Abstract
The study was aimed at analysing the frequency of impulse control disorders (ICDs) and compulsive behaviours (CBs) in patients with Parkinson’s disease (PD) and in control subjects (CS) as well as the relationship between ICDs/CBs and motor, nonmotor features and dopaminergic treatment in PD patients. Data came from COPPADIS-2015, an observational, descriptive, nationwide (Spain) study. We used the validated Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease-Rating Scale (QUIP-RS) for ICD/CB screening. The association between demographic data and ICDs/CBs was analyzed in both groups. In PD, this relationship was evaluated using clinical features and treatment-related data. As result, 613 PD patients (mean age 62.47 ± 9.09 years, 59.87% men) and 179 CS (mean age 60.84 ± 8.33 years, 47.48% men) were included. ICDs and CBs were more frequent in PD (ICDs 12.7% vs. 1.6%, p < 0.001; CBs 7.18% vs. 1.67%, p = 0.01). PD patients had more frequent previous ICDs history, premorbid impulsive personality and antidepressant treatment (p < 0.05) compared with CS. In PD, patients with ICDs/CBs presented younger age at disease onset, more frequent history of previous ICDs and premorbid personality (p < 0.05), as well as higher comorbidity with nonmotor symptoms, including depression and poor quality of life. Treatment with dopamine agonists increased the risk of ICDs/CBs, being dose dependent (p < 0.05). As conclusions, ICDs and CBs were more frequent in patients with PD than in CS. More nonmotor symptoms were present in patients with PD who had ICDs/CBs compared with those without. Dopamine agonists have a prominent effect on ICDs/CBs, which could be influenced by dose.
Similar content being viewed by others
Introduction
Impulse control disorders (ICDs) and compulsive behaviours (CBs) are known conditions that frequently appear in patients with Parkinson’s disease (PD). Within the ICDs spectrum, pathological gambling, compulsive shopping, hypersexuality and compulsive eating are included. CBs are those related to the performing of aimless repetitive stereotyped complex tasks, known as punding, hobbysm, excessive and aimless wandering (“walkabout”) and dysregulation dopaminergic syndrome (DDS)1.
The etiopathology of these disorders is not simple. Demographic factors, premorbidities, comorbidities and genetic factors can influence the risk of ICDs and CBs2,3,4,5. Comorbidities such as tobacco consumption and depression are well-established factors linked to ICDs. However, the directionality of the association between ICDs and affective disorders is not still clear6,7,8. Epidemiologic factors such as sex, age and age at onset could influence the incidence of ICDs and CBs1. Nevertheless, it is controversial whether these disorders are exclusively linked to the first years of disease evolution when patients still have an integrum ventral striatum2, or whether they are associated with longer exposure to dopamine treatment3. On the other hand, estimated prevalence rates across countries range from 10 to 39%4. This wide range depends on the study characteristics, but also on the population features, being therefore important the assessment of specific populations.
In addition, it is known that dopaminergic replacement therapy (DRT) plays an important role in ICDs and CBs, given their prevalence in untreated de novo patients is the same as in control subjects (CS)8. Thus, under external dopaminergic stimulation by DRT, the regulation of mesocorticolimbic pathway activity might be inappropriate, inducing an aberrant response to reward tasks and impulsivity traits9. Despite this fact, not all patients undergoing DRT treatment have ICDs or CBs, suggesting the action of a neurobiological substrate leading to individual susceptibility5.
Dopamine agonists (DA) have been shown to be a principal drug involved in ICDs, and the association appears to be dose dependent10,11. However, there is controversy regarding DA dose and ICDs, given it is not fully understood whether the ICDs are associated with the DA dose alone or in combination with the dose of levodopa and other dopaminergic drugs2,3,12.
Parkinsonian features have also been associated with ICDs. Not only can cognitive performance in patients with ICDs be impaired, but executive dysfunction as well as impairment in the reward-related decision process and set shifting can be present6,13,14.
Taking into account the factors influencing ICDs and CBs in PD, we sought to assess clinical features linked to these disorders in the COPPADIS-2015 population of PD and CS. We especially aimed to evaluate the impact of DRT and the influence of clinical features on these disorders in the PD population.
Methods
Participants
The participant data was drawn from the baseline assessment of the COPPADIS-2015, an observational, descriptive, 5-year follow-up, nationwide (Spain) evaluation study15. The project is conducted in accordance with the standards for Good Clinical Practice and the fundamental ethical principles established in the Declaration of Helsinki. Approval of the Ethics Committee at each center was obtained (see Supplementary Material Table 6) and informed consent was obtained for each participant. COPPADIS-2015 was classified by the AEMPS (Agencia Española del Medicamento y Productos Sanitarios) as a Post-authorization Prospective Follow-up study with the code COH-PAK-2014-01.
A total of 694 patients with PD (mean age 62.6 ± 8.9 years, 60.3% men) and 207 CS (mean age 61 ± 8.3 years, 49.5% men) were included in the COPPADIS study15.
Assessments
The Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease Rating Scale (QUIP-RS) was assessed in both patients with PD and CS for screening of ICDs and CBs (cutoff points: gambling ≥ 6, shopping ≥ 8, sex ≥ 8, eating ≥ 7, hobbysm-punding ≥ 7)16. For DDS, we used the investigator criterion, given there is no established cut-off.
Demographic data concerning educational level, marital status, smoking, alcohol intake, premorbid impulsive personality, previous ICDs and family history of ICDs were collected for both groups (patients with PD and CS).
Data analysis
In the PD group, a cross-sectional assessment was performed for each patient according to the study methodology15. Baseline data were used for the analysis.
Subanalyses of the group with an age at disease onset younger than 50 years and of the DA treatment group were also performed.
Parametric univariate tests (t-test and chi-squared test) and nonparametric univariate tests (Wilcoxon test) were used to compare demographics and QUIP-RS results between the CS and the patients with PD. A logistic regression analysis (controlling for sex and age) was also used.
In the PD group analysis, univariate and multivariate analysis were used to compare demographic variables, motor and nonmotor symptoms and dopaminergic treatment variables between patients with PD with and without ICDs/CBs. While controlling for age, age at disease onset and disease progression time, a multivariate linear regression analysis was used for the quantitative variables and a logistic regression analysis was employed for the categorical variables. Finally, the association between ICDs severity and treatment was analysed using the QUIP-RS score as the outcome variable and dopaminergic treatment variables (type of treatment and levodopa-equivalent daily dose) as independent variables in multivariate linear regressions (controlling for age, age at disease onset and disease progression time). All the statistical analyses were conducted in R for Statistical Computing, version 3.5.1.
Results
Participants and prevalence of ICDs and CBs
Within the COPPADIS sample, 613 patients with PD and 179 CS completed the ICDs and CBs evaluation. ICDs and CBs were more frequent in patients with PD compared with the CS (ICDs 12.7% vs. 1.6%, respectively, p < 0.001; CBs 7.18% vs. 1.67%, respectively, p = 0.01). The most frequent ICD was compulsive eating in both groups and hobbysm-punding in terms of CBs (Fig. 1).
The analysis split by sex showed a higher presence of hypersexuality in male patients with PD (4.5% vs. 0.16%, p < 0.001). In the CS, the presence of hypersexuality was higher in the male participants, but it was not significant (0.55% vs. 0%, p = 0.85).
Demographic characteristics of the participants with PD and CS
The demographic characteristics of the sample are shown in Table 1. Patients with PD were older than the CS (62.47 ± 9.09 vs. 60.84 ± 8.33 years, respectively; p = 0.004) and were more often men (59.87% vs. 47.48%, respectively; p = 0.004). Premorbid impulsive personality (8.65% vs. 1.1%, respectively; p < 0.001) and previous ICDs (16.1% vs. 0%, respectively; p < 0.001) were more frequent among patients with PD than CS, in addition to them having a higher rate of antidepressant treatment (25.12% vs. 11.73%, respectively; p < 0.001).
Parkinson’s disease features according to ICDs
In the PD group, those with ICDs had a more frequent previous history of ICDs or CBs (17.95% vs. 7.23%; p = 0.01) and premorbid impulsive personality (17.95% vs. 6.6%; p = 0.002) (Table 2).
PD patients with ICDs were younger at evaluation (59.46 ± 9.7 vs. 62.9 ± 8.92; p = 0.002) and at disease onset (54.28 ± 9.88 vs. 58.42 ± 9.43; p < 0.001) (Table 3).
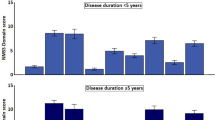
Concerning the PD features according to the presence of ICDs, the motor characteristics were similar in both groups, except for a higher score in the freezing of gait (FOG) scale in patients with ICDs (5.73 ± 5.73 vs. 3.38 ± 4.23; p < 0.001) (Table 3). However, the differences in nonmotor features as well as in the performance and quality of daily living activities were notable. The score on the Non-Motor Symptoms Scale (NMSS) was higher in patients with ICDs (60.37 ± 42.48 vs. 41.33 ± 33.77; p < 0.001). In addition, when the NMSS subdomains were analysed, the cardiovascular, sleep/fatigue, mood/cognition, attention/memory, urinary, sexual and miscellaneous domains had a higher impact on the patients with ICDs (Fig. 2). Along these lines, the Neuropsychiatry Inventory (NPI) showed that, overall, patients with ICDs presented with a more prominent burden of these symptoms (8.6 ± 10.28 vs. 5.48 ± 7.32; p = 0.005). In terms of mood, patients with ICDs showed higher rates of depression (48.97% vs 65.38%, p = 0.004). Analyzing the subtype of depression according to the Beck Depression Inventory, patients with ICDs showed a higher ratio of subclinic and minor depression compared to those without ICDs. Anxiety, euphoria and irritability were the features specifically associated with patients with ICDs.
Mean nonmotor symptoms scale domain score in patients with Parkinson's disease according to the presence of impulse control disorders. Multivariate linear regression adjusted by age, age at disease onset and years of disease evolution. *Statistically significant (p < 0.05). ICD impulse control disorders.
Apart from the NMSS sleep score, the Parkinson’s Disease Sleep Scale showed that patients with ICDs presented with poorer sleep quality, as shown by a lower score (102.58 ± 25.84 vs 116.83 ± 25.63; p < 0.001).
The cognitive status did not differ between groups. After assessing the separate domains of the Parkinson’s Disease Cognitive Rating Scale (PD-CRS), we did not find differences between the patients with and without ICDs.
The quality of life as measured by the 39-item Parkinson’s disease Questionnaire (PDQ-39) was poorer in patients with ICDs (23.38 ± 14.93 vs. 15.64 ± 12.36; p < 0.001). Analysing the domains of the PDQ-39, ICDs had a higher impact on patients in terms of mobility, activities of daily living, body discomfort, communication, cognition and emotional well-being (Fig. 3). ICDs were also associated with a poorer score on the performance of daily activities, as shown by a higher motor items score in the Unified Parkinson's Disease Rating Scale (UPDRS) part II in off condition (2.66 ± 1.79 vs. 3.34 ± 2.27; p = 0.009) (Table 3).
39 items Parkinson's disease Questionaire (PDQ-39) domains in Parkinson's disease patients according to the presence of impulse control disorders. Score expressed by a percentage (0–100%). Multivariate linear regression adjusted by age, age at disease onset and years of disease evolution. *Statistically significant (p < 0.05). ICD impulse control disorders.
In terms of CBs, the results were similar to those observed for the ICDs (Supplementary Material Table 1). There were few exceptions for the age, age at onset, disease progression time and the score of the UPDRS part II in off that were similar in the group of CBs positive and negative.
Regarding dopaminergic treatment, we analysed patients receiving DA treatment, levodopa and/or monoamine oxidase-B inhibitors. After controlling for levodopa-equivalent dose, we found that ICDs were more frequent in patients on DA treatment and the agonist dose was related to the presence of ICDs (237.65 ± 194.72 vs. 174.72 ± 164.92; p = 0.02) (Table 4).
To analyse the influence of the type of DA, we compared not taking any agonists with the risk linked to each type of agonist. For this analysis, we observed that Rotigotine had the lowest risk (OR 2.45; 95% CI 1.06–5.8; p = 0.03) and Ropirinole the highest (OR 2.82; 95% CI 1.25–6.57; p = 0.013) (Table 5). Nevertheless, the analysis of ICDs risk among agonists did not show statistically significant differences.
To examine the influence of ICDs severity according to treatment, we analysed the QUIP-RS total score with dopaminergic treatment characteristics. As expected, we observed that patients with PD undergoing DA treatment had a significantly greater QUIP-RS total score compared with the patients with PD without agonist treatment, controlling for age, age at disease onset and disease progression time (5.47 ± 9.24 vs. 2.26 ± 5.78, respectively; F = 19.39; p < 0.001). When we compared the type of DA with the non-use of DA and the severity of ICDs/CBs using the QUIP-RS total score, Ropinirole was associated with the greatest significant increase in severity (Beta = 4.40; 95% CI 2.44–6.35; F = 7.411; p < 0.001). This result remained significant after correcting for confounding variables such as age, age at disease onset and disease progression time (Beta = 3.83; 95% CI 1.62–6.04; F = 4.773; p < 0.001). Moreover, the increased QUIP-RS total score in patients with ICDs undergoing Ropinirole treatment was significant (Beta = 5.97; 95% CI 1.51–10.43; F = 105.6; p < 0.001), but not for patients with other types of DA (Fig. 4a). The results were the same when correcting for confounding variables (age, age at disease onset and disease progression time )(Beta = 5.99; 95% CI 1.38–10.59; F = 62.35; p < 0.001). On the other hand, we observed that the QUIP-RS total score was significantly increased according to DA equivalent dose (Beta = 0.853; 95% CI 0.41–1.30; F = 9.02; p < 0.001) and daily levodopa equivalent dose (Beta = 0.274; 95% CI 0.07–0.47; F = 6.89; p < 0.01), but not with levodopa dose alone (Fig. 4b).
For the subanalysis of the group with an age at onset younger than 50 years, this group presented with a higher rate of ICDs (19.78%) and differences on the cultural level. Other features such as a premorbid impulsive personality, non-motor symptoms according to NMSS and BDI and poorer quality of life were similar to the entire sample. In the same line with the global analysis, a higher rate in the FOG scale was shown in the group under 50 years old (Supplementary Material, Tables 4 and 5). In the DA treatment group, the results were also similar except for poorer motor performance in off in patients with ICDs (22.31 ± 10.43 vs. 25.46 ± 13.16; p = 0.02) (Supplementary Material, Tables 2 and 3).
Discussion
The objective of the present study was to assess ICDs and CBs in patients with PD and CS, as well as the clinical features linked to these disorders in both groups. We found that in the patients with PD, ICDs and CBs were more frequent compared with the CS (ICDs 12.7% vs. 1.6%, respectively; p < 0.001; CBs 7.18% vs. 1.67%, respectively; p = 0.01). The proportion of ICDs found in our sample was similar to that reported in the DOMINION study2, which is the largest PD prevalence study to date, in which they found ICDs in 13.2% of cases, in accordance with our study and with other European works17. This result is in contrast to the high rate of ICDs found in some other European studies, including in the Spanish population3,10,11. The prospective Italian study, ICARUS, had found a rate of ICDs at the basal evaluation of 29.3%, which was higher than in our baseline assessment in COPPADIS3. These discrepancies could partially be explained by the sociocultural differences in both groups and by the differing tools used for ICDs screening. In the ICARUS study, the Minnesota Impulsive Disorders Interview (MIDI) and QUIP were used, whereas in COPPADIS, the QUIP-RS was applied. Following the MDS recommendations, QUIP-RS has more accurate clinimetric properties for application in PD, being “recommended,” whereas MIDI lacks some criteria validity when compared with the QUIP-RS18. In addition, ICDs screening using QUIP could overestimate ICDs frequency since Papay et al. found that, up to 40% of patients who scored positive for ICDs on the QUIP did not fulfil the diagnosis of ICDs19. However, the QUIP-RS used in COPPADIS has stricter ICDs diagnostic criteria; thus, the proportion found in our study could be more accurate for the identification of ICDs.
On the other hand and in contrast to two Spanish studies, Vela et al. had found an ICDs estimation that reached 58.3% in their cohort of early-onset PD; and in their study of patients undergoing DA treatment, García-Ruiz et al. found this rate increased up to 39.1%10,11. In COPPADIS, when we analysed these two groups separately, the prevalence of ICDs reached 15.87% in patients with PD undergoing DA treatment and 19.78% in patients with an age at disease onset younger than 50 years. Again, these differences could be justified by the different estimation of ICDs provided by QUIP as screening tool that could overestimate the prevalence of ICDs19.
Accounting for the features in PD and CS, we observed that a previous history of ICDs as well as premorbid impulsive personality were more frequent in patients with PD than in CS. A higher rate of antidepressant treatment was also shown in patients with PD, which could be an intrinsic feature of PD, given a higher burden of nonmotor symptoms is present compared with CS, and consequently, depression is also more frequent7,8.
In the PD group, as previous studies have reported, those who had ICDs were younger and their disease onset was earlier2, 3,10,11,20. On the other hand, and in contrast to previous studies, in our cohort, ICDs were not related to a longer disease duration, nor a higher exposure to DRT3,21,22. This result is interesting, given previous studies had hypothesised that the presence of ICDs in young patients could be explained by a dysfunction of the mesocorticolimbic loop due to the influence of the DRT on a relatively preserved ventral striatum23. However, recent studies have supported the contrary version given that, reduced dopamine synthesis capacity in the nucleus accumbens as well as reduced functional connectivity in this area have been shown9,24. Consequently, the higher rate of ICDs in young patients could not be fully explained by the neurodegenerative pattern progression. Genetic factors could instead be involved in the early disease onset and ICDs in these patients5,25.
In patients with PD who have ICDs, a premorbid history of ICDs and impulsive personality were more frequent, according to observations in previous studies developed in European countries and worldwide2,17,26.
Concerning motor performance, in our case and according to the vast majority of studies, the motor status did not differ in patients with ICDs3,10,11. However, it is interesting to note that, although the motor performance according to the UPDRS III was not different according to the presence of ICDs, the scores on the FOG scale were higher in patients with ICDs. This observation was similar in the subgroup of early disease onset, suggesting a shared physiopathological background or due to the perception of the patients since a poorer status was also found in the motor subdomain of the PDQ-39 in patients with ICDs.
The neuropsychiatric sphere is highly impaired in patients with ICDs, as is shown in multiple studies3,14. In COPPADIS, we found a high rate of depression as well as a poorer perception of mood disturbances according to the NMSS, PDQ-39 and NPI. The link between mood and impulsivity is not fully understood because it is not clear whether there is a predisposing factor or whether it is simply a consequence of the ICDs6,7,8. Indeed, it is difficult to elucidate by the comorbidity of both disorders. However, we must take into account that there are genetic forms of PD with prominent nonmotor manifestations, and a common genetic background could therefore underlie both, mood disorders and ICDs27,28,29. In COPPADIS, depression was more frequent in patients with PD compared with CS, which could suggest it as an intrinsic feature of PD rather than a consequence of ICDs. On the other hand, and also correlated with mood disorders, sleep disturbances are usually present in patients with ICDs30,31,32. The relationship could again be bidirectional, as in depression, but it also appears that there is a shared neurobiological substrate, given some genetic risk factors might justify the coexistence of both ICDs and sleep problems33. On the other hand, the intake of antidepressant drugs was not different between patients with ICDs positive or negative in the whole cohort. This observation is important since it could point out to a infradiagnosis of depression in patients with ICDs.
Apart from the impact of neuropsychiatric symptoms and sleep, in line with previous studies3, other nonmotor symptoms were more prominent in patients with ICDs. Urinary and cardiovascular symptoms were more frequent in patients with ICDs, in agreement with the Italian study ICARUS and other series. It is interesting to note that these patients present with a high burden of nonmotor symptoms from different spheres; thus, we should keep this into mind when performing an integral assessment in this particular group of individuals.
Concerning cognitive performance related to ICDs, we did not find any difference between the groups, even when analysing the subgroups of patients undergoing DA treatment or early-onset PD. It contrasts with the abnormalities noted in other studies in which executive dysfunction as well as impairment in the reward-related decision process and set shifting have been noticed13,14,34. In COPPADIS, the global cognition did not differ between groups. Analysing the frontal-subcortical domains of PD-CRS separately, no differences were found. To better understand the reasons for these discrepancies, future neuroimaging studies would be useful to determine the functionality of this area in our cohort.
Regarding the DRT and its influence on ICDs, we found that DA use had greater impact on ICDs; it was dose-dependent and related to severity, which was in line with the observation noted in multiple studies across populations2,10,11,35. However, the risks linked to the type of agonist can be different. We found, according to another Spanish study and a European multicentre study, that Rotigotine had a low risk of developing ICDs, and Ropirinole the highest11,36. As Rizos et al. explained, the association between Rotigotine and ICDs is poorly understood, but it could be associated with its mechanism of action from continuous dopaminergic stimulation37. This possibility is important, given we could consider this in patients with other risk factors for ICDs in the case that DA treatment is required.
On the other hand, it is interesting to highlight the fact that longer levodopa or DA exposure was not related to the appearance of ICDs; thus, ICDs could appear at any time in the disease course. Therefore, clinicians should be aware of and investigate ICDs over time.
This study has some limitations. The presented data correspond to the cross-sectional evaluation at baseline of COPPADIS cohort, nevertheless, the assessment of the ICDs/CBs behaviour over time would be more informative. This issue would be overcome in the future since the follow up of this cohort is on-going and we will obtain the longitudinal data38. On the other hand, following the methodology of the study, the UPDRS part II evaluation only included motor items to assess the phenotype (tremor dominant, postural instability and gait disorders or undetermined). It would be more accurate to evaluate the whole UPDRS part II to establish the handicaps in the performance of the daily activities according this scale. To partially compensate this issue, we assessed the PDQ-39 that also included activities of daily living.
Conclusions
ICDs and CBs are common nonmotor features in patients with PD compared with CS. Some premorbid features such as a previous impulsive personality act as risk factors for developing these disorders. DRT had a large impact on ICDs, and DAs were the drugs with the greatest effect. However, patients with ICDs had a high burden of nonmotor symptoms belonged to mood, cardio-vascular, sleep, urinary domains among others as well as a poor quality of life. Hence, apart from treatment, patients might have a genetic background that could influence the presence of a prominent non-motor phenotype.
References
Weintraub, D., David, A. S., Evans, A. H., Grant, J. E. & Stacy, M. Clinical spectrum of impulse control disorders in Parkinson’s disease. Mov. Disord. 30, 121–127. https://doi.org/10.1002/mds.26016 (2015).
Weintraub, D. et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch. Neurol. 67, 589–595. https://doi.org/10.1001/archneurol.2010.65 (2010).
Antonini, A. et al. ICARUS study: prevalence and clinical features of impulse control disorders in Parkinson’s disease. J. Neurol. Neurosurg. Psych. 88, 317–324. https://doi.org/10.1136/jnnp-2016-315277 (2017).
Eisinger, R. S. et al. Medications, deep brain stimulation, and other factors influencing impulse control disorders in Parkinson’s disease. Front. Neurol. 10, 86. https://doi.org/10.3389/fneur.2019.00086 (2019).
Kraemmer, J. et al. Clinical-genetic model predicts incident impulse control disorders in Parkinson’s disease. J. Neurol. Neurosurg. Psych. https://doi.org/10.1136/jnnp-2015-312848 (2016).
Vriend, C. et al. Depression and impulse control disorders in Parkinson’s disease: two sides of the same coin?. Neurosci. Biobehav. Rev. 38, 60–71. https://doi.org/10.1016/j.neubiorev.2013.11.001 (2014).
Ishihara, L. & Brayne, C. A systematic review of depression and mental illness preceding Parkinson’s disease. Acta Neurol. Scand. 113, 211–220. https://doi.org/10.1111/j.1600-0404.2006.00579.x (2006).
Weintraub, D., Papay, K., Siderowf, A. & Parkinson's Progression Markers Initiative. Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology 80, 176–180. https://doi.org/10.1212/WNL.0b013e31827b915c (2013).
Hammes, J. et al. Dopamine metabolism of the nucleus accumbens and fronto-striatal connectivity modulate impulse control. Brain J. Neurol. 142, 733–743. https://doi.org/10.1093/brain/awz007 (2019).
Vela, L. et al. The high prevalence of impulse control behaviors in patients with early-onset Parkinson’s disease: a cross-sectional multicenter study. J. Neurol. Sci. 368, 150–154. https://doi.org/10.1016/j.jns.2016.07.003 (2016).
Garcia-Ruiz, P. J. et al. Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: a multicentre study. J. Neurol. Neurosurg. Psych. 85, 840–844. https://doi.org/10.1136/jnnp-2013-306787 (2014).
Voon, V. et al. Impulse control disorders in Parkinson disease: a multicenter case–control study. Ann. Neurol. 69, 986–996. https://doi.org/10.1002/ana.22356 (2011).
Santangelo, G., Raimo, S. & Barone, P. The relationship between Impulse Control Disorders and cognitive dysfunctions in Parkinson’s Disease: a meta-analysis. Neurosci. Biobehav. Rev. 77, 129–147. https://doi.org/10.1016/j.neubiorev.2017.02.018 (2017).
Martini, A., Dal Lago, D., Edelstyn, N. M. J., Grange, J. A. & Tamburin, S. Impulse control disorder in parkinson’s disease: a meta-analysis of cognitive, affective, and motivational correlates. Front. Neurol. 9, 654. https://doi.org/10.3389/fneur.2018.00654 (2018).
Santos-Garcia, D. et al. COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015), a global–clinical evaluations, serum biomarkers, genetic studies and neuroimaging–prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression. BMC Neurol. 16, 26. https://doi.org/10.1186/s12883-016-0548-9 (2016).
Weintraub, D. et al. Questionnaire for impulsive-compulsive disorders in Parkinson’s disease-rating scale. Mov. Disord. 27, 242–247. https://doi.org/10.1002/mds.24023 (2012).
Callesen, M. B., Weintraub, D., Damholdt, M. F. & Moller, A. Impulsive and compulsive behaviors among Danish patients with Parkinson’s disease: prevalence, depression, and personality. Parkinsonism Relat. Disord. 20, 22–26. https://doi.org/10.1016/j.parkreldis.2013.09.006 (2014).
Evans, A. H. et al. Scales to assess impulsive and compulsive behaviors in Parkinson’s disease: critique and recommendations. Mov. Disord. 34, 791–798. https://doi.org/10.1002/mds.27689 (2019).
Papay, K. et al. Patient versus informant reporting of ICD symptoms in Parkinson’s disease using the QUIP: validity and variability. Parkinsonism Relat. Disord. 17, 153–155. https://doi.org/10.1016/j.parkreldis.2010.11.015 (2011).
Biundo, R. et al. Impulse control disorders in advanced Parkinson’s disease with dyskinesia: The ALTHEA study. Mov. Disord. 32, 1557–1565. https://doi.org/10.1002/mds.27181 (2017).
Kim, J. et al. Clinical characteristics of impulse control and repetitive behavior disorders in Parkinson’s disease. J. Neurol. 260, 429–437. https://doi.org/10.1007/s00415-012-6645-9 (2013).
Pontieri, F. E. et al. Sociodemographic, neuropsychiatric and cognitive characteristics of pathological gambling and impulse control disorders NOS in Parkinson’s disease. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 25, 69–76. https://doi.org/10.1016/j.euroneuro.2014.11.006 (2015).
Voon, V., Mehta, A. R. & Hallett, M. Impulse control disorders in Parkinson’s disease: recent advances. Curr. Opin. Neurol. 24, 324–330. https://doi.org/10.1097/WCO.0b013e3283489687 (2011).
Smith, K. M., Xie, S. X. & Weintraub, D. Incident impulse control disorder symptoms and dopamine transporter imaging in Parkinson disease. J. Neurol. Neurosurg. Psych. 87, 864–870. https://doi.org/10.1136/jnnp-2015-311827 (2016).
Cormier-Dequaire, F. et al. Suggestive association between OPRM1 and impulse control disorders in Parkinson’s disease. Mov. Disord. 33, 1878–1886. https://doi.org/10.1002/mds.27519 (2018).
Voon, V. & Fox, S. H. Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch. Neurol. 64, 1089–1096. https://doi.org/10.1001/archneur.64.8.1089 (2007).
Jesus, S. et al. GBA variants influence motor and non-motor features of Parkinson’s disease. PLoS ONE 11, e0167749. https://doi.org/10.1371/journal.pone.0167749 (2016).
Dan, X. et al. Association between common genetic risk variants and depression in Parkinson’s disease: a dPD study in Chinese. Parkinsonism Relat. Disord. 33, 122–126. https://doi.org/10.1016/j.parkreldis.2016.09.029 (2016).
Swan, M. et al. Neuropsychiatric characteristics of GBA-associated Parkinson disease. J. Neurol. Sci. 370, 63–69. https://doi.org/10.1016/j.jns.2016.08.059 (2016).
Scullin, M. K. et al. Sleep and impulsivity in Parkinson’s disease. Parkinsonism Relat. Disord. 19, 991–994. https://doi.org/10.1016/j.parkreldis.2013.06.018 (2013).
O’Sullivan, S. S. et al. Sleep disturbance and impulsive-compulsive behaviours in Parkinson’s disease. J. Neurol. Neurosurg. Psych. 82, 620–622. https://doi.org/10.1136/jnnp.2009.186874 (2011).
Pontone, G., Williams, J. R., Bassett, S. S. & Marsh, L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology 67, 1258–1261. https://doi.org/10.1212/01.wnl.0000238401.76928.45 (2006).
Parekh, P. K., Ozburn, A. R. & McClung, C. A. Circadian clock genes: effects on dopamine, reward and addiction. Alcohol 49, 341–349. https://doi.org/10.1016/j.alcohol.2014.09.034 (2015).
Santangelo, G., Piscopo, F., Barone, P. & Vitale, C. Personality in Parkinson’s disease: clinical, behavioural and cognitive correlates. J. Neurol. Sci. 374, 17–25. https://doi.org/10.1016/j.jns.2017.01.013 (2017).
Corvol, J. C. et al. Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology 91, e189–e201. https://doi.org/10.1212/WNL.0000000000005816 (2018).
Rizos, A. et al. A European multicentre survey of impulse control behaviours in Parkinson’s disease patients treated with short- and long-acting dopamine agonists. Eur. J. Neurol. https://doi.org/10.1111/ene.13034 (2016).
Samuel, M. et al. Management of impulse control disorders in Parkinson’s disease: Controversies and future approaches. Mov. Disord. 30, 150–159. https://doi.org/10.1002/mds.26099 (2015).
Santos Garcia, D. et al. COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015): an ongoing global Parkinson’s disease project about disease progression with more than 1000 subjects included. Results from the baseline evaluation. Eur. J. Neurol. https://doi.org/10.1111/ene.14008 (2019).
Funding
Funding sources: www.curemoselparkinson.org.
Author information
Authors and Affiliations
Consortia
Contributions
S.J.: conception of the manuscript, analysis, writing of the first draft of the manuscript; recruitment and/or evaluation of participants. M.A.L.-E.: analysis, review and critique. A.D.A.: review and critique; recruitment and/or evaluation of participants. C.M.B.: review and critique; recruitment and/or evaluation of participants. J.C.M.-C.: review and critique; recruitment and/or evaluation of participants. A.A.-C.: review and critique; recruitment and/or evaluation of participants. P.S.A.: review and critique; recruitment and/or evaluation of participants. S.N.-P.: review and critique; recruitment and/or evaluation of participants. M.G.A.-L.: review and critique; recruitment and/or evaluation of participants. N.L.A.: review and critique; recruitment and/or evaluation of participants. J.C.S.R.: review and critique; recruitment and/or evaluation of participants. M.I.M.: review and critique; recruitment and/or evaluation of participants. I.G.: review and critique; recruitment and/or evaluation of participants. F.L.B.: review and critique; recruitment and/or evaluation of participants. P. C.I.: review and critique; recruitment and/or evaluation of participants. J.K.: review and critique; recruitment and/or evaluation of participants. J.P.: review and critique; recruitment and/or evaluation of participants. B.P.-S.: review and critique; recruitment and/or evaluation of participants. P.M.-M.: review and critique. Overall supervision. D.S.-G.: conception, organization, and execution of the project; recruitment and/or evaluation of participants; review and critique. M.P.: conception of the manuscript, analysis, review and critique.
Corresponding author
Ethics declarations
Competing interests
S. Jesús has received honoraria from Abbvie, Bial, Merz, UCB, Italfarmaco and Zambon. She holds the competitive contract "Juan Rodés" supported by the Instituto de Salud Carlos III (JR16/00031). She received grants from the Spanish Ministry of Economy and Competitiveness (PI18/01898) and the Consejería de Salud de la Junta de Andalucía (PI-0459-2018). M. A. Labrador-Espinosa: None. A. D. Adarmes has received honoraria from Abbvie and Italfarmaco. C. Méndez del Barrio has received honoraria from Neuraxpharm. J. C. Martínez-Castrillo has received grants/research support from Allergan, Abbvie, Bial, Ipsen, Italfarmaco, Merz, and Zambon; honoraria or consultation fees from Allergan, AbbVie, Bial, Exeltis, Ipsen, Italfarmaco, Merz, Ipsen, Orion, UCB, and Zambon; and company sponsored speaker’s bureau from Allergan, Abbvie, Bial, Krka, Ipsen, Italfarmaco, Merz, UCB, and Zambon. A. Alonso-Cánovas has received honoraria as lecturer in seminars, simposia and teaching courses, scientific advisory, and travel and congress attendance sponsor with Zambon and Abbvie. P. Sánchez Alonso has received honoraria as lecturer in seminars, symposia and teaching courses, scientific advisory, and travel and congress attendance sponsor with Zambon, Abbvie, Bial. S. Novo-Ponte has received unrestricted educational support from Abbvie, Zambon, Novartis. M. G. Alonso-Losada has received honoraria for educational presentations and advice service by Zambon and Bial. N. López Ariztegui has received honoraria for educational presentations and/or advice service by Abbvie, UCB Pharma, Italfarmaco, Zambon, Bial, and Teva. J. CSegundo Rodríguez: None. M. I. Morales: None. I. Gastón has received research support from Abbvie and Zambon and has served as a consultant for Abbvie, Exelts and Zambon. F. Lacruz Bescos: None. P. Clavero Ibarra: None. J. Kulisevsky has received consulting and lecture fees from Roche, Zambon, Teva and Bial. He got research funding fromRoche, Zambon, Ciberned, Instituto de Salud Carlos III and FundacióLa Maratóde TV3. J. Pagonabarraga has served on advisory or speakers' boards, and received honoraria from UCB, Zambon, AbbVie, Italfarmaco, Allergan, Ipsen and Bial, and received grants from CIBERNED and FIS PI14/02058) (Spanish Government grants) and Fundació La Marató de TV3 20142910. B. Pascual-Sedano has received honoraria by Abbvie and UCB Pharma. P. Martinez-Martin Honoraria: from Editorial Viguera for lecturing in courses; International Parkinson and Movement Disorder Society (IPMDS) for management of the Program on Rating Scales; Air Liquide, Abbvie, and HM Hospitales de Madrid for advice in clinic-epidemiological studies. License fee payments for the King's Parkinson's Disease Pain scale. IPMDS grants for attendance to the IPMDS Congress (2018) and for development and validation of the MDS-NMS. D. Santos-García has received honoraria for educational presentations and/or advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva. P. Mir has received honoraria from AbbVie, Abbott, Allergan, Bial, Merz, UCB, and Zambon. He has received grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Subdirección General de Evaluación y Fomento de la Investigación). He also received grants from Fondo Europeo de Desarrollo Regional (FEDER), the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía [CVI-02526, CTS-7685], the Consejería de Salud y Bienestar Social de la Junta de Andalucía [PI-0437-2012, PI-0471-2013], the Sociedad Andaluza de Neurología, the Jacques and Gloria Gossweiler Foundation, the Fundación Alicia Koplowitz, and the Fundación Mutua Madrileña.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jesús, S., Labrador-Espinosa, M.A., Adarmes, A.D. et al. Non-motor symptom burden in patients with Parkinson’s disease with impulse control disorders and compulsive behaviours: results from the COPPADIS cohort. Sci Rep 10, 16893 (2020). https://doi.org/10.1038/s41598-020-73756-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73756-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.