Abstract
Hyperuricemia is prevalent throughout the world. However, a well-designed large-scale epidemiological investigation of hyperuricemia in southwestern China is lacking. A regional representative sample of 10,141 participants were included using multistage, stratified sampling in Chengdu and Chongqing from September 2013 to March 2014. Hyperuricemia was defined as the self-reported of the doctor's diagnosis of hyperuricemia, or serum uric acid > 420 μmol/L in men or serum uric acid > 360 μmol/L in women. The overall age- and sex-standardized prevalence of hyperuricemia among adults aged 35–79 years was 13.5%. Compared with women, the prevalence of hyperuricemia in men was higher (17.3% versus 10.0%). Hypertension, hyperlipidemia, overweight or obesity, central obesity were associated with an increased risk for hyperuricemia both in men and in women. Married men and women were not susceptible to hyperuricemia. Current cigarette smoking was an associated risk factor of hyperuricemia only in women. Hyperuricemia has become a major health problem among urban adults aged 35–79 years in southwestern China, and special attention should be paid to men. Comorbidities associated with hyperuricemia and causality worth further investigation.
Similar content being viewed by others
Introduction
Uric acid is a natural product generated from purine metabolism. Hyperuricemia can result from the overproduction or underexcretion of uric acid in human1,2. In the human evolutionary perspective, higher uric acid concentrations may have a survival advantage during the period of starvation in the past3,4,5. Although it is inconclusive that hyperuricemia is both a protective and causative factor in non-communicable diseases (e.g., cardiovascular disease6,7, neurodegenerative disease8,9), hyperuricemia is the cause of gout1,3,10. Elevated levels of serum uric acid (SUA) has been observed throughout the world, and hyperuricemia is prevalent both in developed and developing countries11,12,13,14,15.
Due to rapid industrialization, urbanization, and aging, the burden of non-communicable diseases is striking in China16. In 2009–2010, the prevalence of hyperuricemia among Chinese adults was 8.4%, approximately 92.9 million adults with hyperuricemia14. According to regional data in 2018 released from National Bureau of Statistics of China, more than 200 million people reside in southwestern China, approximately a seventh of the Chinese population, contributing to greater than 9520 billion CNY of GDPs. However, a well-designed large-scale epidemiological investigation of hyperuricemia in southwestern China is lacking, with one including 1458 subjects in Tibet Autonomous Region17, and the other including 1416 subjects in Ganzi Tibetan Autonomous Prefecture, Sichuan Province18. We aimed to conduct a large community-based cross-sectional study to assess the prevalence of hyperuricemia among urban adults aged 35–79 years in Chengdu and Chongqing to provide reliable and credible information for the development of a better prevention and control program for hyperuricemia in urban China.
Results
Participant characteristics
The demographic and clinical characteristics of the study participants are shown in Table 1. Of the 10,141 participants, 3447 were males and 6694 were females, and men had a higher mean age than women (P < 0.001). The majority (91.2%) of the study participants were married, but only 23.6% of the subjects had a high school education and above. Compared with women, men had higher education levels and higher personal monthly incomes (P < 0.001). Besides, men had a higher prevalence of drinking, smoking and regular exercise (P < 0.001). Men had higher WC, SBP, DBP, SUA (P < 0.001), while women had higher BMI, 2hPG, TC, HDL-C, LDL-C (P < 0.001). Moreover, women had higher TG than men (P = 0.006). There was no sex difference in FPG (P = 0.439).
Serum uric acid level and hyperuricemia
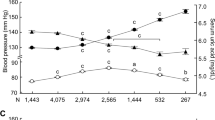
As shown in Fig. 1A, the mean SUA level in men was 350.2 μmol/L (SD = 80.6) for aged 35–44 years, 344.9 μmol/L (SD = 82.8) for aged 45–54 years, 328.6 μmol/L (SD = 70.5) for aged 55–64 years, 346.7 μmol/L (SD = 88.0) for aged 65–79 years, respectively. In women, the mean SUA level was 253.4 μmol/L (SD = 64.0) for aged 35–44 years, 255.1 μmol/L (SD = 62.1) for aged 45–54 years, 269.1 μmol/L (SD = 69.0) for aged 55–64 years, 280.2 μmol/L (SD = 74.6) for aged 65–79 years, respectively. There was statistical significance in the SUA level between the age bands among men and women (P < 0.001). In addition, the crude overall prevalence of hyperuricemia among adults aged 35–79 years was 12.5%. The age- and sex-standardized prevalence of hyperuricemia was 13.5%. Compared with women, the prevalence of hyperuricemia in men was higher (17.3% versus 10.0%, P < 0.001). The prevalence of hyperuricemia increased with advancing age in women (P < 0.001, Fig. 1B). However, there was no increasing trend of hyperuricemia prevalence between the age bands in men (P = 0.994).
Comorbidities of hyperuricemia
Among persons affected with hyperuricemia (Table 2), 55.3%, 27.4, 44.1%, 61.1%, 44.3% had hypertension, diabetes mellitus, hyperlipidemia, overweight/obesity, and central obesity, respectively. Findings stratified by sex indicated 56.0%, 21.7%, 41.8%, 58.7%, 36.6% of men with hyperuricemia had hypertension, diabetes mellitus, hyperlipidemia, overweight/obesity, and central obesity, respectively. 54.6%, 32.5%, 46.7%, 63.6%, 51.2% of women with hyperuricemia had hypertension, diabetes mellitus, hyperlipidemia, overweight/obesity, and central obesity, respectively.
Associated factors of hyperuricemia
In the univariable logistic regression model analysis (Table 3), age, marital status, comorbidities of hypertension, hyperlipidemia, kidney disease, overweight or obesity, central obesity, were associated with the risk of hyperuricemia. In women, age, marital status, education, smoking, comorbidities of hypertension, diabetes mellitus, hyperlipidemia, overweight or obesity, central obesity, menopause, were associated with the risk of hyperuricemia. The multivariable logistic regression model showed that hypertension, hyperlipidemia, overweight or obesity, central obesity were positively associated with hyperuricemia, and being married was negatively associated with hyperuricemia in men. Compared with aged 35–44 years, people aged 55–64 years had lower risks of hyperuricemia in men. In women, advanced age, current cigarette smoking, hypertension, diabetes mellitus, hyperlipidemia, overweight or obesity, central obesity, menopause, were positively associated with higher risks of hyperuricemia. However, being married had lower risks of hyperuricemia than being divorced, widowed, or single.
Discussion
This large-scale population-based study from southwestern China was designed to investigate hyperuricemia and associated risk factors in urban adults aged 35–79 years in Chengdu and Chongqing from September 2013 to March 2014. Overall, the age- and sex-standardized prevalence of hyperuricemia was 13.5%. We showed differences in hyperuricemia prevalence between men (17.3%) and women (10.0%). Associated risk factors for hyperuricemia in men included hypertension, hyperlipidemia, overweight or obesity, central obesity. Advanced age, current cigarette smoking, hypertension, diabetes mellitus, hyperlipidemia, overweight or obesity, central obesity, menopause were identified as associated risk factors for hyperuricemia in women. Furthermore, being married was an associated protective factor both in men and women, although to varying degrees.
In this study, our estimated prevalence of hyperuricemia among adults aged 35–79 years is higher than the national estimated prevalence of hyperuricemia. Data from the China National Survey of Chronic Kidney Disease in 2009–2010 showed the prevalence of hyperuricemia among Chinese adult aged 18 years and above was 8.4%14. A larger national investigation from the China Health and Retirement Longitudinal Study in 2011 indicated the prevalence of hyperuricemia among adults aged 45 years and above was 6.4%15. Differences in the prevalence of hyperuricemia are in part due to the different socioeconomic context, age composition of subjects enrolled. As previous studies shown, the prevalence of hyperuricemia among Chinese adults is higher in economically developed areas and urban areas14,15. Thus, it is not surprising that the result in this study is much higher than the estimated prevalence using national investigations included less-developed areas and rural areas14,15.
The serum uric acid concentrations and the prevalence of hyperuricemia were higher in men than in women, verified in different studies12,13,14,15. The potential biological mechanism underlying the differences between the sexes might be the uricosuric effects of estrogen in premenopausal women3,10,19. Following the menopause, serum uric acid concentrations will increase in women3,10. As indicated in this study and previous studies, menopause is an associated risk factor of hyperuricemia in women, independently of age and other covariates19,20. Furthermore, the age-associated increase in the prevalence of hyperuricemia in women might be partly explained by menopause, and other age-related factors need more evidence to be confirmed20. However, the effect of advanced age on hyperuricemia in men was not observed in this study, which is not consistent from other studies15,21,22,23. The lowest prevalence of hyperuricemia was observed in men aged 55–64 years, identical with a previous study24, which might be associated with the phase of retirement and more attention on health in Chinese men 24. More prospective cohort studies should be conducted to confirm the risk of hyperuricemia caused by advanced age in men.
Some epidemiological studies have shown that cigarette smoking was associated with lower levels of serum uric acid, as a result of oxidative stress induced by the long-term exposure to cigarette smoking25,26,27. In this study, current cigarette smoking was an associated risk factor of hyperuricemia in women rather than men, although to varying degrees, consistent with the result from the China Health and Retirement Longitudinal Study in 201115. The disparity might be elucidated by fewer pack-years in women15,28. Moreover, married men and women were not susceptible to hyperuricemia. The protective effect of marriage on risk of hyperuricemia may result from spousal interaction on health monitoring (e.g., health behavior, dietary pattern)29,30.
Similar to previous observational epidemiological studies, hypertension, hyperlipidemia, overweight or obesity, central obesity were associated with an increased risk for hyperuricemia14,15,22,23,31,32. Besides, diabetes mellitus was found to be associated with hyperuricemia only in women. In contrast, some studies have shown that higher serum uric acid was an associated risk factor for hypertension, diabetes mellitus, and obesity33,34,35,36,37. However, Mendelian randomization studies do not support the causality between serum uric acid and hypertension, diabetes mellitus38,39,40,41. Thus, further studies are still needed to confirm whether there is the bi-direction causality between hyperuricemia and cardiovascular diseases risk factors (e.g., hypertension, diabetes mellitus).
Because this study was cross sectional, we might suffer from many potential biases or reverse causation between associated risk factors and hyperuricemia. Some significant information was not available, such as dietary intake information and family history of hyperuricemia. Kidney disease was based on self-reported history, rather than estimated glomerular filtration rate, which might cause a false-negative result. About 24% of participants with missing values were excluded, which may cause potential selection bias. To minimize this bias, we computed the age- and sex-standardized prevalence using the 2010 census data of China. Finally, participants were enrolled from urban adults in Chengdu and Chongqing, which is likely to overestimate the prevalence of hyperuricemia among adults in southwestern China.
Conclusions
Hyperuricemia has become a major health problem among urban adults aged 35–79 years in southwestern China, and special attention should be paid to men. Comorbidities associated with hyperuricemia and causality worth further investigation.
Methods
Participants
The study protocol has been published, approved by the ethics committee of the Second People’s Hospital of Chengdu (NO 2013015), the methods in the study were in accordance with relevant guidelines, and a written informed consent was obtained from all participants42. Using three-stage (district-subdistrict-community) sampling procedures, five representative urban communities were randomly selected from five districts, including Jinjiang, Qingyang, Longquan district in Chengdu, and Yubei, Jiangbei district in Chongqing. The inclusion criteria for this study were residents aged 35–79 years who had lived in the community for more than five years. The exclusion criteria were people with histories of secondary hypertension, mental illness, malignant tumors, renal failure requiring dialysis, or who refused to participate in the inquiry. In total, 13,378 participants were enrolled using multistage, stratified sampling, from September 2013 to March 2014. Data collection was completed by more than 30 trained investigators. A structured questionnaire and anthropometric measurements were conducted, including demographic characteristics (i.e. sex, age, education, marital status), health-related lifestyles (i.e. current cigarette smoking, alcohol drinking, regular physical exercise), chronic disorders (i.e. hypertension, diabetes, dyslipidemia, hyperuricemia), blood biomarkers (i.e. serum uric acid, fasting plasma glucose). We excluded 3237 participants, who had missing demographic characteristics (i.e. income, marital status), anthropometric measurement parameters (i.e. blood pressure, height), blood biomarkers (i.e. serum uric acid, fast blood glucose). Hence, we included 10,141 participants in the study.
Diagnostic standards
According to Dietary guide for hyperuricemia and gout patients (Chinese standard, WS/T 560-2017), hyperuricemia was defined as serum uric acid > 420 μmol/L in men or serum uric acid > 360 μmol/L in women. The self-reported of the doctor's diagnosis of hyperuricemia was also diagnosed with hyperuricemia. Current cigarette smoking was defined as having smoked more than 100 cigarettes in one’s lifetime. Alcohol drinking was defined as consumption of more than 30 g of alcohol per week for more than 1 year. Regular physical exercise was defined as participation in more than 30 min of moderate or vigorous activity per day for more than 3 days per week43. Hypertension was defined as the self-reported history of hypertension or systolic blood pressure ≥ 140 mmHg and (or) diastolic blood pressure ≥ 90 mmHg. Diabetes was defined as the self-reported history of diabetes or fasting blood glucose ≥ 7.0 mmol/L and (or) OGTT 2-h post-load glucose ≥ 11.1 mmol/L. Based on the Criteria of weight for adults (Chinese standard, WS/T 428-2013), general obesity was determined by the BMI, categorized using the Chinese specific cutoff values as underweight (< 18.5), normal (18.5–23.9), overweight (24.0–27.9) and obesity (≥ 28.0). Central obesity was defined as WC ≥ 90 cm for men and ≥ 85 cm for women. Dyslipidemia was defined as total cholesterol ≥ 6.2 mmol/L, and/or LDL cholesterol ≥ 4.1 mmol/L, and/or HDL cholesterol < 1.0 mmol/L, and/or TG ≥ 2.3 mmol/L, and/or the self-reported history of dyslipidemia, based on the Chinese guidelines for the management of dyslipidemia in adults44. History of kidney disease was defined as the self-reported of the doctor's diagnosis of kidney disease. In women, menopause was defined as her menstrual periods had stopped at least 1 year.
Statistical analysis
Categorical data were presented as absolute numbers with the percentage, and Pearson’s Chi-Square test was used to detect the difference between the sexes. Except for triglyceride (TG), continuous data were presented as means with the standard deviation (SD), and Student’s t test was used to detect the difference between the sexes. Triglyceride was presented as medians with the interquartile range because of its skewed distribution, and the Wilcoxon rank sum test was used to detect the difference between the sexes. Besides, one-way analysis of variance (ANOVA) was used to detect the difference in serum uric acid level between the age bands, and the Cochran-Armitage test was used to test the trend in hyperuricemia prevalence between the age bands. A univariable logistic regression model and a multivariable logistic regression model were used to estimate the odds ratios and 95% confidence interval to explore the associated risk factors of hyperuricemia. For menopause in women, there were 210 missing values, so we created a dump variable for missing values. All statistical analyses were performed using Statistical Product and Service Solutions (SPSS, version 23.0).
References
Dalbeth, N., Merriman, T. R. & Stamp, L. K. Gout. Lancet (London, England) 388, 2039–2052. https://doi.org/10.1016/s0140-6736(16)00346-9 (2016).
Ichida, K. et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 3, 764. https://doi.org/10.1038/ncomms1756 (2012).
Johnson, R. J. et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am. J. Kidney Dis. 71, 851–865. https://doi.org/10.1053/j.ajkd.2017.12.009 (2018).
Oda, M., Satta, Y., Takenaka, O. & Takahata, N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol. Biol. Evol. 19, 640–653. https://doi.org/10.1093/oxfordjournals.molbev.a004123 (2002).
Kratzer, J. T. et al. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl. Acad. Sci. USA 111, 3763–3768. https://doi.org/10.1073/pnas.1320393111 (2014).
Keenan, T. et al. Causal assessment of serum urate levels in cardiometabolic diseases through a mendelian randomization study. J. Am. Coll. Cardiol. 67, 407–416. https://doi.org/10.1016/j.jacc.2015.10.086 (2016).
Kleber, M. E. et al. Uric acid and cardiovascular events: a mendelian randomization study. J. Am. Soc. Nephrol. JASN 26, 2831–2838. https://doi.org/10.1681/ASN.2014070660 (2015).
Simon, K. C. et al. Mendelian randomization of serum urate and parkinson disease progression. Ann. Neurol. 76, 862–868. https://doi.org/10.1002/ana.24281 (2014).
Kia, D. A. et al. Mendelian randomization study shows no causal relationship between circulating urate levels and Parkinson’s disease. Ann. Neurol. 84, 191–199. https://doi.org/10.1002/ana.25294 (2018).
Kuo, C. F., Grainge, M. J., Zhang, W. & Doherty, M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat. Rev. Rheumatol. 11, 649–662. https://doi.org/10.1038/nrrheum.2015.91 (2015).
Smith, E. & March, L. Global prevalence of hyperuricemia: a systematic review of population-based epidemiological studies. Arthritis Rheumatol. 67, 2690–2692 (2015).
Zhu, Y., Pandya, B. J. & Choi, H. K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 63, 3136–3141. https://doi.org/10.1002/art.30520 (2011).
Trifiro, G. et al. Epidemiology of gout and hyperuricaemia in Italy during the years 2005–2009: a nationwide population-based study. Ann. Rheum Dis. 72, 694–700. https://doi.org/10.1136/annrheumdis-2011-201254 (2013).
Liu, H., Zhang, X. M., Wang, Y. L. & Liu, B. C. Prevalence of hyperuricemia among Chinese adults: a national cross-sectional survey using multistage, stratified sampling. J. Nephrol. 27, 653–658. https://doi.org/10.1007/s40620-014-0082-z (2014).
Song, P. et al. Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci. Rep. 8, 4314. https://doi.org/10.1038/s41598-018-22570-9 (2018).
Zhou, M. et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. The Lancet 387, 251–272. https://doi.org/10.1016/s0140-6736(15)00551-6 (2016).
Zhang, Q. et al. The prevalence of gout and hyperuricemia in middle-aged and elderly people in Tibet Autonomous Region, China: A preliminary study. Medicine Baltimore 99, e18542. https://doi.org/10.1097/MD.0000000000018542 (2020).
Zhang, X. et al. The prevalence of hyperuricemia and its correlates in Ganzi Tibetan Autonomous Prefecture, Sichuan Province, China. Lipids Health Dis. 17, 235. https://doi.org/10.1186/s12944-018-0882-6 (2018).
Cho, S. K., Winkler, C. A., Lee, S. J., Chang, Y. & Ryu, S. The prevalence of hyperuricemia sharply increases from the late menopausal transition stage in middle-aged women. J. Clin. Med. https://doi.org/10.3390/jcm8030296 (2019).
Hak, A. E. & Choi, H. K. Menopause, postmenopausal hormone use and serum uric acid levels in US women—The Third National Health and Nutrition Examination Survey. Arthritis Res. Ther. 10, R116. https://doi.org/10.1186/ar2519 (2008).
Yu, S. et al. Prevalence of hyperuricemia and its correlates in rural Northeast Chinese population: from lifestyle risk factors to metabolic comorbidities. Clin. Rheumatol. 35, 1207–1215. https://doi.org/10.1007/s10067-015-3051-6 (2016).
Cui, L. et al. Prevalence and risk factors of hyperuricemia: results of the Kailuan cohort study. Mod.. Rheumatol. 27, 1066–1071. https://doi.org/10.1080/14397595.2017.1300117 (2017).
Dong, X. et al. Epidemiology and prevalence of hyperuricemia among men and women in Chinese rural population: The Henan Rural Cohort Study. Mod Rheumatol. https://doi.org/10.1080/14397595.2019.1660048 (2019).
Qiu, L. et al. Prevalence of hyperuricemia and its related risk factors in healthy adults from Northern and Northeastern Chinese provinces. BMC Public Health 13, 664. https://doi.org/10.1186/1471-2458-13-664 (2013).
Haj Mouhamed, D. et al. Effect of cigarette smoking on plasma uric acid concentrations. Environ. Health Prevent. Med. 16, 307–312. https://doi.org/10.1007/s12199-010-0198-2 (2011).
Wang, W. & Krishnan, E. Cigarette smoking is associated with a reduction in the risk of incident gout: results from the Framingham Heart Study original cohort. Rheumatology (Oxford, England) 54, 91–95. https://doi.org/10.1093/rheumatology/keu304 (2015).
Gee Teng, G., Pan, A., Yuan, J.-M. & Koh, W.-P. Cigarette Smoking and the Risk of Incident Gout in a Prospective Cohort Study. Arthritis Care Res. Hoboken 68, 1135–1142. https://doi.org/10.1002/acr.22821 (2016).
Fröhlich, M. et al. Independent association of various smoking characteristics with markers of systemic inflammation in men: Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95). Eur. Heart J. 24, 1365–1372. https://doi.org/10.1016/s0195-668x(03)00260-4 (2003).
Kiecolt-Glaser, J. K. & Wilson, S. J. Lovesick: how couples’ relationships influence health. Annu. Rev. Clin. Psychol. 13, 421–443. https://doi.org/10.1146/annurev-clinpsy-032816-045111 (2017).
Kendler, K. S., Lönn, S. L., Salvatore, J., Sundquist, J. & Sundquist, K. Effect of marriage on risk for onset of alcohol use disorder: a longitudinal and co-relative analysis in a swedish national sample. Am. J. Psychiatry 173, 911–918. https://doi.org/10.1176/appi.ajp.2016.15111373 (2016).
Zhang, Y. et al. Higher triglyceride level predicts hyperuricemia: A prospective study of 6-year follow-up. J. Clin. Lipidol. 12, 185–192. https://doi.org/10.1016/j.jacl.2017.10.009 (2018).
Ali, N. et al. Prevalence of hyperuricemia and the relationship between serum uric acid and obesity: A study on Bangladeshi adults. PLoS ONE 13, e0206850. https://doi.org/10.1371/journal.pone.0206850 (2018).
Feig, D. I., Madero, M., Jalal, D. I., Sanchez-Lozada, L. G. & Johnson, R. J. Uric acid and the origins of hypertension. J. Pediatr. 162, 896–902. https://doi.org/10.1016/j.jpeds.2012.12.078 (2013).
Johnson, R. J. et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 62, 3307–3315. https://doi.org/10.2337/db12-1814 (2013).
Grayson, P. C., Kim, S. Y., LaValley, M. & Choi, H. K. Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis Care Res. (Hoboken) 63, 102–110. https://doi.org/10.1002/acr.20344 (2011).
Miao, X.-P. et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS ONE 8, e56864. https://doi.org/10.1371/journal.pone.0056864 (2013).
Masuo, K., Kawaguchi, H., Mikami, H., Ogihara, T. & Tuck, M. L. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 42, 474–480. https://doi.org/10.1161/01.HYP.0000091371.53502.D3 (2003).
Yang, Q. et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ. Cardiovasc. Genet. 3, 523–530. https://doi.org/10.1161/CIRCGENETICS.109.934455 (2010).
Sluijs, I. et al. A mendelian randomization study of circulating uric acid and type 2 diabetes. Diabetes 64, 3028–3036. https://doi.org/10.2337/db14-0742 (2015).
Pfister, R. et al. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia 54, 2561–2569. https://doi.org/10.1007/s00125-011-2235-0 (2011).
Kottgen, A. et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 45, 145–154. https://doi.org/10.1038/ng.2500 (2013).
Huang, X. B. et al. Prevalence, awareness, treatment, and control of hypertension in southwestern China. Sci. Rep. 9, 19098. https://doi.org/10.1038/s41598-019-55438-7 (2019).
Yang, W. et al. Serum lipids and lipoproteins in Chinese men and women. Circulation 125, 2212–2221. https://doi.org/10.1161/CIRCULATIONAHA.111.065904 (2012).
Joint committee for guideline, r. ,. Chinese guidelines for the management of dyslipidemia in adults. J. Geriatric Cardiol. JGC 15(1–29), 2018. https://doi.org/10.11909/j.issn.1671-5411.2018.01.011 (2016).
Acknowledgements
We sincerely thank all the staff and participants for their immense contributions.
Author information
Authors and Affiliations
Contributions
X.B.H., W.Q.Z., and W.W.T. conceived and designed the study, analysed the data and drafted the manuscript. X.B.H., Y.L., Y.N., C.H., J.X.L., Y.J.Y., and R.H.X. participanted in the data collection and checking. R.H.X., and T.D.W. advised on the interpretation of results and were responsible for the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, XB., Zhang, WQ., Tang, WW. et al. Prevalence and associated factors of hyperuricemia among urban adults aged 35–79 years in southwestern China: a community-based cross-sectional study. Sci Rep 10, 15683 (2020). https://doi.org/10.1038/s41598-020-72780-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72780-3
This article is cited by
-
Dyslipidemia and hyperuricemia: a cross-sectional study of residents in Wuhu, China
BMC Endocrine Disorders (2024)
-
Prevalence and influencing factors of hyperuricemia in middle-aged and older adults in the Yao minority area of China: a cross-sectional study
Scientific Reports (2023)
-
The effect of lipid metabolism disorder on patients with hyperuricemia using Multi-Omics analysis
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.