Abstract
Understanding how threatened species are distributed in space and time can have direct applications to conservation planning. However, implementing standardized methods to monitor populations of wide-ranging species is often expensive and challenging. In this study, we used baited remote underwater video stations (BRUVS) to quantify elasmobranch abundance and distribution patterns across a gradient of protection in the Pacific waters of Costa Rica. Our BRUVS survey detected 29 species, which represents 54% of the entire elasmobranch diversity reported to date in shallow waters (< 60 m) of the Pacific of Costa Rica. Our data demonstrated that elasmobranchs benefit from no-take MPAs, yet large predators are relatively uncommon or absent from open-fishing sites. We showed that BRUVS are capable of providing fast and reliable estimates of the distribution and abundance of data-poor elasmobranch species over large spatial and temporal scales, and in doing so, they can provide critical information for detecting population-level changes in response to multiple threats such as overfishing, habitat degradation and climate change. Moreover, given that 66% of the species detected are threatened, a well-designed BRUVS survey may provide crucial population data for assessing the conservation status of elasmobranchs. These efforts led to the establishment of a national monitoring program focused on elasmobranchs and key marine megafauna that could guide monitoring efforts at a regional scale.
Similar content being viewed by others
Introduction
Developing cost-effective approaches for assessing the population status of large marine predators is crucial given the rapid rate at which some species are declining1,2,3. Both coastal and pelagic fisheries have played a major role in the global decline of elasmobranch species, with an estimated one-quarter of the world’s sharks and rays being currently threatened with extinction4,5. Moreover, substantial habitat loss, degradation of critical habitats (e.g. nursery, reproductive and foraging grounds) and climate-driven changes may also impact elasmobranch populations at different spatial and temporal scales4,6,7. Therefore, detailed knowledge about elasmobranch distribution patterns and habitat use, particularly how populations of wide-ranging threatened species may be connected8,9,10,11, remains an essential step to adequately assess their status and trends. Ultimately, understanding how threatened species are distributed in space and time can have direct applications to marine conservation planning12. However, developing or implementing standardized methods to monitor populations at multiple scales is often expensive and challenging.
Over the last decade, advances in video technology have led to the production of smaller, more robust, and inexpensive cameras that are becoming a popular among researchers seeking to survey marine life13. Remote underwater video survey methods can avoid many of the biases and ecological impacts associated with traditional and extractive sampling methods14,15,16. For example, they can sample over a wide range of habitats that are not suitable for fishing17,18, and they are not as restricted by depth and time as most diver-operated methods15,19. Recorded footage also provides valuable observations of species’ behaviors in their natural environment20, which may have a powerful outreach and educational potential21.
The use of baited remote underwater video stations (BRUVS) is perhaps one of the most accessible, highly replicated, non-destructive and effective tool for quantifying fish assemblages, species-habitat associations and anthropogenic impacts across large spatial scales19,22,23. Using bait increases the probability of detecting predators in the environment, since the resulting bait plume can trigger bait-search behaviors in nearby species24,25. Therefore, this technique has the potential to provide relatively fast baseline data on sharks and rays, an ecologically and economically important group for which basic information on distribution and population trends is often lacking4,26.
In some countries of the Eastern Tropical Pacific (ETP) such as Costa Rica, there are major information gaps on elasmobranch population trends, despite a large number of species being threatened and in urgent need of conservation attention4,27. Shark landings data from Costa Rica are also scarce and unreliable28, and there is limited enforcement of existing management regulations, which has hindered effective conservation actions at national and international levels27. Moreover, a long-term study based on diving observations reported significant declines in the abundance of two pelagic sharks (the Scalloped hammerhead shark Sphyrna lewini and the silky shark Carcharhinus falciformis) in Cocos Island National Park and World Heritage Site of Costa Rica29, highlighting that even remote and isolated marine protected areas (MPAs) of the ETP can be susceptible to illegal fishing29,30. Therefore, implementing reliable and affordable surveying techniques capable of providing fast baseline data on elasmobranch populations across areas with different levels of protection and enforcement remains crucial to developing sound conservation approaches in the ETP.
Understanding what factors shape elasmobranch diversity and distribution can also have important implications for their management and conservation. The effect of environmental drivers on elasmobranch distribution patterns, abundance and species richness has been widely studied in other regions31,32,33, but only a few studies have been conducted in Costa Rica34,35. For example34, showed that elasmobranch assemblages from Isla del Coco are negatively associated with the El Niño Southern Oscillation (ENSO) event, whereas35 found that depth was one of the main drivers shaping demersal elasmobranch distribution along the continental shelf of the Pacific of Costa Rica. For a few threatened elasmobranchs such as the scalloped hammerhead shark and the largetooth sawfish Pristis pristis, there has been recent efforts to understand what factors shape their distribution and abundance patterns36,37,38. However, major information gaps remain for most species, particularly those demersal and reef-associated that are found along the continental shelf.
Establishing long-term monitoring programs capable of mapping the spatial distribution of elasmobranch species over large scales, as well as understanding the role that spatial management and environmental drivers play is crucial to detect population level changes in response to major threats such as fishing, habitat degradation and climate change. This study used BRUVS to quantify and monitor elasmobranch abundances and distribution patterns in the Pacific of Costa Rica. Specifically, we (1) determined how elasmobranch assemblages were distributed across inshore and offshore sites of the Pacific of Costa Rica; and (2) investigated changes in species richness and abundance in relation to protection status (e.g. no-take vs. open-fishing sites), habitat composition and environmental drivers (e.g. water temperature and depth). These efforts led to the establishment of a national monitoring program focused on elasmobranchs and other key marine megafauna in Costa Rica, which may strengthen management and conservation approaches in the region.
Results
From the 430 BRUVS deployed across all sampling sites (Fig. 1), we detected a total of 29 elasmobranch species from 9 families (Table 1). Elasmobranchs were sighted in 87% of all BRUVS, and the number of species recorded per station varied from 1 to 7 (mean ± SD: 2.3 ± 1.3 species). The most commonly sighted species (i.e. species that were detected in more than 30% of the BRUVS deployed where they are known to occur) were the whitetip reef shark (Triaenodon obesus), the marble ray (Taeniurops meyeni), the Scalloped hammerhead (Sphyrna lewini) and the Galapagos shark (Carcharhinus galapagensis). Based on the different metrics of MaxN used to describe the relative abundance of elasmobranch species, the cownose ray (Rhinoptera steindachneri) (sum: 117; max = 76; mean ± SD: 10.6 ± 22.1), S. lewini (sum: 696; max = 70; mean ± SD: 7.4 ± 11.1) and T. obesus (sum: 674; max = 24; mean ± SD: 3.7 ± 3.5) were the most abundant elasmobranchs (Table 1). Species like R. steindachneri and S. lewini, for example, showed the maximum MaxN values, with 76 and 70 individuals detected on a single frame, respectively (Table 1). Based on the International Union for Conservation of Nature (IUCN) Red List, a large proportion of elasmobranch species recorded by BRUVS were threatened (66%).
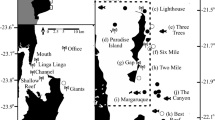
Map of Costa Rica (Central America) showing sampling sites selected to survey elasmobranchs. Color markers indicate the location of the main (yellow: A–F) and additional sampling sites (black: G–K). Red dots represent the location of baited remote underwater videos stations at the main sampling sites. Additional sampling sites: (G) Culebra Bay, (H) Flamingo, (I) Nicoya Península, (J) Marino-Ballena, (K) Golfo Dulce. The bathymetry within the economic exclusive zone is shown in the larger map. Sampling sites were plotted using base and raster layers from the Costa Rican Geographic Information System (GIS) Atlas open-access project (https://hdl.handle.net/2238/6749) in ArcMap 10.4 (ESRI, Redlands, California).
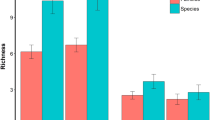
Elasmobranchs were detected at 10 of the 11 sampling sites (Fig. 1), with most sites except for Cocos and Caño Islands detecting a larger number of ray species than sharks (Fig. 2). However, only five coastal sites (Murciélago Islands, Santa Elena Bay, Loros Island, Bajo Rojo and Caño Island) and one offshore site (Cocos Island) were included in the analyses due to low sampling effort (sites with < 15 BRUVS; Table 2). The number of elasmobranch species recorded at these sites ranged from 7 species in Caño Island and Bajo Rojo to 15 species in Murciélago Islands, despite a four-fold increase in sampling effort at Cocos Island relative to coastal sites (Bajo Rojo and Loros Island; Table 2, Fig. 2). Based on the ECDF of species sightings, about 72–97% of all time to first sighting (TFS) events occurred by 75 min soak time across sites, and from 88 to 98% of recorded TFS events occurred by 90 min soak time across sites (Fig. S1). In Santa Elena Bay, Bajo Rojo and Caño Island, soak times of 75 min resulted in 72–82% TFS events, whereas the other sites ranged from 87 to 97%).
The proportion of elasmobranch species that were filter-feeders, small predators and large predators varied across sites (Table 2). A higher proportion of large predators were detected in not-take MPAs such as Cocos Island (58%), Caño Island (43%) and Murciélago Islands (30%). Sites with no protection status (Bajo Rojo, Loros Island and Santa Elena Bay) had a large proportion of small predators. Filter-feeders were recorded opportunistically across sites (Table 2). In addition, more than half of elasmobranch species recorded at the main sampling sites were threatened (> 60%), except for Caño Island (31%).
Overall, BRUVS recorded more elasmobranchs in the North Pacific (21 species) than in Cocos Island (12 species) and the South Pacific (9 species) regions (Fig. 3). However, we also deployed more BRUVS in the North Pacific (N = 203) relative to other regions (Cocos Island: N = 158; South Pacific: N = 69). The frequency of occurrence also varied among regions, with all BRUVS deployed at Cocos Island detecting at least a single elasmobranch species. In the North and South Pacific regions, the frequency of occurrence was 78% and 84%, respectively.
Elasmobranch assemblages across sites
Differences in elasmobranch assemblages were detected among sites (F5,65 = 21.3, p < 0.001; R2 = 0.64). Pairwise comparisons revealed that elasmobranch assemblages from no-take MPAs (Cocos Island, Caño Island and Murciélago Island) were significantly different from each other and were also different from open-fishing sites (Bajo Rojo, Loros Island and Santa Elena Bay), which shared similar assemblages (Fig. 4). Relative abundance of elasmobranchs also differed among sites, but mean MaxN hr−1 of rays was higher than sharks in open-fishing sites relative to no-take MPAs, particularly for Cocos and Murciélago islands (Fig. 5). The cluster analysis revealed a significant separation of samples by region, with all samples from Cocos Island and the North Pacific region forming two distinct clusters (Fig. 6). Interestingly, most of the samples from Caño Island clustered together with samples from Cocos Island; only a few samples shared more similarities with the North Pacific. In addition, in many of the samples from the South Pacific (e.g. Caño Island), BRUVS recorded a high abundance of T. obesus and at least one tiger shark (Galeocerdo cuvier), which are both dominant species in Cocos Island. The heatmap also revealed that S. lewini and T. obesus tend to be detected in the same stations of Cocos Island, whereas coastal batoids such as the longtail stingray (Hypanus longus), the round stingray (Urobatis halleri) and A. laticeps co-occurred at stations from the North Pacific (Fig. 6).
A heatmap with dendrograms showing hierarchical clustering of elasmobranch relative abundance (MaxN hr−1) by site (row samples) and by the level of species co-occurrence (column samples). A species-site matrix was constructed to examine how the samples (i.e. BRUVS deployed on the same site and date were pooled together and treated as single independent samples) clustered by site. Ten species were removed from the analysis (Diplobatis ommata, Narcine entemedor, Styracura pacifica, Urotrygon aspidura, Mobula Japonica, M. munkiana, M. tarapacana, Rhincodon typus, Pseudobatos glauca, P. planiceps) due to low frequency of occurrence. Heat map allows identifying dominant species based on their relative abundance (more intense red colors). Samples were further classified by region (right-side plot) and the two main resulting clusters (green and blue colors) are highlighted.
Drivers of elasmobranch abundance and diversity
Based on maximum likelihood ratio tests and AIC, the best fitted GLM model that explained elasmobranch species richness included region, habitat protection, taxonomical group and depth (Table S1). The best fitted model that explained the relative abundance (MaxN hr−1) of elasmobranchs included the habitat PC2 scores (rock/turf to sand/rubble), in addition to the same predictors that explained elasmobranch richness (Table S1). Poisson GLM models revealed significant interactions between region × elasmobranch group and protection × elasmobranch group for both species’ richness and abundance (Table 3). Overall, there was a significantly higher shark diversity and relative abundance at Cocos Island relative to the other regions. In contrast, ray diversity and relative abundance was significantly higher in the North Pacific region (Fig. 7). In the South Pacific, BRUVS recorded a similar species richness and abundance for both sharks and rays. Habitat protection also had an effect on elasmobranch species richness and abundance, but only shark species seemed to benefit from MPAs, whereas the diversity and abundance of rays was significantly higher at open fishing sites (Table 3, Fig. 7). Depth had a significant positive effect on elasmobranch richness and abundance, whereas an increase in the PC2 scores partially explained a greater elasmobranch abundance (Table 3).
An ordinal logistic regression model showed that habitat protection had a significant effect on the number of BRUVS that recorded small or large predatory elasmobranchs (t value = 10.3, p < 0.0001). In open-fishing sites, there was a significantly higher probability (70%) of detecting only small predators, whereas in no-take sites the probability of detecting an equal proportion of small/large predators or only large predators was 18% and 50%, respectively (Fig. 8). Moreover, there was a low probability (15%) of detecting small predators in no-take sites.
Discussion
This study adds on previous evidence that BRUVS are capable of providing fast and robust estimates of the distribution, abundance and diversity of elasmobranchs over large spatial and temporal scales19,21, information that is crucial by managers to detect population-level changes in response to multiple threats such as overfishing, habitat degradation and climate change4,6. Based on our findings, BRUVS were able to detect approximately 31% of the entire elasmobranch diversity reported for Costa Rica27. Although our study surveyed a wide variety of coastal and offshore habitats where elasmobranchs are known to occur, due to logistic and budgetary reasons, our sampling was restricted to shallow benthic habitats (< 60 m deep) from the Pacific. When excluding Caribbean elasmobranchs, as well as fully pelagic and bathyal species, our benthic and mid-water BRUVS detected 54% of the entire elasmobranch diversity. Moreover, our data revealed that while large predators can benefit from no-take MPAs, they were relatively uncommon or completely absent from open-fishing sites.
In the Pacific of Costa Rica, elasmobranch assemblages have been previously described using a wide range of fishery-dependent and independent methods27. However, with the exception of a few fishery-dependent studies that surveyed elasmobranchs over the entire continental shelf and economic exclusive zone (EEZ) of Costa Rica16,35,40, most studies have been restricted to relatively small areas, including Cocos Island41,42, Golfo Dulce in the South Pacific43, and Tárcoles in the Central Pacific44. Fishery surveys usually provide valuable information on elasmobranch assemblages and their distribution, particularly for species that are harder to detect using traditional survey approaches45,46. For example16, found 24 elasmobranch species from 9 families associated to the longline fishery, whereas33 found 25 demersal elasmobranchs from 14 families in the trawling fishery. Both of these studies reported similar species richness, but different composition compared to our study (Table 4, Table S2). Although most families captured by long-line and trawling were detected in the current study (Table 4), only nine of these species were present across studies (Table S2). The rest were either obligate pelagic or demersal species that are rare or absent from shallow reef habitats (Table S2)27. While a combination of BRUVS and fishery-dependent surveys can detect elasmobranch species on a wide range of habitats, extractive fishing techniques alone suffer from gear-specific sampling bias, are usually not suitable in hard bottoms, are limited to open-fishing areas or may be detrimental to the survival of threatened species14,47.
Elasmobranch studies in Costa Rica using fishery-independent methods have also reported similar species richness and composition compared to our study, at least at local scales (Table 4, Table S2). For example39, used data collected by divers over 21 years to investigate elasmobranch population trends at Cocos Island, whereas38 analyzed video footage from submarine surveys between 2006 and 2012 to examine elasmobranch assemblages in deep waters. All the species recorded by39 were also detected in our study (Table S2). However, due to the capability of the submarine to survey deeper waters (up to 330 m)38, reported four elasmobranch species that were not detected by our BRUVS (Table S2). Our study also reported more elasmobranch species at Caño Island than traditional underwater visual surveys48, which are often biased by divers’ experience and limited by time, depth and weather conditions15. In contrast, BRUVS provide a relatively cost-effective survey method that enhances predator detection by using bait and overcomes fish avoidance issues with divers25,49,50. Moreover, permanent footage generated through BRUVS can be used to answer specific behavioral questions51 or shared with other researchers to support regional and global conservation efforts such as the one lead by the Global FinPrint Project22.
Few studies have used BRUVS to assess shark assemblages in the ETP region, and those were restricted to remote and isolated islands52,53. For example52, reported 10 species of sharks from 4 families in the Galapagos archipelago (Ecuador), whereas53 detected 8 species of sharks from 2 families in Revillagigedo Islands (Mexico). Similar trends in terms of species composition were observed in Cocos Island, in which our BRUVS were able to detect 8 species of sharks from 2 families. However, sampling effort at Cocos Island (158 BRUVS) was considerably lower than in the Galapagos archipelago (629 BRUVS) and relatively similar to Revillagigedo Islands (112 BRUVS deployed). Cocos Island is also 22 and 78 times smaller than Revillagigedo and Galapagos Islands, respectively. The Galapagos archipelago also has some species from the family Triakidae and Heterodontidae that are absent from Cocos and Revillagigedo Islands52. These findings further highlight the efficiency of using BRUVS to survey and monitor elasmobranch species at remote and isolated islands from the ETP, some of which are recognized as UNESCO World Heritage sites.
Numerous studies have demonstrated that shark abundances are generally greater inside than outside not-take MPAs19,22,54, which is in agreement with our findings. However, BRUVS deployed at Caño and Murciélago islands had significantly less species and lower abundances compared to Cocos Island. Differences among MPAs may be related to size, human accessibility and levels of enforcement, which have been previously identified as key drivers of MPA efficiency towards elasmobranch protection55,56,57. Cocos Island, for example, is a relatively pristine, remote and diverse island that currently has one of the largest fish biomass in the Eastern Tropical Pacific58,59. Moreover, given its status as a UNESCO World Heritage Site, more resources for surveillance and enforcement are allocated to Cocos Island than any other MPA in Costa Rica. In contrast, Caño and Murciélago are smaller inshore islands that are in close proximity to important fishing towns, and have limited resources and personnel, which results in higher levels of illegal fishing60.
Illegal fishing is a major threat affecting elasmobranchs, even in remote protected areas such as Cocos Island, where ensuring compliance is challenging30,61,62. Moreover, the increase in foreign pelagic fleets with higher fishing capacity and poor landing statistics remain an ongoing issue in the EEZ of Costa Rica28,63, and may be linked to recent population declines of threatened pelagic species in the ETP42. Therefore, despite the relative pristine conditions of Cocos Island, better management strategies for highly mobile elasmobranch species that move beyond reserve boundaries are necessary to allow population recovery and more effective protection schemes62,64.
Anthropogenic impacts such as habitat loss and degradation represent additional threats in coastal areas, which are generally exposed to multiple chronic stressors56,65, and therefore, may be important drivers of elasmobranch population declines4,38. Efforts towards monitoring key coastal habitats for elasmobranchs are needed, as some of these may also function as critical habitats (e.g. nursery, reproductive and/or feeding grounds) for threatened species36,66,67. In Costa Rica, rapid coastal development and changes in land use practices are already impacting the health of coral reefs and reef fish communities in some areas68,69. However, despite their close proximity to human centers and limited resources for surveillance, Caño and Murciélago islands are still two of the healthiest and most productive areas in Costa Rica, and provide numerous ecosystem services for coastal communities48,70,71.
The absence of large predators from marine habitats is often considered an indicator of fishing pressure72,73, while their presence may be critical to restoring or maintaining ecosystem function and health74,75. Based on our findings, the proportion of large predators (> 1.5 m total length) was greater inside no-take sites, whereas small predators were more diverse and dominated the elasmobranch assemblage in open-fishing sites. Moreover, larger species that may be defined as true apex predators such as the tiger (Galeocerdo cuvier), bull (Carcharhinus leucas), silky (C. falciformis) and Galapagos (C. galapagensis) sharks were exclusively detected inside coastal and offshore no-take reserves. All open-fishing sites included in this study (Loros Island, Bajo Rojo and Santa Elena Bay) were located in the North Pacific of Costa Rica, an area that has been historically exposed to strong pressure from semi-industrial and artisanal fisheries28,76. Declining shark populations since the 1990s have likely resulted in changes in fish community structure and composition, as well as less productive and more degraded habitats69,70. Furthermore, anecdotal information from local stakeholders indicate that apex predators (e.g. S. lewini, C. leucas, C. limbatus and G. cuvier) were often captured in gillnets and coastal long-lines in the late 1980s and early 1990s, but currently are uncommon in open-fishing sites from the North Pacific (Lara. A. & Lara. M., pers. comm).
The decline of large sharks from coastal habitats in Costa Rica may explain why ray diversity and abundance peaked in open-fishing sites. This observation is consistent with the hypothesis of “mesopredator release”, where removal of top predators may propagate down the food web leading to an increase in the abundance of mesopredators, a process that can have negative ecological and economic consequences at the ecosystem level22,77. Predators can also induce habitat shifts in prey that rely on crypsis and refuging51, which may explain common interactions between some rays (e.g. Urobatis halleri and Hypanus longus) and the BRUVS at open-fishing sites. Although there is some evidence that the absence or decline of top predators from coastal habitats in Costa Rica may result in shifts in community structure and function69, more studies are still needed to further elucidate major drivers influencing these patterns. Increasing the number of BRUVS deployed in no-take and open-fishing sites from the Central and South Pacific regions may help clarify whether fishing and/or other drivers are responsible of the shifts in predator abundance.
Environmental factors such as temperature, depth and habitat composition are also key drivers of elasmobranch assemblages31,78,79, but these drivers had little or no contribution in our study. Based on our results, there was a positive effect of depth on both elasmobranch species richness and abundance, which is consistent with findings from other studies19,80. Changes in temperature, light level and productivity associated to depth may explain some of the observed trends in elasmobranch assemblages78. For example, Scalloped hammerhead sharks have been shown to select cooler temperatures below the thermocline81. Due to logistic limitations associated with strong trade winds, we could not survey Murciélago Island during the upwelling season. Therefore, the temperature range between surveyed sites was probably not wide enough to observe differences among elasmobranch assemblages. Greater seasonal replication at each site (at least twice per year to cover dry and rainy seasons) would provide the possibility to assess seasonal variation and the effect of periodic climate events such as ENSO on elasmobranch assemblages. Furthermore, implementing BRUVS deeper than 60 m (maximum depth recorded in this study) would probably increase the number of species detected and provide valuable data about changes on species compositions with wider depth gradients. The effect of habitat and other environmental drivers such as productivity, oceanographic conditions and prey availability on elasmobranch assemblages should be further investigated at the site level to better understand potential anthropogenic impacts.
Overfishing has been identified as the most significant and widespread threat affecting elasmobranch species at a national27 and global scales1,4,5. Some efforts made in Costa Rica within national and coastal waters, are crucial at reducing fishing impacts on elasmobranch populations, and could potentially lead to their recovery. For example, a recent partnership between the government of Costa Rica and the private sector led to the installation of a marine radar in Cocos Island in 2016, which has proven to be instrumental to achieve a better control and surveillance of fishing activities in the island. However, conservation actions and more effective management are still needed to reduce fishery impacts on threatened elasmobranchs that use the EEZ in their migratory route between oceanic islands of the ETP and the mainland11,37,81. In addition, in recent years the use of coastal gillnets in the North Pacific region has been largely reduced (M. Lara comm. pers), and since 2013, the government of Costa Rica stopped issuing new trawl fishing licenses; the last one expired in August 2019. Moreover, Costa Rica has a network of 20 MPAs, which protect approximately 17.5% of national waters60, but only 0.9% of the EEZ, where fishing pressure remains high16. Therefore, in order to achieve elasmobranch conservation goals, it is necessary to reduce fisheries bycatch, improve landing statistics, increase the level of enforcement and resources allocated to current MPAs, and in some cases apply the precautionary approach27.
Recent IUCN assessments of pelagic and endemic species from the ETP revealed that the number of threatened elasmobranchs in Costa Rica increased from 1727 to 53% (43 species)39. These alarming statistics demonstrated that most elasmobranch species that were “Data Deficient” are now listed as threatened when more information became available4. Moreover, some species that were highly abundant in our surveys due to their schooling behavior such as S. lewini changed from Endangered to Critically Endangered based on recent assessments by the IUCN Red List37. Our study showed that over half of the species detected by BRUVS (66%) were threatened39; therefore, a well-designed BRUVS survey may provide crucial information on relative abundance trends for assessing the conservation status of elasmobranchs.
BRUVS have been widely recognized as a suitable sampling method to answer key questions on elasmobranchs19,22,51; however there are some biases associated that are important to recognize. For example, BRUVS are less effective in turbid waters with low visibility, they attract large predators, which may reduce detectability of smaller species, their bait plume dispersion is unknown, and thus our knowledge of the sampling area, and they tend to have a restricted field of view compared to observers in the water14. Another limitation from our study was that smaller fishes were able to take some of the bait from the containers, thus reducing the effect of the bait plume82. Our study is mainly focused on predator species, therefore, the use of bait with its associated biases is necessary to increase detectability of cryptic and threatened species with low natural abundances25. Furthermore, video samples with low visibility were not considered in the analysis overcoming turbidity biases associated with BRUVS. Finally, our survey inside MPAs required a non-destructive approach like BRUVS, thus the advantages of this technique may outweigh the limitations.
To our knowledge, this is the first study using BRUVS that provides a detailed assessment of elasmobranch distribution and abundances in both coastal and offshore sites from the ETP region. With considerably less sampling effort than traditional fishery-dependent and independent methods, our study was able to survey key coastal and offshore sites from Pacific waters of Costa Rica, and recorded a greater number of elasmobranch species. Comparisons with other studies in the ETP further recognize the value of using BRUVS as an accessible technique capable of overcoming the logistical constraints of long-term surveys for elasmobranch populations in the region.
Our results further demonstrate the benefits of no-take MPAs for large predators and confirm the negative impacts of humans on elasmobranch assemblages in near coastal habitats. However, some economically important or threatened species that are generally found in fully pelagic or deep-water demersal habitats were not detected by the BRUVS, which are often an important target of trawling and long-line fisheries27. Therefore, we recommend that further surveys should also include the use of deep-water benthic and pelagic-BRUVS to achieve a more comprehensive assessment of key habitats for elasmobranch species, as well as for identifying potential connectivity routes between oceanic islands and coastal habitats83.
This study is currently being used a reference baseline for developing a national monitoring protocol of elasmobranchs and key marine megafauna in Costa Rica. A standardized methodology using BRUVS is presented in a logistically and economically feasible protocol that promotes the participation of park-rangers in field work, data processing, video analysis and data interpretation. By doing so, we reduce the costs of fieldwork and promote the long-term application of the protocol by stake-holders. The conservation status of elasmobranchs and other large pelagic fishes included as focal species in the protocol is evaluated through a set of indicators in order to detect short term changes and provide early alerts that translate into faster management actions by decision makers. This protocol was first tested at Cocos Island National Park, and subsequently adopted by other MPAs in Costa Rica. A summary of the proposed standards regarding experimental design using BRUVS is presented at Table S3 and Table S4. Although we acknowledge that ideal sampling designs depend on specific research questions, habitats and species surveyed84, here we provide general guidelines to standardize BRUVS surveys on reef-associated elasmobranch populations in tropical marine ecosystems. The use and optimization of this protocol by other countries, especially in Latin America could significantly increase the limited ecological data available for many threatened and/or migratory species leading to more effective management and conservation approaches at the regional level.
Methods
Sampling sites
This study was conducted at 10 coastal sites from the continental shelf of Costa Rica and in Cocos Island, an oceanic island located approximately 500 km southwest from the mainland (Fig. 1). Coastal sites were distributed in the North and South Pacific regions, and included a wide range of structurally complex and diverse habitats (e.g. rocky/coral reefs, sandy/muddy bottoms and macroalgae) from near-shore islands to small islets, underwater pinnacles, and a large bay. We classified the 11 sampling sites into “main study sites” (those sites with > 15 BRUVS deployed) and “additional sites” (< 15 BRUVS deployed) (Table S5). Additional sites were only used to summarized general patterns of species richness across regions.
In the North Pacific, we sampled four main sites (Loros Island, Bajo Rojo, Santa Elena Bay and Murciélago Islands) across a stretch of ocean of 65 km, approximately 1–6 km off the coast. All of these sites are located in the Guanacaste Marine Conservation Area (ACG), a region that is known for its high biodiversity and coastal productivity60,86. Murciélago Islands consist of five small islands and ten islets which represent the largest group of coastal islands in Costa Rica and the only area that we sampled in the North Pacific region that has been fully protected since 1987 (463.91 km2)48,60. Santa Elena Bay has an area of approximately 7.3 km2, and contains a variety of critical habitats for fish, including coral and rocky reefs, mudflats, sandy bottoms, mangroves, and estuaries87. Santa Elena Bay was declared a marine management zone in 2018, where only sport and artisanal hook-and-line fishing are allowed in some areas of the bay. Isla Loros and Bajo Rojo are areas completely open to sport and artisanal fishing activities, including hook-line and compressor fishing. Coral diversity and distribution in the North Pacific region is influenced by a seasonal upwelling event between December and April, combined with strong wave action87,88. The upwelling brings cool nutrient-rich water from the bottom, enhancing coastal productivity and biodiversity87. During the upwelling, water temperature can drop from an annual average of 30–15 °C88,89.
In the South Pacific region, we sampled Caño Island, a 2.9 km2 island located 15 km off the coast that was declared a marine reserve (55.3 km2) in 198760. The island has one of the most diverse coral reef formations in the Pacific south of Costa Rica 90,91 characterized by five fringing coral reef flats ranging in size from 0.008 to 0.042 km2, covered by crustose coralline algae, isolated live colonies of pocilloporids and poritids, and microatolls of massive coral Porites lobata92. The shallow sections of the reef are structured mainly by physical factors (e.g. wave action, temperature and salinity fluctuations, and low tide exposure), whereas the deeper sections are influenced by biological interactions such as bioerosion, damselfish algal lawns, and corallivores92.
Cocos Island is an oceanic island located 500 km southwest of mainland Costa Rica (5°52′N, 87°06′W) (Fig. 1). Given the high level of endemism and biodiversity, Cocos Island was declared a National Park in 1978 and an UNESCO World Heritage site in 1997. The MPA has a surface area of approximately 2011 km260, and has one of the largest fish biomasses in the tropics58,59,93. Cocos Island has a complex bottom morphology94, with a high variety of habitats including, sandy and rocky bottoms at various depths and extensive coral reefs mainly found at the north side of the island inside two of the largest bays along the coast86,95. The southern side of the island is more exposed to the currents and waves typically coming from the southwest96 and is characterized mainly by rocks covered in barnacles and other invertebrates97. This oceanic island has a marked seasonality due to the influence of the North Equatorial Countercurrent (NECC), the main west–east current near the equator96,98. During the rainy season (July–November), the effect of the NECC is enhanced, promoting more productivity, lower temperature and stronger currents98. Sea surface temperatures at Isla del Coco range from 24 to 29 °C and are affected every 4–9 years by the ENSO, which can result in temperatures of up to 30.9 °C34.
Sampling design
We used BRUVS to quantify the distribution and abundance of elasmobranch species across a wide range of habitats and depths, following a biologically informed stratification of sites and replicates along the Pacific of Costa Rica. Sampling sites in this study were selected based on the protection status, reef/rocky formations where species may aggregate or because they represented a gradient of fishing intensity and habitat degradation. Within each site, we tried to cover the main habitats available (e.g. coral and rocky reefs, sandy bottoms, rhodolith beds, mangroves, etc.) based on previous studies, local ecological knowledge and guidance from Park Rangers when surveying some of the conservation areas of Costa Rica. Two types of BRUVS were used according to the bottom structure at each surveyed habitat: (1) benthic BRUVS – a pyramid-shaped steel frame that was lying on the seabed (for a graphical representation of the design see Ref.99); and (2) mid-water BRUVS—a triangle-shape steel frame that had a small weight anchored to the bottom and was suspended 1–2 m from the seafloor in the water column by an underwater buoy system (for a graphical representation of the design see Ref.52). Benthic BRUVS were used to record species associated with flat or less irregular seabed, whereas mid-water BRUVS were used to record species around topographically complex structures (e.g. underwater pinnacles) or more irregular bottoms in order to maximize the quality of the video files and BRUVS retrieval. Both BRUVS designs had detachable bait arms, consisting of a metal bar and a PVC mesh cylinder containing 1–1.5 kg of crushed mackerel (Scomber japonicus), a locally sourced bait used by the longline fishery of Costa Rica. Polystyrene surface floats were attached by an 8 mm polypropylene rope to facilitate retrieval of steel frames from small boats. Each BRUVS had a single camera (GoPro Hero4) with additional battery pack and was set to record at a rate of 60 frames per second/1080p resolution. BRUVS were deployed during daylight hours (8:00–17:00) at depths ranging from 1 to 60 m (mean ± SD; 15.04 ± 8.9 m) and set approximately 300–500 m apart from each other to ensure sample independence19,99. Soak times (effective time when BRUVS were recording at the bottom) for all deployments varied from 50 to 200 min (mean ± SD: 103 ± 29 min) and BRUVS deployments per site from 37 to 158 (Table S5). Water visibility was generally above 5 m and up to 20 m at Cocos Island, but some of the coastal sites had poor visibility (< 5 m) and consequently were removed from the analyses. A temperature datalogger (ONSET Hobo Pendant) was attached to the steel frame to monitor water temperature at 1-min intervals. We also recorded the unique BRUVS ID (combination of the station No. + GoPro No. + site), location (latitude/longitude), date/time and depth. Sampling sites were plotted using base and raster layers from the Costa Rican Geographic Information System (GIS) Atlas open-access project (https://hdl.handle.net/2238/6749) in ArcMap 10.4 (ESRI, Redlands, California) (Fig. 1).
Video footage was processed and analyzed using the software EventMeasure (SeaGIS) in order to: (1) manage data from field operations and video reading; (2) capture the timing of events; and (3) capture reference images of the seafloor and sharks in the field of view. To avoid recounting individuals, we used the MaxN (i.e. maximum number of individuals from each species observed in a single video frame) as a conservative measure of relative abundance14. All species were identified to the lowest taxonomical level using local fishing guides100 or by consulting an expert if needed.
Habitat structure and composition
Habitat composition was determined by analyzing reference images of substrates from each BRUVS. Reference images were overlapped with a 400 evenly-space red dot matrix that was used to estimate the proportion of each substrate available (i.e. number of dots that felt in each substrate type relative to the total number of overlapping points on identifiable substrates) (Fig. S2). Classification and estimation of each substrate cover percentage was made by two independent observers. The substrate cover, hereafter referred to habitat composition, was categorized in the following eight major habitats: bare rock with encrusting organisms, reef-building coral, bleached coral, rock/turf, macroalgae, sand/rubble (including small rubble), Caulerpa sertularioides (invasive algae), and others (i.e. anemones, barnacles, cyanobacteria, octocoral, sponges, softcoral and tunicates). A qualitative scale (1–3; low to high) was used to assess the degree of topographic complexity, visibility and depth of field (i.e. low values were assigned when footage was obstructed by a rock or other kind of substrate, or when camera was facing down/up) for each reference image (Fig. S3). BRUVS with very low visibility and depth of field (average of both parameters between two observers: < 1.5) were removed from the analyses. A protocol was created in order to standardize the substrate cover analysis methodology and decrease subjectivity among observers (see Table S6 for more details). Although results from this analysis may be restricted by the field of view and visibility of each BRUVS, they provided reliable data to evaluate species-habitat associations at the BRUVS level.
We used a principal component analysis (PCA), by constraining habitat scores, to display only the variation among BRUVS that could be explained by the percent cover of major habitat types101. This reduced the number of habitat components that explained > 70% of the variability amongst BRUVS into two major principal scores: (1) bare rock with incrusting organisms (PC1); (2) rock/turf to sand/rubble (PC2) (Table S7). The PCA was analyzed using the RDA function from the vegan library in R statistical package v.3.6.2102.
Elasmobranch assemblages across sites
General patterns of elasmobranch species composition and relative abundance were examined using BRUVS across 11 sites of the Pacific waters of Costa Rica between December 2016 and June 2019 (Fig. 1). From our surveys, 30 BRUVS had low visibility and/or low depth of field, and therefore, were removed from the analysis. The remaining 430 BRUVS consisted of 362 benthic stations (sampling effort: 604.5 h) and 68 mid-water stations (sampling effort: 106.0 h) (Table S4). Sites with < 15 BRUVS from the North Pacific (Flamingo, Culebra Bay and Nicoya Peninsula) and South Pacific (Marino-Ballena and Golfo Dulce) regions were removed from the analyses.
At the BRUVS level, we determined the proportion of stations that recorded elasmobranchs at each site and region (e.g. Cocos Island, North and South Pacific). Both benthic and mid-water BRUVS detected a similar shark richness (11 and 10 species, respectively), whereas rays were more common on benthic (16 species) than mid-water BRUVS (6 species) (Table S4). Given that fewer mid-water BRUVS (N = 68) were deployed across sites compared to benthic BRUVS (N = 362; Table S3), elasmobranch assemblages from both benthic and mid-water BRUVS were pooled for analyses. Due to logistic reasons, there was some variability in deployment soak times (Table S3). However, most BRUVS had deployments that lasted > 80 min (Table S3). A recent study found that 77 min is an optimal soak time for BRUVS surveys of reef-associated elasmobranchs (95% of species sightings occur between 63 and 77 min), with longer deployments not having significant effects on species richness85. Based on these findings, we used the time to first sighting (TFS—time elapsed between the start of the sampling period and the first record of a particular species in the field of view) to assess any potential issues with different sampling effort (soak times) across sites. First, we calculated the proportion of TFS events for all species recorded at each site by time. Then, we used the empirical cumulative density function (ECDF, a step function) to estimate the fraction of observations of TFS that were less than or equal to a specified value. The ECDF plots were used to find the 75th and 95th percentiles of TFS for all species by site (Fig. S1).
Elasmobranch species captured by our BRUVS were classified as filter-feeders, small predators (species with maximum body sizes < 1.5 m) or large predators (species with maximum body sizes > 1.5 m) in order to examine changes in the trophic structure across sites. Since our study did not use stereo-BRUVS our ability to estimate individual size of elasmobranchs was limited. Therefore, our classification system was based on average size-estimates from underwater visual surveys conducted at the same study sites (Espinoza unpublished data), the maximum body size of the species103 and following the criteria regarding the functional groups classification stated by Refs.104,105. Therefore, any size estimates provided are restricted to the proportion of species detected at the site during underwater visual surveys and did not include any abundance information.
In order to reduce biases associated to bait dispersal among close BRUVS units or the wide-ranging movement patterns of some captured species, BRUVS that were deployed simultaneously at the same site and date were pooled together and treated as independent samples. Therefore, the maximum MaxN of each elasmobranch species per date was summed across sites. To standardize sampling effort across sites, the MaxN was divided by the soak time for each site/date, and expressed as catch per unit effort (CPUE; MaxN hrs−1). A non-metric multidimensional scaling (nMDS) ordination plot followed by a Permutational Multivariate Analysis of Variance (PERMANOVA; 1000 permutations) was used to examine differences in elasmobranch assemblages among sites. The function ordiellipse from the vegan library in R v.3.6.2102 was used to display the standard deviation of points from each site. A wrapper function (pairwise.adonis) that returns adjusted p values for multilevel pairwise comparisons from the vegan library in R v.3.6.2 was used to determine sites that were significantly different from each other. A Bray–Curtis dissimilarity matrix was constructed with the transformed elasmobranch species CPUE in columns (squared root MaxN hrs−1) and sites/dates in rows to reduce the influence of highly abundant species106. Using the same species-site matrix from nMDS analysis, we used the function annHeatmap2 from the Heatplus library in R v.3.6.2102 to examine how the samples (i.e. BRUVS deployed at each site on different dates) clustered by site, and also the level of co-occurrence among species. For this analysis, we calculate the Bray–Curtis dissimilarity matrix on the full data set and used an average linkage hierarchical clustering for rows (samples) and columns (species). Ten species were removed from the analysis (Diplobatis ommata, Narcine entemedor, Styracura pacifica, Urotrygon aspidura, Mobula Japonica, M. munkiana, M. tarapacana, Rhincodon typus, Pseudobatos glauca, P. planiceps) because of their low frequency of occurrence (they were sighted in less than 5% of the stations). The resulting heat map allowed identifying dominant species, as well as clustering among rows and columns.
Drivers of elasmobranch abundance and diversity
Poisson and negative binomial generalized linear models (GLMs) were used to assess how species richness and relative abundance (MaxN) were influenced by region (e.g. Isla del Coco, North and South Pacific), habitat protection (no-take vs. open fishing sites), elasmobranch group (sharks vs. rays), environmental drivers (temperature and depth) and habitat composition (e.g. PC1 and PC2). Based on our data, Poisson models outperformed negative binomial models. Only Isla del Coco, Islas Murciélago and Isla del Caño were considered true no-take MPAs, as some types of recreational and artisanal fishing are still allowed in Santa Elena Bay. Sampling effort (hr) was included as an offset to account for variability in soak time at each site. The performance of Poisson models were compared using maximum likelihood ratio tests and Akaike’s information criterion (AIC) of nested models. To determine the importance of the predictors from selected models, the difference in AIC with and without each term was computed using likelihood ratio tests. These analyses were done using the libraries pscl, MuMIn and lmtest from R v.3.6.2102.
An ordinal logistic regression model was used to investigate the effect of habitat protection on the number of BRUVS that recorded small or large predators using the polr from the MASS library in R v.3.6.2102. For this analysis, all elasmobranch species classified as small or large predators were summed across BRUVS from sampling sites belonging to the same category of habitat protection, and a presence-absence matrix was created for these two predatory groups (small and large predators). Filter-feeder species were excluded from the analyses. From the presence-absence matrix, we classified the predatory groups as − 1 (if only small predators were detected at each station), 0 (if there were both small and large predators detected at each station) or 1 (if only large predators were detected). Following the ordinal logistic analysis, we calculated the predicted probabilities of each event.
References
McCauley, D. J. et al. Marine defaunation: animal loss in the global ocean. Science 80(347), 247–254 (2015).
Myers, R. A. & Worm, B. Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283 (2003).
Myers, R. A. & Worm, B. Extinction, survival or recovery of large predatory fishes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 13–20 (2005).
Dulvy, N. K. et al. Extinction risk and conservation of the world’s sharks and rays. Elife 3, e00590–e00590 (2014).
Davidson, L. N. K., Krawchuk, M. A. & Dulvy, N. K. Why have global shark and ray landings declined: Improved management or overfishing?. Fish Fish. 17, 438–458 (2016).
Chin, A., Kyne, P. M., Walker, T. I. & McAauley, R. B. An integrated risk assessment for climate change: analysing the vulnerability of sharks and rays on Australia’s Great Barrier Reef. Glob. Change Biol. 16, 1936–1953 (2010).
Dulvy, N. K. et al. Ghosts of the coast: global extinction risk and conservation of sawfishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 26, 134–153 (2014).
Espinoza, M., Heupel, M. R., Tobin, A. J. & Simpfendorfer, C. A. Evidence of partial migration in a large coastal predator: Opportunistic foraging and reproduction as key drivers?. PLoS ONE 11, e0147608 (2016).
Papastamatiou, Y. P. et al. Telemetry and random-walk models reveal complex patterns of partial migration in a large marine predator. Ecology 94, 2595–2606 (2013).
Williams, J. J. et al. Mobile marine predators: an understudied source of nutrients to coral reefs in an unfished atoll. Proc. R. Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rspb.2017.2456 (2018).
Ketchum, J. T. et al. Inter-island movements of scalloped hammerhead sharks (Sphyrna lewini) and seasonal connectivity in a marine protected area of the eastern tropical Pacific. Mar. Biol. 161, 939–951 (2014).
Espinoza, M., Lédée, E. J., Simpfendorfer, C. A., Tobin, A. J. & Heupel, M. R. Contrasting movements and connectivity of reef-associated sharks using acoustic telemetry: implications for management. Ecol. Appl. 25, 2101–2118 (2015).
Mallet, D. & Pelletier, D. Underwater video techniques for observing coastal marine biodiversity: a review of sixty years of publications (1952–2012). Fish. Res. 154, 44–62 (2014).
Cappo, M., Speare, P. & Death, G. Comparison of baited remote underwater video stations (BRUVS) and prawn (shrimp) trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. J. Exp. Mar. Biol. Ecol. 302, 123–152 (2004).
Caldwell, Z. R., Zgliczynski, B. J., Williams, G. J. & Sandin, S. A. Reef fish survey techniques: assessing the potential for standardizing methodologies. PLoS ONE 11, e0153066 (2016).
Dapp, D., Arauz, R., Spotila, J. R. & O’Connor, M. P. Impact of Costa Rican longline fishery on its bycatch of sharks, stingrays, bony fish and olive ridley turtles (Lepidochelys olivacea). J. Exp. Mar. Biol. Ecol. 448, 228–239 (2013).
Bicknell, A., Godley, B., Sheehan, E., Votier, S. & Witt, M. Camera technology for monitoring marine biodiversity and human impact. Front. Ecol. Environ. 14, 424–432 (2016).
Consoli, P. et al. Fish distribution and habitat complexity on banks of the Strait of Sicily (central Mediterranean Sea) from remotely-operated vehicle (ROV) exploration. PLoS ONE 11, e0167809 (2016).
Espinoza, M., Cappo, M., Heupel, M. R., Tobin, A. J. & Simpfendorfer, C. A. Quantifying shark distribution patterns and species-habitat associations: implications of Marine Park Zoning. PLoS ONE 9, e106885 (2014).
Barley, S. C., Mehta, R. S., Meeuwig, J. J. & Meekan, M. G. To knot or not? Novel feeding behaviours in moray eels. Mar. Biodivers. 46, 703–705 (2016).
Struthers, D. P., Danylchuk, A. J., Wilson, A. D. M. & Cooke, S. J. Action cameras: bringing aquatic and fisheries research into view. Fisheries 40, 502–512 (2015).
Speed, C. W., Rees, M. J., Cure, K., Vaughan, B. & Meekan, M. G. Protection from illegal fishing and shark recovery restructures mesopredatory fish communities on a coral reef. Ecol. Evol. https://doi.org/10.1002/ece3.5575 (2019).
Jabado, R. W., Al Hameli, S. M., Grandcourt, E. M. & Al Dhaheri, S. S. Low abundance of sharks and rays in baited remote underwater video surveys in the Arabian Gulf. Sci. Rep. 8, 1–11 (2018).
Westerberg, H. & Westerberg, K. L. Properties of odour plumes from natural baits. Fish. Res. 110, 459–464 (2011).
Dorman, S. R., Harvey, E. S. & Newman, S. J. Bait effects in sampling coral reef fish assemblages with stereo-BRUVs. PLoS ONE 7, e41538 (2012).
Sherley, R. B. et al. Estimating IUCN Red List population reduction: JARA—a decision-support tool applied to pelagic sharks. Conserv. Lett. https://doi.org/10.1111/conl.12688 (2019).
Espinoza, M., Díaz, E., Angulo, A., Hernández, S. & Clarke, T. M. Chondrichthyan diversity, conservation status, and management challenges in Costa Rica. Front. Mar. Sci. 5, 1–15 (2018).
Trujillo, P., Cisneros-Montemayor, A. M., Harper, S., Zylich, K. & Zeller, D. Reconstruction of Costa Rica’s marine fisheries catches, 1950–2010. Fish. Bethesda 31, 1–16 (2015).
Arias, A. & Pressey, R. L. Combatting illegal, unreported, and unregulated fishing with information: a case of probable illegal fishing in the tropical Eastern Pacific. Front. Mar. Sci. 3, 1–4 (2016).
López-Garro, A., Ilena, Z., Frank, M., Geiner, G.-D. & Maikel, P.-M. pesca ilegal en el Parque Nacional Isla del Coco, Costa Rica. Rev. Biol. Trop. 64, 249–262 (2016).
Heupel, M. R. & Simpfendorfer, C. A. Importance of environmental and biological drivers in the presence and space use of a reef-associated shark. Mar. Ecol. Prog. Ser. 496, 47–57 (2014).
Drymon, J. M., Powers, S. P., Dindo, J., Dzwonkowski, B. & Henwood, T. A. Distributions of sharks across a continental shelf in the northern Gulf of Mexico. Mar. Coast. Fish. Dyn. Manag. Ecosyst. Sci. 2, 440–450 (2010).
Poulakis, G. R., Stevens, P. W., Timmers, A. A., Wiley, T. R. & Simpfendorfer, C. A. Abiotic affinities and spatiotemporal distribution of the endangered smalltooth sawfish, Pristis pectinata, in a south-western Florida nursery. Mar. Freshw. Res. 62, 1165–1177 (2011).
Sibaja-cordero, J. A. Tendencias espacio-temporales de los avistamientos de fauna marina en los buceos turísticos (Isla del Coco, Costa Rica). Revista 56, 113–132 (2008).
Clarke, T. M., Espinoza, M., Ahrens, R. & Wehrtmann, I. S. Elasmobranch bycatch associated with the shrimp trawl fishery off the pacific coast of Costa Rica, Central America. Fish. Bull. 114, 1–17 (2016).
Zanella, I. & López-Garro, A. Abundancia, reproducción y tallas del tiburón martillo Sphyrna lewini (Carcharhiniformes: Sphyrnidae) en la pesca artesanal de Golfo Dulce, Pacífico de Costa Rica. Rev. Biol. Trop. 63, 307–317 (2015).
Nalesso, E. et al. Movements of scalloped hammerhead sharks (Sphyrna lewini) at Cocos Island, Costa Rica and between oceanic islands in the Eastern Tropical Pacific. PLoS ONE 14, e0213741 (2019).
Valerio-Vargas, J. & Espinoza, M. A beacon of hope: distribution and current status of the largetooth sawfish in Costa Rica. Endanger. Species Res. 40, 231–242 (2019).
IUCN. The IUCN Red List of Threatened Species. Version 2019–3 (2020). Available at: https://www.iucnredlist.org.
Whoriskey, S., Arauz, R. & Baum, J. K. Potential impacts of emerging mahi-mahi fisheries on sea turtle and elasmobranch bycatch species. Biol. Conserv. 144, 1841–1849 (2011).
Cortés, J. et al. Elasmobranchs observed in deepwaters (45–330m) at Isla del Coco National Park, Costa Rica (Eastern Tropical Pacific). Rev. Biol. Trop. 60, 257–273 (2012).
White, E. R., Myers, M. C., Flemming, J. M. & Baum, J. K. Shifting elasmobranch community assemblage at Cocos Island-an isolated marine protected area. Conserv. Biol. 29, 1186–1197 (2015).
López-Garro, A. & Zanella, I. Tiburones y rayas capturados por pesquerías artesanales con línea de fondo en el Golfo Dulce, Costa Rica. Rev. Biol. Trop. 63, 183–198 (2015).
López-Garro, A., Arauz-Vargas, R., Ilena, Z. & Le-Foulgo, L. Análisis de las capturas de tiburones y rayas en las pesquerías artesanales de Tárcoles, Pacífico Central de Costa Rica. Rev. Ciencias Mar. y Costeras 1, 145–157 (2009).
Bizzarro, J. J., Smith, W. D., Márquez-Farías, J. F., Tyminski, J. & Hueter, R. E. Temporal variation in the artisanal elasmobranch fishery of Sonora, Mexico. Fish. Res. 97, 103–117 (2009).
Dulvy, N. K. et al. Fishery stability, local extinctions, and shifts in community structure in skates. Conserv. Biol. 14, 283–293 (2000).
White, J., Simpfendorfer, C. A., Tobin, A. J. & Heupel, M. R. Application of baited remote underwater video surveys to quantify spatial distribution of elasmobranchs at an ecosystem scale. J. Exp. Mar. Biol. Ecol. 448, 281–288 (2013).
Salas, E., Sánchez-Godínez, C. & Montero-Cordero, A. Peces marinos de la Reserva Biológica Isla del Caño: Estructura de las comunidades de peces de arrecife y lista taxonómica actualizada de los peces costeros. Rev. Biol. Trop. 63, 97–116 (2015).
Cappo, M., Harvey, E., Malcolm, H., Speare P. Potential of video techniques to monitor diversity, abundance and size of fish in studies of marine protected areas. In Beumer, J. P., Grant, A., Smith, D. C., editors, Aquatic protected areas. What works best and how do we know? Cairns ed. Vol. 1. Queensland: University of Queensland. 2003. p. 455–464.
Lindfield, S. J., Harvey, E. S., McIlwain, J. L. & Halford, A. R. Silent fish surveys: bubble-free diving highlights inaccuracies associated with SCUBA-based surveys in heavily fished areas. Methods Ecol. Evol. 5, 1061–1069 (2014).
Bond, M. E. et al. Top predators induce habitat shifts in prey within marine protected areas. Oecologia 190, 375–385 (2019).
Acuña-Marrero, D. et al. Spatial patterns of distribution and relative abundance of coastal shark species in the Galapagos Marine Reserve. Mar. Ecol. Prog. Ser. 593, 73–95 (2018).
Lara-Lizardi, F. Distribution Patterns of Sharks in the Revillagigedo Archipelago and Their Connectivity in the Eastern Tropical Pacific. Tesis de Maestria. (Instituto Politecnico Nacional, 2018).
Bond, M. E. et al. Reef sharks exhibit site-fidelity and higher relative abundance in marine reserves on the Mesoamerican Barrier Reef. PLoS ONE 7, e32983 (2012).
Edgar, G. J. et al. Global conservation outcomes depend on marine protected areas with five key features. Nature 506, 216–220 (2014).
Cinner, J. E. et al. Gravity of human impacts mediates coral reef conservation gains. Proc. Natl. Acad. Sci. U.S.A. 115, E6116–E6125 (2018).
Juhel, J.-B. et al. Reef accessibility impairs the protection of sharks. J. Appl. Ecol. 55, 673–683 (2018).
Friedlander, A. M. et al. The shallow-water fish assemblage of Isla del Coco National Park, Costa Rica: structure and patterns in an isolated, predator-dominated ecosystem. Rev. Biol. Trop. 60, 321–338 (2012).
Fourriére, M. et al. Energy flow structure and role of keystone groups in shallow water environments in Isla del Coco, Costa Rica, Eastern Tropical Pacific. Ecol. Model. 396, 74–85 (2019).
Alvarado, J. J., Cortés, J., Esquivel, M. F. & Salas, E. Costa Rica’s marine protected areas: status and perspectives. Rev. Biol. Trop. 60, 129–142 (2012).
Arias, A., Pressey, R. L., Jones, R. E., Álvarez-Romero, J. G. & Cinner, J. E. Optimizing enforcement and compliance in offshore marine protected areas: a case study from Cocos Island, Costa Rica. Oryx 50, 18–26 (2014).
Graham, N. A. J., Spalding, M. D. & Sheppard, C. R. C. Reef shark declines in remote atolls highlight the need for multi-faceted conservation action. Aquat. Conserv. Mar. Freshw. Ecosyst. 20, 543–548 (2010).
Dent, F. & Clarke, S. State of the global market for shark products. FAO Fisheries and Aquaculture Technical Paper 590. Food and Agriculture Organization of the United Nations, Rome, Italy (2015).
Hooker, S. K. et al. Making protected area networks effective for marine top predators. Endanger. Species Res. 13, 203–218 (2011).
De’ath, G., Fabricius, K. E., Sweatman, H. & Puotinen, M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. U.S.A. 109, 17995–17999 (2012).
McKinney, J. A., Hoffmayer, E. R., Wu, W., Fulford, R. & Hendon, J. M. Feeding habitat of the whale shark Rhincodon typus in the northern Gulf of Mexico determined using species distribution modelling. Mar. Ecol. Prog. Ser. 458, 199–211 (2012).
Norton, S. L. et al. Designating critical habitat for juvenile endangered smalltooth sawfish in the United States. Mar. Coast. Fish. 4, 473–480 (2012).
Alvarado, J. J. et al. Cuando la conservación no puede seguir el ritmo del desarrollo: Estado de salud de los ecosistemas coralinos del Pacífico Norte de Costa Rica. Rev. Biol. Trop. 66, 280–308 (2018).
Arias-Godínez, G. et al. Spatial and temporal changes in reef fish assemblages on disturbed coral reefs, north Pacific coast of Costa Rica. Mar. Ecol. 40, e12532 (2019).
Beita-Jiménez, A., Alvarado, J. J., Mena, S. & Guzmán-Mora, A. G. Benefits of protection on reef fish assemblages in a human impacted region in Costa Rica. Ocean Coast. Manag. 169, 165–170 (2019).
Cortés, J., Jiménez, C. E., Fonseca, A. C. & Alvarado, J. J. Status and conservation of coral reefs in Costa Rica. Rev. Biol. Trop. 58(Suppl 1), 33–50 (2010).
Stevens, J., Bonfil, R., Dulvy, N. K. & Walker, P. A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 57, 476–494 (2000).
Sandin, S. A. et al. Baselines and degradation of coral reefs in the Northern Line Islands. PLoS ONE 3, e1548 (2008).
Heithaus, M. R. et al. Seagrasses in the age of sea turtle conservation and shark overfishing. Front. Mar. Sci. 1, 1–6 (2014).
Roff, G. et al. The ecological role of sharks on Coral Reefs. Trends Ecol. Evol. 31, 395–407 (2016).
Villalobos-Rojas, F., Herrera-Correal, J., Garita-Alvarado, C., Clarke, T. & Beita-Jiménez, A. Actividades pesqueras dependientes de la ictiofauna en el Pacífico Norte de Costa Rica. Rev. Biol. Trop. 62, 119–138 (2014).
Ruppert, J. L. W., Travers, M. J., Smith, L. L., Fortin, M.-J. & Meekan, M. G. Caught in the middle: combined impacts of shark removal and coral loss on the fish communities of coral reefs. PLoS ONE 8, e74648 (2013).
Schlaff, A. M., Heupel, M. R. & Simpfendorfer, C. A. Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Rev. Fish Biol. Fish. 24, 1089–1103 (2014).
Rizzari, J. R., Frisch, A. J. & Magnenat, K. A. Diversity, abundance, and distribution of reef sharks on outer-shelf reefs of the Great Barrier Reef, Australia. Mar. Biol. 161, 2847–2855 (2014).
Goetze, J. S. et al. Drivers of reef shark abundance and biomass in the Solomon Islands. PLoS ONE 13, 1–16 (2018).
Bessudo, S. et al. Residency of the scalloped hammerhead shark (Sphyrna lewini) at Malpelo Island and evidence of migration to other islands in the Eastern Tropical Pacific. Environ. Biol. Fishes 91, 165–176 (2011).
Hardinge, J., Harvey, E. S., Saunders, B. J. & Newman, S. J. A little bait goes a long way: the influence of bait quantity on a temperate fish assemblage sampled using stereo-BRUVs. J. Exp. Mar. Bio. Ecol. 449, 250–260 (2013).
Bouchet, P. J. & Meeuwig, J. J. Drifting baited stereo-videography: a novel sampling tool for surveying pelagic wildlife in offshore marine reserves. Ecosphere 6, 137 (2015).
Whitmarsh, S. K., Fairweather, P. G. & Huveneers, C. What is Big BRUVver up to? Methods and uses of baited underwater video. Rev. Fish Biol. Fish. 27, 53–73 (2017).
Currey-Randall, L. M., Cappo, M., Simpfendorfer, C. A., Farabaugh, N. F. & Heupel, M. R. Optimal soak times for Baited remote underwater video station surveys of reef-associated elasmobranchs. PLoS ONE 15, e0231688 (2020).
Wehrtmann, I. S. & Cortés, J. Marine Biodiversity of Costa Rica, Central America (Springer, Berlin, 2009).
Cortés, J. Comunidades coralinas y arrecifes del Area de Conservación Guanacaste, Costa Rica. Rev. Biol. Trop. 44–45, 623–625 (1997).
Alfaro, E. et al. Climate and subsurface sea temperature in Bahía Culebra, Costa Rica. Rev. Biol. Trop. 60, 159–171 (2012).
Stuhldreier, I., Sánchez-Noguera, C., Roth, F., Cortés, J., Rixen, T. & Wild, C. Upwelling increases net primary production of corals and reef-wide gross primary production along the Pacific coast of Costa Rica. Front. Mar. Sci. 2, 113 (2015).
Quesada Alpízar, M. A. & Cortés, J. Los ecosistemas marinos del Pacífico sur de Costa Rica: estado del conocimiento y perspectivas de manejo. Rev. Biol. Trop. 54, 101–145 (2006).
Cortés, J. et al. Ambientes y organismos marinos de la Reserva Biológica Isla del Caño, Área de Conservación Osa, Costa Rica. Serie Técnica: Apoyando los esfuerzos en el manejo y protección de la biodiversidad tropical. No. 13. TNC, San José, Costa Rica. 48 p (2009).
Guzman, H. M. & Cortes, J. Coral reef community structure at Caño Island, Pacific Costa Rica. Mar. Ecol. 10, 23–41 (1989).
Fourriére, M., Alvarado, J. J., Ayala Bocos, A. & Cortés, J. Updated checklist and analysis of completeness of the marine fish fauna of Isla del Coco, Pacific of Costa Rica. Mar. Biodivers. https://doi.org/10.1007/s12526-016-0501-6 (2016).
Lizano, O. G. Rasgos morfológicos alrededor de la Isla del Coco y de sus montes submarinos vecinos, Pacífico de Costa Rica. Rev. Biol. Trop. 60, 43–51 (2012).
Guzman, H. M. & Cortes, J. Cocos Island (Pacific of Costa Rica) coral reefs after the 1982–83 El Niño disturbance. Rev. Biol. Trop. 40, 309–324 (1992).
Lizano, O. G. Dinámica de aguas alrededor de la Isla del Coco, Costa Rica. Rev. Biol. Trop. 56, 31–48 (2008).
Alvarado, J. J. et al. Coral reefs of Isla del Coco National Park, Costa Rica: structure and comparison (1987–2014). Rev. Biol. Trop. 64, S153–S176 (2016).
Acuña-González, J., García-Céspedes, J., Gómez Ramírez, E., Vargas-Zamora, J. & Cortés, J. Parámetros físico-químicos en aguas costeras de la Isla del Coco, Costa Rica (2001–2007). Rev. Biol. Trop. 56, 49–56 (2008).
Cappo, M., De’ath, G. & Speare, P. Inter-reef vertebrate communities of the Great Barrier Reef Marine Park determined by baited remote underwater video stations. Mar. Ecol. Prog. Ser. 350, 209–221 (2007).
Robertson, D. R. & R Allen, G. Shorefishes of the Tropical Eastern Pacific online information system. Version 2.0 Smithsonian Tropical Research Institute, Balboa, Panama (2015).
Syms, C. Principal components analysis. In Encyclopedia of Ecology (eds Jorgenses, S. E. & Fath, B. D.) 2940–2949 (Elsevier, Amsterdam, 2008).
R Development Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2019) URL https://www.R-project.org/.
Froese, R. & Pauly, D. FishBase. Available at: https://fishbase.org. (2019)
Heupel, M. R., Knip, D. M., Simpfendorfer, C. A. & Dulvy, N. K. Sizing up the ecological role of sharks as predators. Mar. Ecol. Prog. Ser. 495, 291–298 (2014).
Roff, G. et al. The Ecological Role of Sharks on Coral Reefs. Trends Ecol. Evol. XX, 1–13 (2016).
Clarke, K. R. & Gorley, R. N. Primer Version 5 (2001).
Acknowledgements
This project was financially supported by Universidad de Costa Rica (VI-UCR No. B8600, B8186 and B7146). We are also thankful for the external support from the Global FinPrint, PADI Foundation, IdeaWild, National Geographic (NGS-54004C-18), Fundación Pacífico, Asociación Costa Rica por Siempre, Waitt Foundation, Migramar, Conservación Internacional and Fundación Amigos de la Isla del Coco (FAICO). We would like to thank M. Marrero (Mape), F. Chirino, D. Solís, M. A. Arriaga, M. Eisele, D. Masís, J. Valerio, N. Moore, B. Farias, F. Arias, C. Zuñiga, O. Vega, N. Goebel, S. Mena, B. Naranjo, G. Ugarte and many other students and assistants that help us with the fieldwork and video analysis. We also would like to thank Costa Rica Dive and Surf, Diving Center Cuajiniquil, Snorkeling Cuajiniquil, ConnectOcean Dive Centre and Swimming Academy, Undersea Hunter Group and Costa Rica Wildlife Foundation for their support during our field surveys. Special thanks to J. Alvarado (Chepe) from CIMAR-UCR for his support during early stages of the project and G. Golfin from the Área de Conservación Marina Cocos (ACMC) for all his support in expanding this monitoring to the rest of the ETP islands. We also would like to thank L. Currey-Randall (AIMS) and M. Cappo for the code that was used to reproduce one of the figures. This study was conducted under the following permits PI-044-2017, INV-ACOSA-001-17, INV-ACOSA-022-19, 05-2019-I-ACMC.
Author information
Authors and Affiliations
Contributions
M.E. acquired the funding for this project and was in charge of the administration and field logistic coordination. M.E., T.A.-A., I.C.-Z., I.C. and M.C. collected the data in the field. M.E., T.A.-A., I.C.-Z. and M.C. completed the video analysis. M.E. did all statistical analyses of the manuscript. M.E., T.A.-A., I.C.-Z. and M.C. wrote the manuscript text. M.E. prepared all figures for publication. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Espinoza, M., Araya-Arce, T., Chaves-Zamora, I. et al. Monitoring elasmobranch assemblages in a data-poor country from the Eastern Tropical Pacific using baited remote underwater video stations. Sci Rep 10, 17175 (2020). https://doi.org/10.1038/s41598-020-74282-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74282-8
This article is cited by
-
Do reef fish assemblages benefit from a marine protected area in the north Pacific coast of Costa Rica?
Environmental Biology of Fishes (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.