Abstract
We aimed to compare retinal vascular density in Optical Coherence Tomography Angiography (OCT-A) between patients hospitalized for acute coronary syndrome (ACS) and control patients and to investigate correlation with angiogenesis biomarkers. Patients hospitalized for an acute coronary syndrome (ACS) in the Intensive Care Unit were included in the “high cardiovascular risk” group while patients without cardiovascular risk presenting in the Ophthalmology department were included as “control”. Both groups had blood sampling and OCT-A imaging. Retina microvascularization density in the superficial capillary plexus was measured on 3 × 3 mm angiograms centered on the macula. Angiopoietin-2, TGF-β1, osteoprotegerin, GDF-15 and ST-2 were explored with ELISA or multiplex method. Overall, 62 eyes of ACS patients and 42 eyes of controls were included. ACS patients had significantly lower inner vessel length density than control patients (p = 0.004). A ROC curve found that an inner vessel length density threshold below 20.05 mm−1 was moderately associated with ACS. Significant correlation was found between serum levels of angiopoietin-2 and osteoprotegerin, and retinal microvascularization in OCT-A (R = − 0.293, p = 0.003; R = − 0.310, p = 0.001). Lower inner vessel length density measured with OCT-A was associated with ACS event and was also correlated with higher concentrations of angiopoietin-2 and osteoprotegerin.
Similar content being viewed by others
Introduction
In spite of the improvements in diagnosis and treatment of cardiovascular diseases (CVD), aging of population and urbanization make CVD one of the world’s major disease burdens1,2. Indeed, CVD remain a main cause of premature deaths and disability worldwide, with an estimated 16.7 million deaths in 2010, and projections show an overwhelming 23.3 million by 20303.
Cardiovascular risk factors such as hypertension, hypercholesterolemia, and diabetes mellitus lead to systemic inflammation, vascular and cardiac oxidative stress, which contribute to coronary dysfunction and microvascular impairment4. Thus, coronary macro and microvascular alterations are closely associated and together contribute to the pathophysiology of myocardial ischemia5. The assessment of myocardial microvascularization is then of major interest in order to estimate the risk of acute coronary events; however, only invasive procedures, using intra vascular contrast agents, are currently available6.
Conversely, retinal microvascularization is easy to assess and can be studied through non-invasive exams7. Additionally, the retinal vascular network seems to reflect the systemic vascular status, and its study may even predict cardiovascular mortality8,9,10,11,12. Indeed, changes in the retinal vasculature, such as narrowing or tortuosity, have been associated with elevated blood pressure, heart failure or arterial stiffness index9,10,11,12,13.
Optical Coherence Tomography-Angiography (OCT-A) is a non-invasive method used to obtain detailed imaging of retina and choroidal vascularization without intravascular dye14. This device allows the quantitative evaluation of vascular parameters, such as the superficial capillary plexus (SCP) vessel density and foveal avascular zone (FAZ) area15. We previously showed in a pilot study that inner vessel length density in the SCP measured with OCT-A was associated with cardiovascular risk profiles in patients hospitalized for an acute coronary syndrome (ACS)16. However, these preliminary results need more supporting evidence with control patients. Moreover, histological analysis confirming the link between reduced vessel density in retina and myocardium is difficult and invasive17. Thus in this study, we aimed to reinforce our analysis of systemic microvascularization through biomarkers of angiogenesis and inflammation (angiopoietin-2, TGF-β1, osteoprotegerin, GDF-15 and ST-2). The purpose of this study was therefore to compare and try to seek for associations between retinal microvascularization with OCT-A and systemic microvascularization biomarkers in patients with a high cardiovascular risk profile and control subjects.
Results
Study population
We included 69 eyes of ACS patients from Cardiac Intensive Care Units and 53 eyes of patients from the Ophthalmology department. Eighteen eyes were excluded because their OCT-A exams were of poor quality or blood sample collection was not performed. Finally, 62 eyes of ACS patients and 42 eyes of control patients were kept for analysis (Fig. 1). The two groups were similar in age (p = 0.500) but there were less males in control group (p = 0.026). In the high cardiovascular risk group, 30 patients had hypertension (48%), 8 had diabetes (13%), 23 were smokers and had hypercholesterolemia (37%), 24 were obese (39%). Forty patients (65%) had a ST-segment Elevation Myocardial Infarction (STEMI) (Table 1).
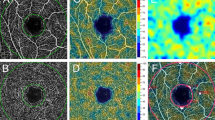
Retinal vascular density analysis
Table 2 shows the quantitative data for retinal microvasculature assessed with OCT-A. There was a significant difference in retinal vascular density between ACS and control patients. Inner vessel length density in ACS patients was lower than in control subjects, respectively 19.76 mm−1 ± 1.77 and 20.67 mm−1 ± 1.33 (p = 0.004). A significant difference was also found for the inner perfusion density between ACS and control patients with respectively a mean measured at 0.360 ± 0.027 and 0.373 ± 0.024 (p = 0.011).
The analysis of ROC curve demonstrated the performance of the inner vessel length density to classify patients in the high cardiovascular group (area under the ROC curve 0.662; 95% CI 0.559–0.766; p < 0.001). From this ROC curve analysis (Fig. 2), an inner vessel length density value lower than 20.05 mm−1 was associated with ACS, with a specificity of 64% and a sensibility of 55%.
ROC curve showing sensitivity and specificity of inner vessel length density measured with Optical Coherence Tomography-Angiography for the occurrence of Acute Coronary Syndrome. Area under the ROC curve = 0.662; 95% CI 0.559–0.766; p < 0.001. An inner vessel length density value lower than 20.05 mm-1 was associated with ACS, with a specificity of 64% and a sensibility of 55%.
Angiogenesis biomarkers analysis and correlations
Our preliminary stability assays showed a good stability at 24 h compared to baseline either with ELISA method for TGF-β1 and angiopoietin-2 measurements and with multiplex method for GDF-15, osteoprotegerin and ST-2 (Fig. 3).
Serum levels of angiopoietin-2 and osteoprotegerin were significantly higher in ACS patients than controls with a mean respectively at 2814 ± 912 pg/mL versus 1962 ± 618 pg/mL (p < 0.001), and 1060 ± 467 pg/mL versus 828 ± 308 pg/mL (p = 0.006) (Fig. 4).
Serum levels of ST2 tended to be higher in ACS patients than controls with a mean respectively at 25,359 ± 42,250 pg/mL versus 13,435 ± 7,632 pg/mL (p = 0.074). No difference in TGF- β1 nor GDF-15 serum levels was found between ACS and control patients (respectively p = 0.700 and p = 0.240).
Age was significantly correlated with inner vessel length density and inner perfusion density (respectively R = − 0.260, p = 0.008 and R = − 0.256, p = 0.009). A significant but moderate correlation was found between angiopoietin-2 and both inner vessel length density and inner perfusion density (respectively R = − 0.293, p = 0.003 and R = − 0.297, p = 0.002) (Fig. 5). Furthermore, osteoprotegerin showed a similar moderate correlation with inner vessel length density and inner perfusion density (respectively R = − 0.310, p = 0.001 and R = − 0.309, p = 0.001).
Discussion
The main results of our study show that patients presenting with an ACS were more likely to have a lower inner vessel length density and that an inner vessel length density defined as less than 20.05 mm−1 was moderately associated with ACS. Moreover, angiopoetin-2 and osteoprotegerin circulating levels were associated with retinal vasculature parameters.
Our study showed that inner vessel length density assessed through OCT-A imaging was significantly lower in patients with ACS as compared to control patients matched for age. This result strengthens the hypothesis that retinal vasculature status does not act independently and can reflect a systemic cardio vascular alteration. Cheung et al. found that a narrower retinal arteriolar caliber was associated with left ventricular remodeling, independently of traditional risk factors and coronary atherosclerotic burden18. Seidelmann et al. also found that retinal vascular changes conferred long-term risk of mortality, ischemic stroke and coronary heart disease9. A low inner vessel length density evaluated with OCT-A was also involved in iodinated contrast agent induced acute kidney injury (AKI) following revascularization in ACS19. In obstructive sleep apnea–hypopnea syndrome patients, vessel densities in the parafoveal areas on OCT-A decreased with greater disease severity20. In the EYE-MI study, our group previously highlighted the association between inner vessel length density in OCT-A imaging and cardiovascular risk profile16. We also demonstrated that inner vessel length density was independent from systemic hemodynamic variables21. In the present study, patients presenting an ACS showed similar inner vessel length density as those in the EYE-MI study, measured respectively at 19.76 mm−1 and 19.70 mm−1. Our results support a link between low inner vessel length density measured with OCT-A and ACS. Moreover, we showed that an inner vessel length density below 20.05 mm−1 was moderately associated with ACS. A recent study also detected that inner vessel length density was decreased in patients with coronary heart disease compared to controls. Their findings were interesting because they correlated OCT-A data with coronary artery angiography and observed that greater coronary artery stenosis was negatively associated with retinal and choroidal vessel density22. This result is similar to what was found for diabetes mellitus in which OCT-A was used to identify pre-clinical diabetic retinopathy features23. Concerning FAZ analysis in our study, no difference was found in FAZ between ACS and control patients which can be explained by the fact this area varies considerably in healthy eyes24. Thus, it has been proposed that monitoring a single subject’s FAZ area enlargement in diabetic retinopathy is more informative in early stages of diabetic retinopathy than using measurements of FAZ alone25.
This study also showed a relation between OCT-A data and systemic biomarkers. The biomarkers chosen in this study were shown to be involved in angiogenesis and microvascular inflammation in both myocardium and retina26,27,28. Osteoprotegerin and its ligands system play an active role in pathological angiogenesis and inflammation in microvascularization29. They are involved in the endothelial homeostasis and muscular smooth cells alteration that leads to vascular calcification29. Thus, osteoprotegerin was associated with the risk of future coronary artery disease in apparently healthy men and women independently of established cardiovascular risk factors30. Angiopoietin-2 is a well-recognized vascular destabilizing factor, and higher levels of angiopoietin-2 were associated with decreased capillary density in a model of myocardial ischemia31. It appears to be a biomarker of poor outcome after ACS through exacerbating hypoxia and myocardial infarction32. In our study we showed that reduced inner vessel length density measured with OCT-A was associated with higher levels of osteoprotegerin and angiopoietin-2 blood levels. These correlations remained moderate regarding the sample size of our study. These findings highlight a link between retinal vascular density measurements made with OCT-A and blood circulating factors. Therefore, these results suggest a pathophysiological rationale to our hypothesis that retinal capillary rarefaction in OCT-A can be considered as a good reflect of systemic cardiovascular alteration. OCT-A analysis is not only beneficial for providing imaging of retina microvascularization but can also be associated with angiogenesis biomarkers modification. Thus, OCT-A could be a useful parameter to consider together with other pre-existing cardiovascular risk factors to assess high cardiovascular risk profiles and improve the screening of these patients in primary prevention.
We acknowledge several limitations to this study. First, many patients had to be excluded due to poor OCT-A quality33. Second, only participants able to have a retinal examination were included. Patients with the most severe ACS were therefore not analyzed, which could have introduced a selection bias. Third, the groups’ gender differences could also constitute a selection bias. Fourth, concerning biomarkers analysis, ACS is associated with a modification of systemic biomarkers kinetics which could be a confounding factor between retinal vascular density and biomarkers’ blood levels. Fifth, in our study, we collected ACS patients’ blood immediately after the admission in order to limit the effect of the acute cardiovascular event on the biomarkers’ kinetic. Indeed, significant modification of biomarkers’ kinetic after ACS have been shown to appear several hours or days after32. Moreover, kinetic biomarkers and their interpretation on vascularization is difficult to define due to their multiple interactions with each other and the clinical state of the tissue. Thus, the same biomarker can be pro-angiogenic or anti-angiogenic depending on the situation as demonstrated it with angiopoietin-2 in retina34. Sixth, in our study, we did not use axial length to scale individual images. In future studies, we should take into account axial length in order to correct for magnification error. Moreover, we should investigate the effect of signal strength and image processing method on our association35.
In conclusion, lower inner vessel length density measured with OCT-A was associated with ACS. Our findings also suggest a moderate correlation between angiopoietin-2 and osteoprotegerin and inner vessel length density. Thus, OCT-A seems to be a promising and useful assessment tool to assess cardiovascular risk, reflecting systemic microvascularization’s status. The clinical relevance of these findings deserves further studies.
Methods
Design and population of the study
This cross-sectional study included patients from the Coronary Care Unit and the Ophthalmology department of Dijon University Hospital from November 2017 to May 2019. Two groups of patients were assessed: high cardiovascular risk (ACS patients) and controls without self-declared cardiovascular risk (Ophthalmology patients). The study complied with the Declaration of Helsinki, Dijon University Hospital ethics committee gave prospective approval for the research protocol (N°2017-A02095-48, ClinicalTrials.gov identifier: NCT03551717) and written informed consent was obtained from every patient. This study followed the STROBE statement according to the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) guidelines36.
Patients presenting with ACS, with or without ST-segment elevation, were included in the high cardiovascular risk group if their health condition allowed them to perform an ophthalmologic consultation. Patients older than 40 years old without cardiovascular risk (no diabetes, no arterial hypertension, no smoking, no previous cardiovascular event) presenting for a cataract surgery or refractive consultation were included from the Ophthalmology department. The non-inclusion criteria were: preexisting retinal disease (vascular and degenerative macular diseases, glaucoma, diabetic retinopathy, epiretinal membrane), patients under guardianship, patients without national health insurance, patients who refused to take part in the study, and patients with ongoing hemodynamic instability. We excluded myopic eyes with axial length > 26 mm because of retinal microvascular density modification with ocular elongation37. Patients from the Cardiology department benefited from an examination of the retinal microvasculature using OCT-A within the first forty eight hours of the cardiovascular event.
Cardiovascular data and collection
In patients hospitalized for an ACS, data were collected from medical records of the RICO (obseRvatoire des Infarctus de Côte d’Or) survey. The design and methods of the RICO have been detailed previously38. In brief, age, sex, previous high blood pressure, previous diabetes, obesity, treated hypercholesterolemia, family history of coronary heart disease (CHD), smoking, personal cardiovascular and chronic kidney failure history, hemodynamic features and biological parameters (creatinine, blood glucose, HbA1c, troponin, Brain Natriuretic Peptid) were recorded.
Description of retinal microvasculature with OCT-A
OCT-A was performed by technicians after mydriasis was obtained with tropicamide 0.5% (Thea, Clermond-Ferrand, France). During OCT-A exam, a cardiologist was present for monitoring patients’ heart rhythm and hemodynamic status if necessary. Axial length was measured using the IOLMaster 500 (Carl Zeiss Meditec, Lena, Germany). OCT-A images were obtained using a CIRRUS HD-OCT Model 5000 instrument (Carl Zeiss Meditec, Lena, Germany). The Angioplex v10 software was used to measure and collect retinal vascular features in the Superficial Capillary Plexus (SCP) and to measure the Foveal Avascular Zone (FAZ). We automatically measured two vascular densities with the Angioplex software: perfusion density and vessel length density. Perfusion density (area, percentage) represents the total area of perfused vasculature per unit area ([white pixels / (white + black pixels)] X 100), and vessel length density (length, mm − 1) represents the total length of perfused vasculature per unit area. All densities were measured in each sector of the SCP from the internal limiting membrane to the inner plexiform layer. The inner vessel length density and perfusion density are the average of the four sectors (superior, nasal, inferior sector and temporal sectors) in the SCP. We previously described these technical aspects16. The device automatically provides measurements for the foveal, parafoveal, and both areas together using the Early Treatment of Diabetic Retinopathy Study grid on en face OCT-A images. These regions are respectively presented as central, inner and full area in the Supplementary Fig. S1. In our study, we retained for analysis 3X3 mm angiograms centered on the macula. As quantitative studies on retinal microvasculature were shown to be repeatable16,39,40,41, the measurements were made on both eyes, but only one eye was retained for analysis according to the following procedure: (1) the angiogram with the highest quality of acquisition was retained; (2) images with a signal strength < 7/10 were excluded; (3) in single-eye patients, the functional eye was selected.
Description of biomarkers pre-analytical protocol
Blood samples were collected at admission for Cardiology patients, and during OCT-A examination for Ophthalmology patients and allowed to clot into serum gel separation tubes. Serum was obtained after centrifugation and then separated into aliquots that were frozen at − 80 °C. Blood samples were stored at + 4 °C for a maximum of 24 h before centrifugation if necessary. Different biomarkers involved in angiogenesis and atherosclerosis were evaluated by enzyme-linked immunosorbent assay (ELISA Quantitine and Multiplex kits, Bio-Techne, Lille, France): angiopoietin-2, TGF-β1, osteoprotegerin, GDF-15 and ST-2. Indeed, angiogenic biomarkers such as angiopoietin-2 and TGF- β1 play a key role in physiology but also cardiovascular remodeling42,43. Osteoprotegerin, GDF-15 and ST-2 have been shown to represent independent biomarkers of cardiovascular diseases44,45,46.
Every assay was performed in duplicate, and a control inter-plate standard, composed of a mix of different patients’ serum, was used for standardization. Before taking any measurements in patients’ samples, we performed a stability assay to make sure the results were not affected by being stored for up to 24 h at 4 °C before centrifugation, sampling and freezing. For that purpose, 2 separate blood tubes were collected in ACS (n = 4) and controls patients (n = 4) and were centrifuged either within one hour after collection, or left 24 h at + 4 °C before processing. All biomarkers were analyzed for stability with appropriate methods in this study.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or medians with interquartile range depending on their distribution. The dichotomous variables were expressed as numbers (percentages). For continuous variables, normality was verified with a Kolmogorov test. The unit was one eye per participant. In ACS patients’ clinical profiles, retina vasculature parameters measured through OCT-A, and serum biomarkers levels were assessed and compared with controls using a Student t-test for continuous variables and the chi-square or Fisher exact test for categorical variables. All tests were bilateral, and significance was set at p < 0.05. A Receiver Operating Characteristics (ROC) curve was used to find a predictable parameter associated with ACS. The correlation between the different biomarkers and OCT-A data was tested by a Pearson test. All analyses were performed using the SPSS 22.0 software (IBM, Chicago, IL, USA).
References
Silverio, A., Cavallo, P., De Rosa, R. & Galasso, G. Big health data and cardiovascular diseases: a challenge for research, an opportunity for clinical care. Front. Med. 6, 36. https://doi.org/10.3389/fmed.2019.00036 (2019).
Zeller, M. et al. Air pollution and cardiovascular and cerebrovascular disease: epidemiologic data. Presse Med. 35, 1517–1522. https://doi.org/10.1016/s0755-4982(06)74844-8 (2006).
Bansilal, S., Castellano, J. M. & Fuster, V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int. J. Cardiol. 201(Suppl 1), S1-7. https://doi.org/10.1016/s0167-5273(15)31026-3 (2015).
Sorop, O. et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc. Res. 114, 954–964. https://doi.org/10.1093/cvr/cvy038 (2018).
Trask, A. J. et al. Dynamic micro- and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. J. Appl. Physiol. 113, 1128–1140. https://doi.org/10.1152/japplphysiol.00604.2012 (2012).
Adjedj, J. et al. Coronary microcirculation in acute myocardial ischaemia: from non-invasive to invasive absolute flow assessment. Arch. Cardiovasc. Dis. 111, 306–315. https://doi.org/10.1016/j.acvd.2018.02.003 (2018).
Matsunaga, D., Yi, J., Puliafito, C. A. & Kashani, A. H. OCT angiography in healthy human subjects. Ophthalmic Surg. Lasers Imaging Retina 45, 510–515. https://doi.org/10.3928/23258160-20141118-04 (2014).
Mimoun, L., Massin, P. & Steg, G. Retinal microvascularisation abnormalities and cardiovascular risk. Arch. Cardiovasc. Dis. 102, 449–456. https://doi.org/10.1016/j.acvd.2009.02.008 (2009).
Seidelmann, S. B. et al. Retinal vessel calibers in predicting long-term cardiovascular outcomes: the atherosclerosis risk in communities study. Circulation 134, 1328–1338. https://doi.org/10.1161/circulationaha.116.023425 (2016).
Nagele, M. P. et al. Retinal microvascular dysfunction in heart failure. Eur. Heart J. 39, 47–56. https://doi.org/10.1093/eurheartj/ehx565 (2018).
Poplin, R. et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2, 158–164. https://doi.org/10.1038/s41551-018-0195-0 (2018).
Arnould, L. et al. Association between the retinal vascular network with Singapore “I” Vessel Assessment (SIVA) software, cardiovascular history and risk factors in the elderly: The Montrachet study, population-based study. PLoS ONE 13, e0194694. https://doi.org/10.1371/journal.pone.0194694 (2018).
Tapp, R. J. et al. Associations of retinal microvascular diameters and tortuosity with blood pressure and arterial stiffness: United Kingdom Biobank. Hypertension 74, 1383–1390. https://doi.org/10.1161/hypertensionaha.119.13752 (2019).
Spaide, R. F., Klancnik, J. M. Jr. & Cooney, M. J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 133, 45–50. https://doi.org/10.1001/jamaophthalmol.2014.3616 (2015).
Coscas, F. et al. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 57, 211–223. https://doi.org/10.1167/iovs.15-18793 (2016).
Arnould, L. et al. The EYE-MI pilot study: a prospective acute coronary syndrome cohort evaluated with retinal optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci. 59, 4299–4306. https://doi.org/10.1167/iovs.18-24090 (2018).
Curcio, C. A. & Kar, D. Commentary on Lavia et al.: progress of optical coherence tomography angiography for visualizing human retinal vasculature. Retina 39, 223–225. https://doi.org/10.1097/iae.0000000000002421 (2019).
Cheung, N. et al. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 50, 48–55. https://doi.org/10.1016/j.jacc.2007.03.029 (2007).
Alan, G. et al. Retinal vascular density as a novel biomarker of acute renal injury after acute coronary syndrome. Sci. Rep. 9, 8060. https://doi.org/10.1038/s41598-019-44647-9 (2019).
Yu, J., Xiao, K., Huang, J., Sun, X. & Jiang, C. Reduced retinal vessel density in obstructive sleep apnea syndrome patients: an optical coherence tomography angiography study. Invest. Ophthalmol. Vis. Sci. 58, 3506–3512. https://doi.org/10.1167/iovs.17-21414 (2017).
Arnould, L. et al. Influence of cardiac hemodynamic variables on retinal vessel density measurement on optical coherence tomography angiography in patients with myocardial infarction. J. Fr. Ophtalmol. https://doi.org/10.1016/j.jfo.2019.07.026 (2020).
Wang, J. et al. Retinal and choroidal vascular changes in coronary heart disease: an optical coherence tomography angiography study. Biomed. Opt. Express 10, 1532–1544. https://doi.org/10.1364/boe.10.001532 (2019).
Simonett, J. M. et al. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 95, e751–e755. https://doi.org/10.1111/aos.13404 (2017).
Chui, T. Y., VanNasdale, D. A., Elsner, A. E. & Burns, S. A. The association between the foveal avascular zone and retinal thickness. Invest. Ophthalmol. Vis. Sci. 55, 6870–6877. https://doi.org/10.1167/iovs.14-15446 (2014).
Lynch, G. et al. Within-subject assessment of foveal avascular zone enlargement in different stages of diabetic retinopathy using en face OCT reflectance and OCT angiography. Biomed. Opt. Express 9, 5982–5996. https://doi.org/10.1364/boe.9.005982 (2018).
Van Campenhout, A. & Golledge, J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis 204, 321–329. https://doi.org/10.1016/j.atherosclerosis.2008.09.033 (2009).
Abu El-Asrar, A. M. et al. Osteoprotegerin is a new regulator of inflammation and angiogenesis in proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 58, 3189–3201. https://doi.org/10.1167/iovs.16-20993 (2017).
Patel, J. V., Lim, H. S., Varughese, G. I., Hughes, E. A. & Lip, G. Y. Angiopoietin-2 levels as a biomarker of cardiovascular risk in patients with hypertension. Ann. Med. 40, 215–222. https://doi.org/10.1080/07853890701779586 (2008).
Rochette, L. et al. The role of osteoprotegerin and its ligands in vascular function. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20030705 (2019).
Semb, A. G. et al. Osteoprotegerin and soluble receptor activator of nuclear factor-kappaB ligand and risk for coronary events: a nested case-control approach in the prospective EPIC-Norfolk population study 1993–2003. Arterioscler. Thromb. Vasc. Biol. 29, 975–980. https://doi.org/10.1161/atvbaha.109.184101 (2009).
Zhang, W. et al. The association of depressed angiogenic factors with reduced capillary density in the Rhesus monkey model of myocardial ischemia. Metallomics 8, 654–662. https://doi.org/10.1039/c5mt00332f (2016).
Lee, S. J. et al. Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction. J. Clin. Investig. 128, 5018–5033. https://doi.org/10.1172/jci99659 (2018).
Lavia, C. et al. Vessel density of superficial, intermediate, and deep capillary plexuses using optical coherence tomography angiography. Retina 39, 247–258. https://doi.org/10.1097/iae.0000000000002413 (2019).
Oshima, Y. et al. Different effects of angiopoietin-2 in different vascular beds: new vessels are most sensitive. FASEB J. 19, 963–965. https://doi.org/10.1096/fj.04-2209fje (2005).
Sampson, D. M. et al. Axial length variation impacts on superficial retinal vessel density and foveal avascular zone area measurements using optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci. 58, 3065–3072. https://doi.org/10.1167/iovs.17-21551 (2017).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61, 344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008 (2008).
Li, M. et al. Retinal microvascular network and microcirculation assessments in high myopia. Am. J. Ophthalmol. 174, 56–67. https://doi.org/10.1016/j.ajo.2016.10.018 (2017).
Zeller, M. et al. Prevalence and impact of metabolic syndrome on hospital outcomes in acute myocardial infarction. Arch. Intern. Med. 165, 1192–1198. https://doi.org/10.1001/archinte.165.10.1192 (2005).
Al-Sheikh, M., Tepelus, T. C., Nazikyan, T. & Sadda, S. R. Repeatability of automated vessel density measurements using optical coherence tomography angiography. Br. J. Ophthalmol. 101, 449–452. https://doi.org/10.1136/bjophthalmol-2016-308764 (2017).
La Spina, C., Carnevali, A., Marchese, A., Querques, G. & Bandello, F. Reproducibility and reliability of optical coherence tomography angiography for foveal avascular zone evaluation and measurement in different settings. Retina 37, 1636–1641. https://doi.org/10.1097/iae.0000000000001426 (2017).
Manalastas, P. I. C. et al. Reproducibility of optical coherence tomography angiography macular and optic nerve head vascular density in glaucoma and healthy eyes. J. Glaucoma 26, 851–859. https://doi.org/10.1097/ijg.0000000000000768 (2017).
Akwii, R. G., Sajib, M. S., Zahra, F. T. & Mikelis, C. M. Role of Angiopoietin-2 in vascular physiology and pathophysiology. Cells https://doi.org/10.3390/cells8050471 (2019).
Santibanez, J. F., Quintanilla, M. & Bernabeu, C. TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin. Sci. 121, 233–251. https://doi.org/10.1042/cs20110086 (2011).
Rochette, L. et al. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 182, 115–132. https://doi.org/10.1016/j.pharmthera.2017.08.015 (2018).
Hagstrom, E. et al. Growth Differentiation Factor 15 predicts all-cause morbidity and mortality in stable coronary heart disease. Clin. Chem. 63, 325–333. https://doi.org/10.1373/clinchem.2016.260570 (2017).
Dieplinger, B. & Mueller, T. Soluble ST2 in heart failure. Clin. Chim. Acta 443, 57–70. https://doi.org/10.1016/j.cca.2014.09.021 (2015).
Acknowledgements
The authors thank Perrine Robinet and Helene Tron for providing technical assistance. The authors also thank Alison Hannappe for English revision of the paper.
Funding
None.
Author information
Authors and Affiliations
Contributions
M.A.H, L.A., C.G. wrote the main manuscript text, A.M., B.M., C.V., Y.C. conceived and planned the experiments, C.C.G., M.Z., C.B., F.B. contributed to the interpretation of the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hannappe, MA., Arnould, L., Méloux, A. et al. Vascular density with optical coherence tomography angiography and systemic biomarkers in low and high cardiovascular risk patients. Sci Rep 10, 16718 (2020). https://doi.org/10.1038/s41598-020-73861-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73861-z
This article is cited by
-
Coronary artery disease, its associations with ocular, genetic and blood lipid parameters
Eye (2024)
-
Ocular images-based artificial intelligence on systemic diseases
BioMedical Engineering OnLine (2023)
-
Diabetic choriocapillaris flow deficits affect the outer retina and are related to hemoglobin A1c and systolic blood pressure levels
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.