Abstract
Multidrug-resistant tuberculosis (MDR-TB) is a major threat to the eradication of tuberculosis. TB control strategies need to be adapted to the necessities of different countries and adjusted in high-risk areas. In this study, we analysed the spatial distribution of the MDR- and non-MDR-TB cases across municipalities in Continental Portugal between 2000 and 2016. We used Bayesian spatial models to estimate age-standardized notification rates and standardized notification ratios in each area, and to delimitate high- and low-risk areas, those whose standardized notification ratio is significantly above or below the country’s average, respectively. The spatial distribution of MDR- and non-MDR-TB was not homogeneous across the country. Age-standardized notification rates of MDR-TB ranged from 0.08 to 1.20 and of non-MDR-TB ranged from 7.73 to 83.03 notifications per 100,000 population across the municipalities. We identified 36 high-risk areas for non-MDR-TB and 8 high-risk areas for MDR-TB, which were simultaneously high-risk areas for non-MDR-TB. We found a moderate correlation (ρ = 0.653; 95% CI 0.457–0.728) between MDR- and non-MDR-TB standardized notification ratios. We found heterogeneity in the spatial distribution of MDR-TB across municipalities and we identified priority areas for intervention against TB. We recommend including geographical criteria in the application of molecular drug resistance to provide early MDR-TB diagnosis, in high-risk areas.
Similar content being viewed by others
Introduction
In Europe, the incidence of tuberculosis (TB) has been decreasing since 2008, at a rate of about 5% per year. In 2017 the incidence of TB was 30 new cases per 100,000 population. Although this rate of decline was higher than the global rate of decline of incidence (currently at 2%), it still needs to be improved to achieve the goals of the End TB Strategy1.
Multidrug-resistant tuberculosis (MDR-TB) is defined as TB caused by strains of Mycobacterium tuberculosis resistant to the two most potent first-line anti-TB drugs, isoniazid and rifampicin. It contributes to the difficulty in achieving the goals of the End TB Strategy. MDR-TB treatment requires the use of second-line anti-TB drugs, which are less effective, more toxic and more costly2, with a lower success rates than standard therapy1. Hence, prevention and control of MDR-TB are priorities for elimination of TB3.
Since the 1990’s, when MDR-TB was recognised as a potential threat to TB control, it was considered that in general, drug-resistance was acquired during treatment due to poor quality case management4. Currently, the premise that drug-resistant TB is predominantly acquired has changed. The use of new epidemiological tools, such as modelling and molecular techniques, demonstrates that the majority of MDR-TB cases result from transmission of MDR-TB strains rather than selection of de-novo resistance during previous treatment5,6.
In 2017, in Europe, MDR-TB was reported for 24% of all TB cases with first-line drug susceptibility testing (DST). This proportion was considerably lower among European Union (EU) countries (4%) compared with non-EU countries (28%). Among pulmonary TB cases, 18% of new and 48% of previously treated cases were MDR-TB1. In the same year, in Portugal, the incidence of TB was 16 cases per 100,000 population and 1% of all TB cases were MDR-TB. However, TB incidence was not homogeneous across the country, with 57% of cases in the two largest urban centers, Porto and Lisbon7. These cities were previously identified as the most critical regions for TB incidence8, and pulmonary TB in particular9,10. Adaptation of strategies and interventions to national and local contexts is pivotal for effective TB control11. This can only be achieved with a detailed understanding of the disease distribution across the different regions, along with an epidemiological characterization of the populations affected, paying special attention to the identification of geographical areas or subpopulations with especially high TB burden11. Spatial statistics and disease mapping are effective approaches to investigate the detailed geographical variations in TB incidence12, being particularly relevant in identifying high- and low-risk areas8,13.
In the present study, we analysed the spatial distribution of notification of TB in municipalities in Continental Portugal to identify high-risk areas for MDR- and non-MDR-TB. We also assessed the correlation between the spatial distributions of MDR- and non-MDR-TB, highlighting populations that could be major targets for public health authorities to reduce and prevent the incidence of MDR-TB in Portugal.
Methods
Data collection
We used the national TB Surveillance System (SVIG-TB) as the source of data. We analysed all TB cases notified in Continental Portugal from January 2000 until December 2016. According to national regulations, 2 independent sputum samples are collected and tested. TB diagnosis is done either through positive identification using microscopy and nucleic acid amplification or positive Mycobacterium tuberculosis (Mtb) culture, followed by conventional first-line DST. All tests are performed in laboratories integrated in the national network, periodically certified and checked. All Mtb strains that have shown resistance to isoniazid and rifampicin at the same time should be tested for second-line anti-TB drugs in the TB National Reference Laboratory (Instituto Nacional de Saúde Ricardo Jorge: INSA). In the case of suspicion of MDR-TB (patients with previous TB treatment that report contact with MDR-TB patients, that belong to specific vulnerable populations, or that are health professionals), clinical samples are submitted to molecular testing for detection of isoniazid and rifampicin resistance.
We selected MDR-TB cases (i.e., resistant to at least isoniazid and rifampicin) and divided all TB cases into two groups: MDR- and non-MDR-TB cases. We obtained notifications of MDR- and non-MDR-TB by municipality (n = 278), year of diagnosis, age (5-year age groups) and sex. Population counts by municipality, year, age (5-year age groups) and sex were obtained from Statistics Portugal (https://www.ine.pt/) for the study period.
Demographic and clinical characteristics of each patient, including age, sex, country of origin, health-related behaviours (e.g. drug or alcohol abuse), HIV status, reclusion (prison confinement), community residence (social housing for people with socio-economic vulnerabilities), homelessness, comorbidity (diabetes and silicosis), previous TB treatment and site of disease, were also collected from SVIG-TB.
Statistical analysis
Descriptive statistics [absolute and relative frequencies or median with interquartile range (IQR)], according to the nature of the variables, were used to describe patient characteristics. We compared these characteristics between patient groups using the Chi-squared test (or Fisher’s test, if appropriate) for categorical variables and the Mann–Whitney U-test for continuous variables. In order to control for an effect of the different sample sizes of both groups (MDR and non-MDR, we selected two random samples with 583 cases of the non-MDR to compare with our MDR group).
To estimate age-standardized notification rates in each area and to delimitate high risk and low risk areas, we used hierarchical Bayesian spatial models. These models take into account the spatial autocorrelation and large variance of small areas. To minimize the effect of random fluctuations associated with small number of cases, and because we found no substantial differences in the geographical distribution of non-MDR and MDR TB across our study period, we considered the average rates of the 17-year study period. We assumed that the response variable, cases of TB \(({O}_{i})\) in each \(i\)th area, follows a Poisson distribution where \({E}_{i}\) is the expected number of cases and \({\theta }_{i}\) the relative risk (RR), or Standardized Notification Rate (Eqs. 1 and 2). We used the Portuguese TB notification rates by sex and age group (5-year age groups) as a reference to compute the expected number of cases, according to the indirect method of standardization. The expected number of cases was obtained by summing the product of the age-sex specific notification rates of the standard population (in our study Portugal) by the population by age and sex of each Portuguese municipality.
Here \(\alpha\) is an intercept quantifying the average number of TB cases in the 278 areas. The area specific effect \(s_{i}\) was modelled considering a BYM model14 with a parameterization suggested by Dean et al.15 (Eq. 3)
where \(u_{i}\) is the structured effect and \(v_{i}\) is the unstructured effect. The \(u_{i}\) effect was scaled in order to render the model more intuitive and interpretable16, so that \(\varphi\) expresses the proportion of the spatial effect due to the structured part and 1/\(\tau\) is the marginal variance of \(s_{i}\).
Additionally, we used the function ‘excursions’ to delimitate high risk and low risk areas8,17,18. High-risk areas are those whose standardized notification ratio is significantly above 1 (i.e., above the country’s average) and low risk areas are those whose standardized notification ratio is significantly below 1 (i.e., below the country’s average). This method uses the posterior joint distribution computed from the Integrated Nested Laplace Approximation (INLA) and takes into account the dependence structure, allowing to accurately identify areas where the notification ratio is greater than zero.
To analyse the correlation between MDR-TB and non-MDR-TB, the Pearson correlation coefficient (r and corresponding 95% Credible Intervals, 95% CrI) was computed based on the standardized notification ratios of MDR-TB and non-MDR-TB derived from the previously described models.
To facilitate interpretation, standardized notification ratios were converted into rates per 100,000 inhabitants by multiplying the standardized notification ratios by the crude national notification rates.
Statistical analyses were performed using SPSS version 18.0 (PASW Statistics 18), and p-values below 0.05 were considered statistically significant.
Posterior distributions were obtained using the INLA, which was implemented in the R INLA library19.
Standardized notification rates and high and low risk areas were mapped using ArcMap release 10.5.1. (Environmental Systems Research Institute, Redlands, CA, USA).
Ethical considerations
Ethical approval and informed consent were not required, as the patient data, collected for an official national surveillance system, were anonymized in accordance with the research ethical guidelines in Portugal. Authorization for its use in the present manuscript was given by the National program for Tuberculosis.
Results
We evaluated 53,417 TB cases, notified in Continental Portugal during the study period (2000–2016) (Supplementary Table S1). We identified 583 (1.1%) cases of MDR-TB. We compared demographic and clinical characteristics between MDR- and non-MDR-TB patients. We observed that MDR-TB patients were younger (40.0 years vs. 42.0 years) and were more likely to be foreign-born (27.3% vs. 13.6%), infected with HIV (27.8% vs. 13.1%), alcohol abusers (24.5% vs. 15.0%), injectable drug users (20.3% vs. 10.3%), prisoners (6.2% vs. 2.3%), homeless (3.7% vs. 1.8%) and having a history of previous TB treatment (40.3% vs. 9.9%) than non-MDR-TB patients (Table 1). The same statistical differences were obtained with two randomized samples I and II of the non-MDR-TB with similar size as the MDR-TB group (Supplementary Table S2).
The crude non-MDR-TB notification rate was 31.19 notifications per 100,000 population (95% CrI 30.93–31.46) and the crude MDR-TB notification rate was 0.34 notifications per 100,000 population (95% CrI 0.32–0.37). Geographical differences in reporting were observed.
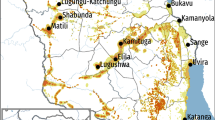
The spatial distribution of the age-standardised notification rates of non-MDR-TB is depicted in Fig. 1A with the delimitation of the high- and low-risk areas given in Fig. 1B. Age-standardized notification rates of non-MDR TB ranged from 7.73 to 83.03 notifications per 100,000 population. We identified 36 high-risk areas, mostly located in Porto and Lisbon metropolitan areas, and also in the southern regions of Alentejo and the Algarve (Fig. 1B). The spatial distribution of the age-standardized notification rates of MDR-TB is shown in Fig. 1C and the delimitation of the high- and low-risk areas is shown in Fig. 1D. Age-standardized notification rates ranged from 0.08 to 1.20 notifications per 100,000 population. Eight high-risk areas for MDR-TB were located mostly in the Lisbon metropolitan area (Fig. 1D). These 8 high-risk areas were also high-risk areas for non-MDR-TB. Only 22% (8/36) of the high-risk areas for non-MDR-TB were high-risk areas for MDR-TB (Supplementary Table S3). In order to confirm the stability of the inferred high-risk areas through the entire dataset, we performed the analysis on a time series across the 17 years. We obtained stable patterns for the geographical locations of risk areas (Supplementary Figs. S1 and S2).
Spatial distribution of the age-standardized notification rates of non-MDR-TB (A) and the corresponding delimitation of the high- and low-risk areas (B). Spatial distribution of the age-standardized notification rates of MDR-TB (C) and the corresponding delimitation of the high- and low-risk areas (D). MDR-TB multidrug-resistant tuberculosis; high-risk areas are those whose standardized notification ratio is significantly above 1 (i.e., above the country’s average); low risk areas are those whose standardized notification ratio is significantly below 1 (i.e., below the country’s average).
We analysed the correlation between MDR- and non-MDR-TB standardized notification ratios and found a moderate correlation (ρ = 0.653; 95% CrI 0.457–0.728) between them.
Since only some areas with a high-risk for non-MDR-TB also have a high-risk for MDR-TB (Supplementary Table S3), we compared demographic and clinical characteristics of the non-MDR-TB patients from high-risk areas for only non-MDR-TB (28 areas) with patients from areas, which are also high-risk areas for MDR-TB (8 areas) to determine factors that could be associated with the risk for MDR-TB. We observed that the patients from high-risk areas for both MDR- and non-MDR-TB were younger (40.0 years vs. 42.0 years) than patients from areas with highest-risk for only non-MDR-TB. Among them, there was a higher proportion of females (34.6% vs. 31.3%), foreign-born patients (25.5% vs. 7.2%), HIV infection (20.9% vs. 12.2%), alcohol abusers (17.4% vs. 13.9%), injectable drugs users (16.3% vs. 10.2%), prisoners (4.0% vs. 1.5%), community residents (4.9% vs. 2.4%), homeless persons (3.3% vs. 1.1%), cases of extra-pulmonary disease (28.3% vs. 24.5%) and cases with a history of previous TB treatment (12.6% vs. 10.0%) (Table 2).
Discussion
In this study, we combined the epidemiological characteristics of MDR- (resistant to at least isoniazid and rifampicin) and non-MDR-TB (all other TB) patients, over a 17-year period, with a detailed spatial description to identify high- and low-risk areas, to obtain a systematic comparison between MDR- and non-MDR-TB high-risk areas across Portugal.
We demonstrated significant heterogeneity in the spatial distribution of the age-standardized notification rates of MDR- and non-MDR-TB at the municipality level. We found a moderate correlation between MDR- and non-MDR-TB standardized notification ratios. We identified 36 high-risk areas for non-MDR-TB and 8 high-risk areas for MDR-TB.
In our study period (2000–2016), the spatial distribution of the age-standardised notification rates of non-MDR-TB ranged from 7.73 to 83.03 notifications per 100,000 population. A high degree of heterogeneity in spatial TB distribution was expected as previously reported in national8,9,10 and international12 spatial studies. The spatial distribution of the age-standardised notification rates of MDR-TB was also heterogeneous (up to fifteen times difference), ranging from 0.08 to 1.20 notifications per 100,000 population across municipalities.
The pronounced spatial heterogeneity of MDR-TB burden has been observed in Moldova (the notified incidence of MDR-TB ranged from 0.5 to 27.2 cases per 100,000 population)20, China (where the proportion of incident MDR-TB cases varied between 3 and 30%)21 and Ethiopia (where the standardized morbidity ratio ranged from 0 to 7.0)22.
We found a moderate correlation between MDR- and non-MDR-TB. We identified 36 high-risk areas for non-MDR-TB and 8 high-risk areas for MDR-TB, which were simultaneously high-risk areas for non-MDR-TB. It was expected that MDR-TB risk areas were comparable with non-MDR-TB risk areas, due to the high probability of acquisition of drug resistance during treatment for TB and the transmission of existing MDR-TB strains in areas with higher rate of transmission of non-MDR-TB. However, only 22% (8/36) of the non-MDR-TB high-risk areas were also MDR-TB high-risk areas. We compared non-MDR-TB patients from high-risk areas for non-MDR-TB with patients from high-risk areas for both MDR- and non-MDR-TB. Among the characteristics which were most common among the patients from high-risk areas for both MDR- and non-MDR-TB, being HIV infected23,24,25,26, being foreign-born6,23, homelessness27 and having history of imprisonment6, consumption of alcohol6,25 and injectable drug use6 have been previously reported as factors associated with MDR-TB development. Previous TB treatment is particularly important risk factor for MDR-TB23,24,25,26,27.
The role of HIV infection as risk factor for MDR-TB has been inconsistent. In several studies, an association between HIV and MDR-TB disease was not significant or was negative27,28. This association was stronger for transmitted than acquired MDR-TB29,30.
Regarding previous TB treatment, in our study, 40% of MDR-TB patients were previously exposed to anti-tuberculosis drugs. These cannot be assumed to have acquired resistance during treatment. As previously described, 61% of the incidence of MDR-TB among previously treated patients resulted from MDR-TB transmissio5. In fact, genetic studies31,32,33 suggested that a high percentage of these cases in Portugal were related with the transmission of two stable MDR-TB clusters.
Regarding hotspots of MDR-TB, 7 out of the 8 high-risk areas are located in the Lisbon metropolitan area. Previously identified MDR-TB genetic clusters revealed evidence of transmission of multidrug-resistant strains in this region31,32,33.
The strengths of this study are the robust statistical methods used to characterise geographic patterns, taking advantage of the epidemiological characterization of the population over a significant amount of time. This allowed the identification of risk areas for MDR-TB, which are areas for priority action and intervention for the existing national TB control program. We complemented the spatial analysis with quality-assured laboratory data and a detailed epidemiological characterization to evaluate potential risk factors for MDR-TB in the TB risk areas. One possible study limitation is its retrospective design using the national notification system, which limited us in the analysis of the study variables.
In conclusion, we found heterogeneity in the spatial distribution of MDR-TB across municipalities in Portugal. We identified priority areas for intervention against MDR-TB. Our findings suggest that in addition to the development of MDR-TB, transmission of MDR-TB strains occurs in these areas. We propose the inclusion of geographical criteria in the application of molecular drug resistance testing, paying particular attention to screening and early MDR-TB diagnosis in these areas and the performance of routine genotyping of all TB isolates to understand the dynamics of MDR-TB emergence and transmission.
Data availability
The epidemiological and geographical datasets generated during the current study are available from the corresponding author on reasonable request.
References
WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Tuberculosis surveillance and monitoring in Europe 2019–2017 data. (WHO Regional Office for Europe, Copenhagen, 2019).
Gunther, G., Gomez, G. B., Lange, C., Rupert, S. & van Leth, F. Availability, price and affordability of anti-tuberculosis drugs in Europe: A TBNET survey. Eur. Respir. J. 45, 1081–1088. https://doi.org/10.1183/09031936.00124614 (2015).
Lonnroth, K. et al. Towards tuberculosis elimination: An action framework for low-incidence countries. Eur. Respir. J. 45, 928–952. https://doi.org/10.1183/09031936.00214014 (2015).
WHO. Anti-tuberculosis drug resistance in the world. WHO/TB/97.229. (Geneva, Switzerland, 1997).
Kendall, E. A., Fofana, M. O. & Dowdy, D. W. Burden of transmitted multidrug resistance in epidemics of tuberculosis: A transmission modelling analysis. Lancet Respir. Med. 3, 963–972. https://doi.org/10.1016/s2213-2600(15)00458-0 (2015).
Dheda, K. et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. https://doi.org/10.1016/s2213-2600(17)30079-6 (2017).
DGS. (Direção-Geral da Saúde, Lisboa, 2018).
Apolinario, D. et al. Tuberculosis inequalities and socio-economic deprivation in Portugal. Int. J. Tuberc. Lung Dis. 21, 784–789. https://doi.org/10.5588/ijtld.16.0907 (2017).
Couceiro, L., Santana, P. & Nunes, C. Pulmonary tuberculosis and risk factors in Portugal: A spatial analysis. Int. J. Tuberc. Lung Dis. 15, 1445–1455. https://doi.org/10.5588/ijtld.10.0302 (2011).
Areias, C., Briz, T. & Nunes, C. Pulmonary tuberculosis space-time clustering and spatial variation in temporal trends in Portugal, 2000–2010: An updated analysis. Epidemiol. Infect. 143, 3211–3219. https://doi.org/10.1017/s0950268815001089 (2015).
WHO. Implementing the End TB Strategy: The Essentials. (WHO, Geneva, 2015).
Shaweno, D. et al. Methods used in the spatial analysis of tuberculosis epidemiology: A systematic review. BMC Med. 16, 193–193. https://doi.org/10.1186/s12916-018-1178-4 (2018).
Sifuna, P. M. et al. Spatial epidemiology of tuberculosis in the high-burden counties of Kisumu and Siaya, Western Kenya, 2012–2015. Int. J. Tuberc. Lung Dis. 23, 363–370. https://doi.org/10.5588/ijtld.18.0245 (2019).
Besag, J., York, J. & Mollié, A. Bayesian image restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math. 43, 1–20 (1991).
Dean, C. B., Ugarte, M. D. & Militino, A. F. Detecting interaction between random region and fixed age effects in disease mapping. Biometrics 57, 197–202 (2001).
Riebler, A., Sørbye, S. H., Simpson, D. & Rue, H. An intuitive Bayesian spatial model for disease mapping that accounts for scaling. arXiv e-prints (2016). https://ui.adsabs.harvard.edu/abs/2016arXiv160101180R.
Bolin, D. & Lindgren, F. Excursion and contour uncertainty regions for latent Gaussian models. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 77, 85–106. https://doi.org/10.1111/rssb.12055 (2015).
Ribeiro, A. I., Krainski, E. T., Carvalho, M. S. & Pina Mde, F. Where do people live longer and shorter lives? An ecological study of old-age survival across 4404 small areas from 18 European countries. J. Epidemiol. Community Health 70, 561–568. https://doi.org/10.1136/jech-2015-206827 (2016).
Rue, H., Martino, S. & Lindgren, F. INLA: Functions which allow to perform a full Bayesian analysis of structured additive models using Integrated Nested Laplace Approximation. R package version 0.0-1404466487. http://www.R-INLA.Org (2009).
Jenkins, H. E. et al. Assessing spatial heterogeneity of multidrug-resistant tuberculosis in a high-burden country. Eur. Respir. J. 42, 1291–1301. https://doi.org/10.1183/09031936.00111812 (2013).
Ding, P., Li, X., Jia, Z. & Lu, Z. Multidrug-resistant tuberculosis (MDR-TB) disease burden in China: A systematic review and spatio-temporal analysis. BMC Infect. Dis. 17, 57. https://doi.org/10.1186/s12879-016-2151-5 (2017).
Alene, K. A., Viney, K., McBryde, E. S. & Clements, A. C. Spatial patterns of multidrug resistant tuberculosis and relationships to socio-economic, demographic and household factors in northwest Ethiopia. PLoS ONE 12, e0171800. https://doi.org/10.1371/journal.pone.0171800 (2017).
Lange, C. et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: A TBNET consensus statement. Eur. Respir. J. 44, 23–63. https://doi.org/10.1183/09031936.00188313 (2014).
Workicho, A., Kassahun, W. & Alemseged, F. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients: A case-control study. Infect. Drug Resist. 10, 91–96. https://doi.org/10.2147/idr.s126274 (2017).
Mesfin, E. A. et al. Drug-resistance patterns of Mycobacterium tuberculosis strains and associated risk factors among multi drug-resistant tuberculosis suspected patients from Ethiopia. PLoS ONE 13, e0197737. https://doi.org/10.1371/journal.pone.0197737 (2018).
Pradipta, I. S., Forsman, L. D., Bruchfeld, J., Hak, E. & Alffenaar, J.-W. Risk factors of multidrug-resistant tuberculosis: A global systematic review and meta-analysis. J. Infect. 77, 469–478. https://doi.org/10.1016/j.jinf.2018.10.004 (2018).
Gunther, G. et al. Multidrug-resistant tuberculosis in Europe, 2010–2011. Emerg. Infect. Dis. 21, 409–416. https://doi.org/10.3201/eid2103.141343 (2015).
Dean, A. S., Zignol, M., Falzon, D., Getahun, H. & Floyd, K. HIV and multidrug-resistant tuberculosis: Overlapping epidemics. Eur. Respir. J. 44, 251–254. https://doi.org/10.1183/09031936.00205413 (2014).
Suchindran, S., Brouwer, E. S. & Van Rie, A. Is HIV infection a risk factor for multi-drug resistant tuberculosis? A systematic review. PLoS ONE 4, e5561. https://doi.org/10.1371/journal.pone.0005561 (2009).
Mesfin, Y. M., Hailemariam, D., Biadgilign, S. & Kibret, K. T. Association between HIV/AIDS and multi-drug resistance tuberculosis: A systematic review and meta-analysis. PLoS ONE 9, e82235–e82235. https://doi.org/10.1371/journal.pone.0082235 (2014).
Oliveira, O. et al. A nationwide study of multidrug-resistant tuberculosis in Portugal 2014–2017 using epidemiological and molecular clustering analyses. BMC Infect. Dis. 19, 567. https://doi.org/10.1186/s12879-019-4189-7 (2019).
Perdigão, J. et al. Tuberculosis drug-resistance in Lisbon, Portugal: A 6-year overview. Clin. Microbiol. Infect. 17, 1397–1402. https://doi.org/10.1111/j.1469-0691.2010.03351.x (2011).
Perdigão, J. et al. Unraveling Mycobacterium tuberculosis genomic diversity and evolution in Lisbon, Portugal, a highly drug resistant setting. BMC Genom. 15, 991. https://doi.org/10.1186/1471-2164-15-991 (2014).
Acknowledgements
Authors would like to thank the National Program for Tuberculosis of the Directorate-General of Health for providing data used in this study. We also thank Professor Carla Nunes for her valuable comments on the manuscript and Dr. John Yaphe for editorial assistance.
Funding
This work was developed under the scope of the project NORTE-01-0145-FEDER-000013, supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER) and project PTDC/SAU-PUB/29521/2017. Olena Oliveira is supported by the project NORTE-08-5369-FSE-000041, financed by the Operational Program NORTE 2020 and co-financed by the European Social Fund through a doctoral grant (UMINHO/BD/47/2016). This study was also supported by FEDER through the Operational Programme Competitiveness and Internationalization and national funding from the Foundation for Science and Technology—FCT (Portuguese Ministry of Science, Technology and Higher Education) under the Unidade de Investigação em Epidemiologia—Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (POCI-01-0145-FEDER-006862; Ref. UID/DTP/04750/2019). Ana Isabel Ribeiro was supported by National Funds through FCT, under the programme of ‘Stimulus of Scientific Employment—Individual Support’ within the contract CEECIND/02386/2018.
Author information
Authors and Affiliations
Contributions
O.O. and A.I.R. conceived and designed the study; O.O., A.I.R. and E.T.K. performed statistical analysis; O.O., T.R., M.C.-N. and R.D. evaluated and interpreted the data; O.O., A.I.R., E.T.K. and T.R. drafted the manuscript and all authors critically reviewed it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oliveira, O., Ribeiro, A.I., Krainski, E.T. et al. Using Bayesian spatial models to map and to identify geographical hotspots of multidrug-resistant tuberculosis in Portugal between 2000 and 2016. Sci Rep 10, 16646 (2020). https://doi.org/10.1038/s41598-020-73759-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73759-w
This article is cited by
-
Drug-resistant tuberculosis: a persistent global health concern
Nature Reviews Microbiology (2024)
-
Use of unsupervised machine learning to characterise HIV predictors in sub-Saharan Africa
BMC Infectious Diseases (2023)
-
Intra-urban variation in tuberculosis and community socioeconomic deprivation in Lisbon metropolitan area: a Bayesian approach
Infectious Diseases of Poverty (2022)
-
Spatial heterogeneity of extensively drug resistant-tuberculosis in Western Cape Province, South Africa
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.