.
Author information
Authors and Affiliations
Contributions
Conception or design: A.C., C.A.C., A.L.V., M.J.P.S. Drafting the article or revising it: A.C., C.A.C., A.L.V., C.B., J.B.M., D.R.P., I.A.O., J.P., M.J.P.S. Providing intellectual content of critical importance to the work described: M.C., J.B., A.M., J.P. Final approval of the version to be published: A.C., C.A.C., A.L.V., C.B., J.B.M., D.R.P., I.A.O., M.C., J.B., A.M., J.P., M.J.P.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coca, A., Arias-Cabrales, C., Valencia, A.L. et al. Validation of a survival benefit estimator tool in a cohort of European kidney transplant recipients. Sci Rep 10, 17109 (2020). https://doi.org/10.1038/s41598-020-74295-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74295-3
This article is cited by
-
An external validation of the Kidney Donor Risk Index in the UK transplant population in the presence of semi-competing events
Diagnostic and Prognostic Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.