Abstract
Studies on the risk factors for intrahepatic cholestasis of pregnancy (ICP) in a population-based cohort are lacking. We assess the prevalence and risk factors of ICP in a Chinese population. In this study, a cohort study was conducted that included 12,200 eligible pregnant women. The overall incidence of ICP in this cohort was 6.06%. With increasing maternal age, the incidence of ICP decreased in women younger than 30 years of age but increased in those older than 30. With increasing pre-pregnancy BMI, the incidence of ICP decreased if the pre-pregnancy BMI was less than 23 kg/m2 but increased if it was 23 kg/m2 or higher. Further analysis showed that the risk of ICP increased when maternal age was < 25 years (Adjusted RR 2.01; 95% CI 1.64–2.47) or ≥ 35 years (Adjusted RR 1.34; 95% CI 1.02–1.76). Furthermore, an increased risk of ICP was associated with pre-pregnancy underweight (adjusted RR 1.27; 95% CI 1.04–1.56), inadequate gestational weight gain (GWG) (adjusted RR 1.58; 95% CI 1.28–1.96), lower maternal education (adjusted RR 2.96; 95% CI 2.35–3.74), multiparity (adjusted RR 1.54; 95% CI 1.23–1.93), and twin/multiple pregnancies (adjusted RR 2.12; 95% CI 1.25–3.58). Maternal age (< 25 or ≥ 35 years), underweight, inadequate GWG, lower maternal education, multiparity, and twin/multiple pregnancies were identified as risk factors of ICP.
Similar content being viewed by others
Introduction
Intrahepatic cholestasis of pregnancy (ICP), also known as obstetric cholestasis, is defined as the presence of pruritus in combination with a total serum bile acid (TBA) level above 10 μmol/L during the second and third trimesters of pregnancy1,2. ICP is a pregnancy-specific liver disease, and the incidence varies from 0.1 to 15.6% depending on geography and ethnicity3. ICP is more common in South Asia, South America and Scandinavia. The etiology of ICP is multifactorial, and may be associated with increased estrogen levels as well as altered expression of hepatobiliary transport proteins during pregnancy4,5. ICP increases the risk of adverse fetal outcomes. Several epidemiological studies have shown that ICP is associated with spontaneous and iatrogenic preterm delivery6,7,8. Several experimental studies have also demonstrated that ICP is a leading cause of stillbirth and neonatal demise9,10,11. Furthermore, many studies show an association between ICP and respiratory distress syndrome, fetal intrauterine growth restriction, a low (< 7) 5-min Apgar score, and meconium-stained fluid12,13,14. A recent randomised control trial in pregnant women with ICP reports that treatment with ursodeoxycholic acid, a common agent for treating ICP, not only does not significantly reduces serum TBA levels and improves pruritus and liver functions, but also does not decrease the occurrence of adverse fetal outcomes15.
ICP is also associated with increased risks of adverse maternal outcomes. Women who have experienced ICP have increased risks of later-life cardiovascular diseases, autoimmune-mediated conditions, diabetes mellitus, hepatobiliary diseases and carcinoma16,17. Epidemiological studies also report that women with ICP are at increased risks for gestational diabetes mellitus, dyslipidemia and pre-eclampsia18,19,20.
To date, most of the studies have focused on the association between ICP and adverse fetal and maternal outcomes. Although the incidence of ICP differs significantly among various countries and ethnicities21,22, studies on the risk factors for ICP in a population-based cohort are lacking.
The objective of the current study was to assess the prevalence and risk factors of ICP in a Chinese population. We found that pregnancy at a young or advanced maternal age, underweight, inadequate GWG, lower maternal education, multiparity, and twins/multiple pregnancies were associated with an increased risk of ICP.
Results
The demographic characteristics and laboratory measurements of participants
The incidence of ICP in this cohort was 6.06% (739/12,200, Table 1). The demographic characteristics of study population are summarized in Table 1. There were significant differences in maternal age, maternal pre-pregnancy BMI, gestational weight gain, maternal education, mode of delivery and parity between control and ICP groups. The mean gestational age was significantly lower in those with ICP as compared with controls (Table 1). There was also a significant difference in the prevalence of twins or multiplets between the two groups (Table 1). However, gravidity and gestational diabetes were not significantly different between the two groups (Table 1). Furthermore, the levels of serum TBA, aspartate transaminase, alanine transaminase, total bilirubin, direct bilirubin and indirect bilirubin were all significantly higher in those with ICP than controls (Table 2).
Association between demographic characteristics as a categorical variable and the risk of ICP
The association between demographic characteristics and the risk of ICP was analyzed (Table 3). Compared to a maternal age range of 25–34 years, younger than 25 or older than 35 was associated with an increased risk of ICP, with an adjusted RR of 2.01 (95% CI 1.64–2.47) and 1.34 (95% CI 1.02–1.76) respectively (Table 3). Referring to pre-pregnancy body weight, underweight was associated with an increased risk of ICP (adjusted RR 1.27; 95% CI 1.04–1.56); however no significant associations were observed between overweight, obesity and the risk of ICP (Table 3). When gestational weight gain (GWG) and the risk of ICP was analyzed, inadequate GWG was associated with an increased risk of ICP (adjusted RR 1.58; 95% CI 1.28–1.96), whereas excessive GWG decreased the risk of ICP (adjusted RR 0.72; 95% CI 0.62–0.92). The association between maternal education and the risk of ICP was also analyzed. Compared to those with high education, mothers with medium and low education had a higher risk of ICP, and adjusted RRs were 1.40 (95% CI 1.10–1.78) and 2.96 (95% CI 2.35–3.74) respectively (Table 3). When parity and gravidity were considered, multiparity increased the risk of ICP compared to primiparity (adjusted RR 1.54; 95% CI 1.23–1.93), but gravidity was not significantly associated with ICP (Table 3). Furthermore, twin or multiple pregnancies were associated with a 2.12 fold increase (adjusted 95% CI 1.25–3.58) of ICP (Table 3).
Association between demographic characteristics as a continuous variable and the risk of ICP
Participants were divided into ten categories according to their pre-pregnancy age, and the incidence of ICP across these age ranges was analyzed (Fig. 1A). The lowest ICP was in those with a maternal age of 27.5–29.9 years (category 5, Fig. 1A). When the maternal age was less than 30 years (categories 1–5, Fig. 1A), increases in age was associated with an decreased risk of ICP. However, when the maternal age was 30 years or higher (categories 6–10, Fig. 1A), increases in age increased the incidence of ICP increased. We further analyzed the association between maternal age as a continuous variable and the risk of ICP. The adjusted RRs for an increase in maternal age of 1 SD were respectively 0.92 (95% CI 0.84–1.02) when all participants were analyzed as a cohort, 0.52 (95% CI 0.44–0.63) for those aged younger than 30 years, and 1.23 (95% CI 1.02, 1.48) for those aged 30 or older (Table 4).
The relationship between ICP frequency and maternal age and BMI. (A) Maternal age in 10 categories. Age range in category 1, younger than 20.0 years of age (n = 98); 2, 20.0–22.4 (n = 617); 3, 22.5–24.9 (n = 1170); 4, 25–27.4 (n = 3117); 5, 27.5–29.9 (n = 2523); 6, 30.0–32.4 (n = 2451); 7, 32.5–34.9 (n = 809); 8, 35.0–37.4 (n = 821); 9, 37.5–39.9 (n = 279); 10, 40 or older (n = 315). (B) Maternal BMI in 8 categories. BMI range in category 1, less than 18.5 kg/m2 (n = 2074); 2, 18.5–19.9 (n = 2718); 3, 20.0–21.4 (n = 2994); 4, 21.5–22.9 (n = 1836); 5, 23.0–24.4 (n = 1189); 6, 24.5–25.9 (n = 673); 7, 26.0–27.4 (n = 329); 8, 27.5 or higher (n = 387).
Participants were also divided into eight categories according to their pre-pregnancy BMI, and the incidence of ICP across these BMI ranges is shown in Fig. 1B. The incidence of ICP was lowest when the pre-pregnancy BMI was between 21.5 and 22.9 kg/m2) (category 4, Fig. 1B). When BMI was less than 23 kg/m2 (categories 1–4), its increase decreased the incidence of ICP (Fig. 1B). However, when the pre-pregnancy BMI was 23 kg/m2 or higher (categories 5–8), its increase increased ICP (Fig. 1B). We further analyzed the association between maternal BMI as a continuous variable and the risk of ICP. Adjusted RRs for an increase in maternal BMI of 1 SD were respectively 0.97 (95% CI 0.90–1.06) among all mothers, 0.81 (95% CI 0.69–0.94) for those with a pre-pregnancy BMI less than 23 kg/m2, and 1.22 (95% CI 1.05–1.42) when the pre-pregnancy BMI was 23 kg/m2 or higher (Table 4). Additionally, we investigated the relationship between GWG as a continuous variable and the risk of ICP, and the adjusted RR for an increase in GWG of 1 SD was 0.73 (95% CI 0.67–0.80) (Table 4).
Discussion
To date, most of the studies have focused on the association between ICP and adverse fetal and maternal outcomes6,7,8,23. Although the differences including in maternal age and prepregnancy BMI were observed between ICP cases and controls23, studies on the risk factors for ICP in a Chinese population are lacking. The current study investigated the prevalence and risk factors of ICP in a Chinese population. Within 12,200 deliveries included in the study, 6.06% of the participants developed ICP. Increases in maternal age decreased the incidence of ICP when the maternal age was less than 30 years but increased it if the maternal age was 30 years or older. With increasing pre-pregnancy BMI, the incidence of ICP decreased when BMI was less than 23 kg/m2 but increased when the BMI was 23 kg/m2 or higher. Logistic regression models showed that in this cohort maternal age below 25 or above 35 years, pre-pregnancy underweight, inadequate GWG, lower maternal education, multiparity, and twins/multiple pregnancies were risk factors of ICP.
The incidence of 6.06% ICP found in the current study was comparable with findings of other cities in China24,25, but it was higher than that reported in the neighboring countries like Punjab Pakistan26. The disease was more common in South America, especially in Chile, where early study reported a 15.1% overall incidence and 24.1% among women of Araucanian Indian descent27. ICP was less common in North America, Central and Western Europe, which has been stable for many28,29. These variations in ICP prevalence might be due to differences in eating habit and nutritional status, geographic location, levels of health services, and differing diagnostic criteria. In addition, the overall incidence of ICP in a primarily Latina Los Angeles population was reported to be 5.6%, which is more than ten times higher than the previously reported prevalence within the United States and suggests an potential association between ICP and ethnicity30. Our current study found a complex association between pre-pregnancy BMI and ICP risks, which may provide an additional explanation to why the incidence of ICP differs among different populations and ethnicities.
Numerous reports agree that a suboptimal maternal age is linked to an increased risk of adverse pregnancy outcomes, such as preeclampsia, cesarean section, miscarriage, preterm delivery, fetal growth restriction, and neonatal mortality31,32,33. However, the association between maternal age and the occurrence of ICP remains unclear. In this cohort, 11.6% (1412/12,200) of deliveries were from women older than 35 years of age. The prevalence of advanced maternal age in our current cohort was lower than that of the European countries in 201634, but was higher than that of China in 200935. In addition, 15.5% (1888/12,200) of deliveries in this cohort were from women younger than 25 years of age, which was comparable with the overall rate of China in 200935. Furthermore, this study found that an advanced as well as young maternal age, after adjustment for other maternal characteristics, was associated with an increased risk of ICP. Specifically, an older maternal age decreased ICP if the woman was younger than 30 years but increased it if she was older than 30. Our studies suggest that a maternal age between 27.5 and 32.5 is most optimal in lowering the risk of ICP.
This study found that twin/multiple pregnancies were associated with an increased risk of ICP. This may be related to higher levels of hormones such as estrogen and progesterone in these pregnancies4,21,36. Estrogen has also been demonstrated to induce cholestasis during pregnancy by inhibiting the expression of hepatic biliary proteins in rodents37. Previous studies have also demonstrated that progesterone metabolites could alter hepatic bile acid homeostasis by impairing the function of the major hepatic bile acid receptors38. Epidemiological studies show that the increased estrogen levels in twin pregnancies are associated with a greater risk of ICP39. Further experimental studies have proven that estrogen could inhibit the utilization of blood sugar, fat decomposition and free fatty acid release, while high free fatty acids induce liver injury and aggravate cholestasis40.
A 12-year population-based cohort in Sweden showed that women with ICP had an increased risk of gestational diabetes compared with normal pregnant women23. However, our study showed that there was no significant difference in gestational diabetes between the two groups. Actually, a previous study indicated that the incidence of gestational diabetes was significantly higher in Caucasian population but not in Asian population18, suggesting an ethnic disparity on the relationship between ICP and gestational diabetes.
An advantage of the current study is that it included a large population-based birth cohort and had adequate power to estimate associations using multivariable analyses. However, the study had three limitations. Firstly, as the cohort included only Chinese population, cautions are needed when the findings are branched out to other ethnic populations. Secondly, as the study was limited to only one hospital, a potential selection bias might not be completely excluded. A third limitation was the lack of information on the history of ICP in participants and their immediate family members, which prevented analyses of genetic susceptibilities to ICP41,42.
In summary, the present study analyzed the prevalence and risk factors of ICP in a Chinese population that included 12,200 eligible pregnant women. In this cohort, the overall incidence of ICP was 6.06%, and the risk factors of ICP were maternal age below 25 or above 35 years, pre-pregnancy underweight, inadequate GWG, lower maternal education, multiparity and twin/multiple pregnancies. Our studies provide new understandings of ICP, which may facilitate the prioritization of medical interventions, resource assignments and policy making. In particular, our results may aid the prediction of pregnancies with a high risk of ICP, providing clinicians with time to plan and strategize their patients’ maternal/fetal surveillance and care.
Materials and methods
Participants
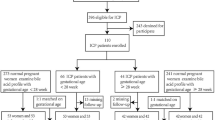
The birth cohort included 13,801 pregnant women who received antenatal care and delivery in the first affiliated Hospital of Anhui Medical University from January 2011 to December 201443. The diagnosis of ICP was based on the presence of pruritus in combination with elevated serum levels of total bile acid (TBA ≥ 10 μmol/L). The study analyzed a total of 12,200 pregnant women, following the exclusion of 897 who withdrew or had no detailed delivery records, and the omission of a further 704 who had no diagnostic records of ICP. Biochemical parameters (aspartate transaminase, alanine transaminase, and bilirubin) were retrieved from the hospital records. The study was approved by the ethics committee of Anhui Medical University (Approval No. 20160010). A written informed consent was obtained from all participants, and all protocols were carried out in accordance with the approved guidelines.
Measurement of serum TBA
Serum TBA levels were measured by an automatic biochemical analyzer (Dirui CS-T300, Ltd, Changchun, China) according to our previous protocol44.
Statistical analysis
The data were analyzed using SPSS 20.0. Normal distribution of variables was assessed with the Shapiro–Wilk test. The mean differences were compared using non-parametric statistics (Mann–Whitney U test). Chi-square test (χ2 test) was used to compare categorical variables or ordinal variables. Crude and adjusted relative risks (RRs) of ICP with 95% confidence intervals (95% CI) were calculated using multiple logistic regression models. A p value of < 0.05 (two-tailed) or a 95% CI not including 1 and 0 (for relative risk) was considered to be statistically significant.
References
Kawakita, T. et al. Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. Am. J. Obstet Gynecol. 213(570), e1–e8 (2015).
Joshi, D., James, A., Quaglia, A., Westbrook, R. H. & Heneghan, M. A. Liver disease in pregnancy. Lancet 375, 594–605 (2010).
Lee, N. M. & Brady, C. W. Liver disease in pregnancy. World J. Gastroenterol. 15, 897–906 (2009).
Williamson, C. & Geenes, V. Intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 124, 120–133 (2014).
Dixon, P. H. & Williamson, C. The molecular genetics of intrahepatic cholestasis of pregnancy. Obstet. Med. 1, 65–71 (2008).
Geenes, V. et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology 59, 1482–1491 (2014).
Kondrackiene, J. et al. Predictors of premature delivery in patients with intrahepatic cholestasis of pregnancy. World J. Gastroenterol. 13, 6226–6230 (2007).
Glantz, A., Marschall, H. U. & Mattsson, L. A. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology 40, 467–474 (2004).
Ovadia, C. et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet 393, 899–909 (2019).
Di Mascio, D. et al. Perinatal death by bile acid levels in intrahepatic cholestasis of pregnancy: a systematic review. J. Matern. Fetal. Neonatal. Med. 19, 1–9 (2019).
Zhang, Y. et al. Maternal bile acid transporter deficiency promotes neonatal demise. Nat. Commun. 6, 8186 (2015).
Chen, W. et al. Obeticholic acid protects against gestational cholestasis-induced fetal intrauterine growth restriction in mice. Oxid. Med. Cell. Longev. 2019, 7419249 (2019).
Herrera, C. A. et al. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J. Matern. Fetal. Neonatal. Med. 31, 1913–1920 (2018).
Puljic, A. et al. The risk of infant and fetal death by each additional week of expectant management in intrahepatic cholestasis of pregnancy by gestational age. Am. J. Obstet. Gynecol. 212(667), e1-5 (2015).
Chappell, L. C. et al. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet 394, 849–860 (2019).
Wikström Shemer, E. A. et al. Intrahepatic cholestasis of pregnancy and cancer, immune-mediated and cardiovascular diseases: a population-based cohort study. J. Hepatol. 63, 456–461 (2015).
Marschall, H. U., Wikström Shemer, E., Ludvigsson, J. F. & Stephansson, O. Intrahepatic cholestasis of pregnancy and associated hepatobiliary disease: a population based cohort study. Hepatology 58, 1385–1391 (2013).
Martineau, M., Raker, C., Powrie, R. & Williamson, C. Intrahepatic cholestasis of pregnancy is associated with an increased risk of gestational diabetes. Eur. J. Obstet. Gynecol. Reprod. Biol. 176, 80–85 (2014).
Martineau, M. G. et al. The metabolic profile of intrahepatic cholestasis of pregnancy is associated with impaired glucose tolerance, dyslipidemia, and increased fetal growth. Diabetes Care 38, 243–248 (2015).
Raz, Y. et al. Severe intrahepatic cholestasis of pregnancy is a risk factor for preeclampsia in singleton and twin pregnancies. Am. J. Obstet. Gynecol. 213(395), e1-8 (2015).
Smith, D. D. & Rood, K. M. Intrahepatic Cholestasis of Pregnancy. Clin. Obstet. Gynecol. 63, 134–151 (2020).
Geenes, V. & Williamson, C. Intrahepatic cholestasis of pregnancy. World J. Gastroenterol. 15, 2049–2066 (2009).
Shemer, E. W., Marschall, H. U., Ludvigsson, J. F. & Stephansson, O. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG 120, 717–723 (2013).
Ge, X. et al. Intrahepatic cholestasis of pregnancy and fetal outcomes: a prospective birth cohort study. Zhonghua. Liu. Xing. Bing. Xue. Za. Zhi. 37, 187–191 (2016).
Jin, W. Y. et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC. Pregnancy Childbirth 16, 60 (2016).
Hafeez, M., Ansari, A., Parveen, S., Salamat, A. & Aijaz, A. Frequency of intrahepatic cholestasis of pregnancy in Punjab Pakistan: a single centre study. J. Pak. Med. Assoc. 66, 203–206 (2016).
Reyes, H. et al. Prevalence of intrahepatic cholestasis of pregnancy in Chile. Ann Intern. Med. 88, 487–493 (1978).
Ozkan, S., Ceylan, Y., Ozkan, O. V. & Yildirim, S. Review of a challenging clinical issue: intrahepatic cholestasis of pregnancy. World J. Gastroenterol. 21, 7134–7141 (2015).
Allen, A. M. et al. The epidemiology of liver diseases unique to pregnancy in a US Community: a population-based study. Clin. Gastroenterol. Hepatol. 14, 287-94.e1–2 (2016).
Lee, R. H., Goodwin, T. M., Greenspoon, J. & Incerpi, M. The prevalence of intrahepatic cholestasis of pregnancy in a primarily Latina Los Angeles population. J. Perinatol. 26, 527–532 (2006).
Pan, L., Fu, Z., Yin, P. & Chen, D. Pre-existing medical disorders as risk factors for preeclampsia: an exploratory case–control study. Hypertens. Pregnancy 38, 245–251 (2019).
Khalil, A., Syngelaki, A., Maiz, N., Zinevich, Y. & Nicolaides, K. H. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet. Gynecol. 42, 634–644 (2013).
Schummers, L. et al. Variation in relationships between maternal age at first birth and pregnancy outcomes by maternal race: a population-based cohort study in the United States. BMJ. Open 9, e033697 (2019).
Claramonte Nieto, M., Meler Barrabes, E., Garcia Martínez, S., Gutiérrez Prat, M. & Serra Zantop, B. Impact of aging on obstetric outcomes: defining advanced maternal age in Barcelona. BMC. Pregnancy Childbirth 19, 342 (2019).
Chen, Y. H. et al. Influent factors of gestational vitamin D deficiency and its relation to an increased risk of preterm delivery in Chinese population. Sci. Rep. 8, 3608 (2018).
Lammert, F., Marschall, H. U., Glantz, A. & Matern, S. Intrahepatic cholestasis of pregnancy: molecular pathogenesis, diagnosis and management. J. Hepatol. 33, 1012–1021 (2000).
Crocenzi, F. A. et al. Estradiol-17beta-D-glucuronide induces endocytic internalization of Bsep in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G449–G459 (2003).
Abu-Hayyeh, S. et al. Prognostic and mechanistic potential of progesterone sulfates in intrahepatic cholestasis of pregnancy and pruritus gravidarum. Hepatology 63, 1287–1298 (2016).
Gonzalez, M. C. et al. Intrahepatic cholestasis of pregnancy in twin pregnancies. J. Hepatol. 9, 84–90 (1989).
Du, Q. et al. Placental gene-expression profiles of intrahepatic cholestasis of pregnancy reveal involvement of multiple molecular pathways in blood vessel formation and inflammation. BMC Med. Genom. 7, 42 (2014).
Dixon, P. H. et al. Contribution of variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut 58, 537–544 (2009).
Zhou, J. et al. Association between bile salt export pump polymorphisms and intrahepatic cholestasis of pregnancy susceptibility: a meta-analysis of case-control studies. Gynecol. Endocrinol. 35, 179–183 (2019).
Chen, Y. H. et al. Pre-pregnancy underweight and obesity are positively associated with small-for-gestational-age infants in a Chinese population. Sci. Rep. 9, 15544 (2019).
Li, L. et al. Continuous association of total bile acid levels with the risk of small for gestational age infants. Sci. Rep. 10, 9257 (2020).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81471467), National College Students’ Innovation and Entrepreneurship Training Program (201910366007), the Scientific Research Foundation of Reserve Candidates for Anhui Provincial Academic and Technological Leaders (2018H204), and the Key Projects of Anhui Provincial Natural Science Research in Colleges and Universities (KJ2019A0224).
Author information
Authors and Affiliations
Contributions
Y.H.C. designed research; X.X.G., M.Y.Y., Y.L., J.Y.L., L.L., W.C., and X.L. conducted data collection and research; Y.H.C. and X.X.G. analyzed data and performed statistical analysis; Y.H.C. and X.X.G. wrote paper; G.N. critically reviewed the data and revised the manuscript; Y.H.C. and G.N. had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, XX., Ye, MY., Liu, Y. et al. Prevalence and risk factors of intrahepatic cholestasis of pregnancy in a Chinese population. Sci Rep 10, 16307 (2020). https://doi.org/10.1038/s41598-020-73378-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73378-5
This article is cited by
-
Maternal and neonatal outcomes of intrahepatic cholestasis of pregnancy after in vitro fertilization
BMC Pregnancy and Childbirth (2024)
-
Predictors of Adverse Fetal Outcomes in Intrahepatic Cholestasis of Pregnancy (ICP): a Narrative Review
Reproductive Sciences (2024)
-
Maternal Exposure to Per- and Polyfluoroalkyl Substances: Implications for Intrahepatic Cholestasis of Pregnancy and Adverse Birth Outcomes
Exposure and Health (2024)
-
Exosomes from Intrahepatic Cholestasis of Pregnancy Induce Cell Apoptosis Through the miRNA-6891-5p/YWHAE Pathway
Digestive Diseases and Sciences (2024)
-
Perinatal outcomes associated with ICP in twin pregnancies were worse than singletons: an almost 5-year retrospective cohort study
BMC Pregnancy and Childbirth (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.