Abstract
Growing evidence indicated that single nucleotide polymorphisms (SNPs) in the apolipoprotein E (APOE) gene are related to increase the risk of many inflammatory-related diseases. However, few genetic studies have associated the APOE gene polymorphism with sepsis. This study was to investigate the clinical relevance of the APOE gene polymorphism in the onset and progression of sepsis. A multicenter case–control association study with a large sample size (601 septic patients and 699 healthy individuals) was conducted. Clinical data showed that the APOEε4 allele was overrepresented among all patients with septic shock (p = 0.031) compared with sepsis subtype, suggesting that APOEε4 allele may associated with increased susceptibility to the progression of sepsis. Moreover, the APOE mRNA levels decreased after lipopolysaccharide (LPS) stimulation in cells in culture. Then 21 healthy individuals to extract PBMC for genotype grouping (APOE4+ group 8; APOE4− group 13) was selected to evaluate the effect on APOE level, and results showed that the expression level of APOE in APOE4+ group and APOE4− group did not differ in mRNA levels after an LPS challenge, but the protein levels in APOE4+ group decreased slower than that in APOE4− group, and this process was accompanied by the upregulation of proinflammatory cytokines. These results provide evidence that the APOEε4 allele might be associated with the development of sepsis and a potential risk factor that can be used in the prognosis of sepsis.
Similar content being viewed by others
Introduction
Sepsis due to infection is a complex disease that results in organ dysfunction, according to the latest definition for sepsis (Sepsis 3.0)1. Although progress in the development of antibiotics and other supportive care therapies, sepsis still causes at least one-third of hospital deaths2,3. The pathophysiological mechanisms that underlie sepsis are unclear, but growing evidence indicates that single nucleotide polymorphisms (SNPs) in genes play a significant role in the pathogenesis of sepsis and even contribute to sepsis susceptibility, progression, and prognosis4,5,6,7,8,9,10,11,12. Therefore, identifying genes that are associated with sepsis and evaluating their effects on gene expression and protein function can contribute to an increased understanding of the mechanism of sepsis occurrence and progression.
Apolipoprotein E (APOE) is a 34 kDa glycosylated protein, with relatively high amounts expressed in brain- and monocyte-derived macrophages13. In addition to its role in cholesterol transport and lipid metabolism, APOE has been shown exert immunomodulatory effects in vitro on both innate and acquired immune responses, as evidenced by its ability to suppress the proliferation of lymphocytes, the generation of cytolytic T-cells, and the stimulation of cultured neutrophils. These functions suggest that APOE has a potential role in various inflammatory-related diseases, including sepsis14,15,16,17,18. Increasing evidence has shown that APOE plays a critical role in the modulation of inflammatory processes by suppressing nuclear factor-κb-driven inflammation and atherosclerosis in monocytes and macrophages19. Recent studies have indicated that APOE knockout mice are highly susceptible to endotoxemia, Listeria monocytogenes and Klebsiella pneumoniae infection20,21. Rensen et al. showed that APOE redirect lipopolysaccharide (LPS) from Kupffer cells to hepatocytes and protect against endotoxemia in rats22. Other studies have demonstrated that the genomic deletion of APOE in mice resulted in an increased inflammatory reaction and high mortality rates following sepsis23,24,25,26. These lines of evidence suggest that APOE may have anti-infective and anti-inflammatory properties, which play a significant role in the pathogenesis of directly inflammatory-related diseases, such as sepsis.
The human APOE gene is located on chromosome 19q13.32 and exhibits polymorphism. There were three common APOE alleles, namely, ε2, ε3, and ε4, encoding the APOE2, APOE3, and APOE4 isoforms, respectively, resulting in six genotypes: APOE2/2, APOE2/3, APOE2/4, APOE3/3, APOE3/4, and APOE4/418,27,28,29,30. Numerous of studies have shown that APOE gene polymorphisms result in genetic predisposition to various inflammation-related disease, such as Alzheimer’s disease31, coronary heart disease32, and multiple sclerosis33. However, the clinical relationship between APOE polymorphism and the development of sepsis is not well known, and clinical observations have been unsystematic.
The present study used multicenter data to investigate the association between APOE gene polymorphism and sepsis. In total, 601 septic patients and 699 healthy subjects from three regions—northern, central, and southern China—were included in the study to evaluate the clinical relevance of the APOE polymorphism in the susceptibility and progression of sepsis and explore the relationship between polymorphism in this gene and sepsis.
Results
Clinical characteristics of patients and healthy controls
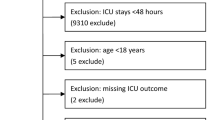
In total, 699 healthy controls and 601 septic patients from three areas (Harbin, Wuhan, and Zhanjiang city) in China were enrolled in the study. The demographic characteristics of the 601 studied patients are shown in Table 1. The mean ages of the sepsis subtype and septic shock patients were 62.4 ± 0.3 years and 61.6 ± 0.4 years, respectively. There were no significant differences in age or sex distributions between the sepsis subtype and septic shock patients. The primary source of blood infection was lung infection. The most common type of infection was Gram-negative bacterial infections, which accounted for 33.4% of sepsis subtype cases and 34.2% of septic shock cases. The most common pathogens identified was Acinetobacter baumannii. The mean ages of the 699 healthy controls was 62.3 ± 0.5 years, including 365 male and 334 female. No significant differences in age and gender were observed between all sepsis patients and controls (Fig. 1).
Flowchart of subject inclusion into clinical analysis cohort. Main procedures of clinical studies include sample collection, primary analysis and subgroup analysis. The participants included 282 sepsis patients from the Affiliated Hospital of Guangdong Medical College in southern China (Zhanjiang, China), 293 sepsis patients from the Center Hospital of Wuhan in central China (Wuhan, China) and 219 sepsis patients from Harbin Medical University in northern China (Harbin, China). Total healthy controls on admission is 713. In total, 601 sepsis patients and 699 healthy controls were included in the primary analysis.
The association between APOE gene polymorphism and sepsis susceptibility
The APOE genotype was successfully determined for all subjects. Genotypic distributions of APOE were consistent with Hardy–Weinberg equilibrium in the all sepsis patient and control groups (Table 2). The genotype distributions of APOE polymorphisms in the cases and controls of three groups are shown in Supplementary Table S1, the data shows that healthy volunteers and septic patients from three regions of Zhanjiang, Wuhan and Harbin have no statistically significant differences among the six genotypes of APOE. At the same time, we also analysis the genotype distributions of APOE polymorphisms in all patients and healthy controls in Table 3, the data shows that, there is also no differences among the six genotypes of APOE between patients and healthy controls. The patients were further separated into two subgroups according to genotype, regardless of clinical diagnosis: those possessing at least one APOEε4 allele (2/4, 3/4, or 4/4, named the APOE4+ group) and those lacking APOEε4 alleles (2/2, 2/3, or 3/3, named the APOE4− group)34. However, no significant differences in the APOE genotypes frequencies were observed between septic patients and healthy controls from Zhanjiang, Wuhan and Harbin in Supplementary Table S1 (all p > 0.05). However, Although not statistically significant, we observed that the frequency of carrying APOEε4 allele in all patients with sepsis got higher trend than that of all healthy controls (Table 3) (p = 0.077).
The association between APOE gene polymorphism and sepsis progression
We further divided the septic patients into two subgroups—of sepsis subtype and septic shock—based on the severity of sepsis, to assess the effect of APOE SNPs on the progression of sepsis. As presented in the Supplementary Table S2, there is no differences between the genotypes of APOE in the sepsis subtype group and septic shock group from three regions. Next, we analyzed whether the APOE genotype is different between the total septic patients and septic shock patients. As shown in Table 4, the proportions of E2/E2, E2/E3, E2/E4, E3/E4, and E4/E4 genotypes in all septic shock group were higher than those in the sepsis subtype group, with OR values of 1.314, 1.227, 3.722, 1.321 and 1.313 respectively, significantly higher frequency of E3/E3 genotype was observed in the sepsis subtype subgroup, APOE3/E3 genotype is protective compared to all other genotypes (OR = 0.65). Next, we divided all sepsis subtype subgroup and septic shock into two groups (APOE4+ and APOE4−) according to a specific allele. As presented in Table 4, in the APOE4+ group, more individuals had septic shock (p = 0.031, OR = 1.560) than sepsis subtype. We also divided all sepsis subtype subgroup and septic shock into APOE2+ and APOE2‒ or APOE3+ and APOE3‒ group in the Table 5, we found that in the APOE2+ group, there is a tendency to increase the development of sepsis (p = 0.066, OR = 1.490); In the APOE3+ group, lower individuals had septic shock (p = 0.028, OR = 0.368) than sepsis subtype. These findings suggest that carrying APOEε4 allele may have a role in promoting the progression of sepsis from sepsis subtype to septic shock.
The association between APOE gene polymorphism and 30-day mortality in patients with sepsis genotypes
The genotypic frequency distributions of the APOE in the two subgroups: APOE4+ and APOE4− groups or APOE2+ and APOE2‒ groups were stratified by 30-day mortality for further evaluation. Statistically significant difference was found between the 30-day surviving and non-surviving patients carrying the APOE4+ genotype (Supplementary Table S3, p = 0.041). Furthermore, Kaplan–Meier survival analysis showed that the 30-day survival of patients in the APOE4+ group (n = 117) was worse than that of patients in the APOE4− group (n = 484) (log-rank test 5.073, p = 0.024; Fig. 2A). Nevertheless, no difference was found between the 30-day surviving and non-surviving patients carrying the APOE2+ genotype (Supplementary Table S3, p = 0.053) and Kaplan–Meier survival analysis showed that no significant differences were observed in the APOE2+ and APOE2‒ group (log-rank test 1.512, p = 0.219; Fig. 2B).
Kaplan–Meier survival analysis in all sepsis patients. The effect of APOE4+ and APOE4− genotype on the 30-day survival of all 601 patients from the Affiliated Hospital of Guangdong Medical University, the Center Hospital of Wuhan, and Harbin Medical University was assessed using Kaplan–Meier survival analysis (A); The effect of APOE2+ and APOE2− genotype on the 30-day survival of all 601 patients was assessed using Kaplan–Meier survival analysis (B).
APOE is downregulated while expressions of inflammatory cytokines are elevated in sepsis patients and LPS-stimulated monocytes and macrophages in vitro
We randomly selected 90 septic patients and 28 healthy individuals to evaluate the plasma APOE level in vivo. The APOE mRNA and protein levels in septic patients were significantly lower than those of the healthy controls (Fig. 3A, B, p = 0.024 and p < 0.001, respectively), while the levels of TNF-α, IL-6, and IL-1β were elevated in sepsis patients (Fig. 3C). To assess the transcriptional and translational changes in APOE under the sepsis condition, we also measured APOE mRNA expression and protein production of PBMCS extracted from another 21 healthy controls under LPS stimulation. As shown in Fig. 3D–F, the mRNA expression and protein production of APOE were significantly decreased in LPS-stimulated PBMCs (p = 0.016 and p = 0.002, respectively), while the levels of TNF-α, IL-6, and IL-1β were elevated. Furthermore, RAW264.7 cells were challenged with LPS and the resulting mRNA and protein levels of APOE and expressions of inflammatory cytokines were detected. As shown by qPCR, the decrease in APOE after the LPS challenge occurred in a dose-dependent manner and reached a low point at 1000 ng/mL LPS. Thus, 1000 ng/mL was selected as the best LPS concentration for subsequent LPS challenge experiments using RAW264.7 cells (Fig. 3G). Next, we assessed the expression of the APOE protein in RAW264.7 cells after 1000 ng/mL LPS stimulation for 24 h. As shown in Fig. 3H, after LPS stimulation, the expression of the APOE protein decreased, while the levels of inflammatory factors TNF-α, IL-6, and IL-1β increased (p < 0.05) (Fig. 3I).
The APOE expressions and related inflammatory factors in sepsis patients and LPS-stimulated monocytes and macrophages in vitro. The APOE mRNA expression level, plasma concentration of APOE and related inflammatory factors in all sepsis patients (n = 90) and healthy controls (n = 28) (A–C); the APOE mRNA expression and the supernatant concentration of APOE in PBMCs from another 21 healthy individuals under 1000 ng/mL lipopolysaccharide (LPS) stimulation for 24 h (D,E); the related inflammatory factors of PBMCs under LPS (1000 ng/mL) stimulation for 6 h (F); dose responding viability of raw264.7 cells after 6, 12 and 24 h treatment with LPS (G);The effects of LPS on the expression of APOE in cultured raw264.7 cells for 24 h was detected by western blot analysis (H), because the strips were placed too close at the time, there was no long enough image, the original image is in the supplementary Figure S1; The related inflammatory factors of raw264.7 cells under LPS (1000 ng/mL) stimulation for 6 h (I); The error bar represents standard error of the mean. *p < 0.05; **p < 0.01; ***p < 0.001.
The effect of APOE gene polymorphisms on the expression of APOE and related proinflammatory cytokines in LPS-stimulated PBMCs
Next we measured the mRNA and protein levels of APOE with different genotypes under the sepsis condition. An LPS challenge experiment was performed using PBMCs from the blood samples of healthy people, and the results are shown in Fig. 4. There was no difference in APOE mRNA levels among PBMCs with different genotypes, regardless of whether or not cells were stimulated by LPS (Fig. 4A, p > 0.05). After LPS stimulation, the supernatants APOE protein level in the APOE4− group was lower than that in the control (Fig. 4B, p < 0.001), whereas the protein level in the APOE4+ group showed no difference compared with the control (Fig. 4B, p > 0.05), however, the supernatants protein level in the APOE4+ group was higher than that in the APOE4− group (Fig. 4B, p < 0.01). To confirm the effect of different APOE genotypes on proinflammatory cytokines, we also measured the expression levels of TNF-α, IL-6, and IL-1β in LPS-stimulated PBMCs. In the supernatants of LPS-stimulated PBMCs isolated from 21 healthy volunteers with different APOE genotypes, the results showed that after LPS stimulation, the TNF-α and IL-6 levels in the APOE4+ group were higher than those in the APOE4− group (Fig. 4C, p = 0.006 and p = 0.037, respectively), while the IL-1β level did not differ between the APOE4+ and APOE4− groups (Fig. 4C, p > 0.05). These results indicated that APOEε4 carriers may suffer a more excessive inflammatory response in sepsis.
The APOE expression and related inflammatory factors with different APOE genotypes in LPS-stimulated monocytes in cells in culture. The APOE mRNA expression and the supernatant concentration in PBMCs from another 21 healthy individuals under 1000 ng/mL lipopolysaccharide (LPS) stimulation for 24 h in APOE4+ and APOE4− group (A,B); the related inflammatory factors in PBMCs under LPS (1000 ng/mL) stimulation for 6 h in APOE4+ and APOE4− group (C); the error bar represents standard error of the mean. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Gene polymorphisms are critical for determining a predisposition to sepsis susceptibility and progression and could be used as potential therapeutic targets for the treatment of sepsis8,9,35,36,37,38,39,40. In vivo and in vitro studies have confirmed that APOE and APOE gene polymorphisms play a critical role in the modification of immune responses and inflammation, which, in turn, may contribute to the development of sepsis14,18,20. In this study, we investigated the association between sepsis and APOE in a multicenter case–control study. Our clinical data showed that patients in the APOE4+ group demonstrated a tendency toward an increased risk of sepsis, because the occurrence of sepsis is affected by many factors, whether the APOE gene polymorphism is related to the occurrence of sepsis needs to be further explored in a more uniform background and more samples, and a significant correlation was found between APOE4+ patients and increased sepsis mortality compared with APOE4−. Moreover, in the 30-day survival curve, significant differences in the APOE genotype were observed between surviving and non-surviving patients, with a worse outcome found for APOEε4 carriers compared with non-carriers. In addition, APOE mRNA and the plasma protein levels decreased under the sepsis condition. Further analysis revealed that the APOE protein level in the APOE4− group was lower than that in the APOE4+ group. This result is consistent with our clinical data, which indicate that the presence of the APOEε4 allele may be a susceptibility gene for sepsis and a potential prognostic indicator.
APOE is a polymorphic protein that is involved in the transformation and metabolism of lipoproteins and the regulation of systemic inflammation41. APOE gene polymorphism is associated with many diseases, especially neurodegenerative diseases such as Alzheimer’s disease42. Previous studies have shown that patients with APOE3 deficiency are more likely to present with hyperlipidemia43. APOE3 protects porcine proximal tubular cells from gentamicin-induced injury44 and have protective effect in Alzheimer's disease45, this is consistent with our research results, which show that the proportion of APOE E3/E3 genotype in sepsis subtype is significantly higher than that in the septic shock group, suggesting that the APOE E3/E3 genotype is protective. Studies have also demonstrated that targeted replacement mice expressing human APOE4 transgenes had increased plasma inflammatory factors, hepatic injury, and splenic lymphocyte apoptosis after a systemic lipopolysaccharide challenge46,47. The volunteers carrying the APOE4 allele had higher plasma TNF-α and IL-6 levels than those carrying the APOE3 allele after intravenous lipopolysaccharide administration48. These results suggest that the APOEε4 allele may act as a pro-inflammatory factor. As a disease closely related to inflammation, sepsis was studied in association with the polymorphism of APOE gene in the present study, and our results suggest that the APOEε4 allele contributed to aggravating the progression of sepsis from sepsis subtype to septic shock. Moreover, APOEε4 carriers had a lower 30-day survival rate, suggesting that the APOEε4 allele acted as a risk factor of mortality following sepsis.
Missense mutations at exons rs429358 and rs7412 underlie the three APOE allelic isoforms: ε2, ε3, and ε441. The molecular basis of APOE polymorphism is cysteine–arginine interchanges at residues 112 and 158: E2 (Cys112–Cys158), E3 (Cys112–Arg158), and E4 (Arg112–Arg158)49. Previous studies have shown that the substitutions at positions 112 and 158 affect salt bridge formation within the protein, which ultimately affects receptor-binding activity and lipoprotein ‘preference’ for the APOE protein49,50. The different transportability of the APOE3 and APOE4 proteins with arginine affects the ability of macrophages to produce nitric oxide34. These findings support the view that the effects of the APOE3 and APOE4 genetic polymorphisms on disease risk are likely attributable to functional differences in the translated proteins. In this study, we confirmed that APOE mRNA levels were decreased in the blood during sepsis, which is consistent with the results of our in vitro experiments using LPS-stimulated PBMCs and RAW264.7 cells. This result is supported by a previous study that reported a decrease in APOE mRNA in 279 pediatric patients with bacterial infections51. We found that the APOE protein level was lower in patients compared with the controls; this is inconsistent with previous studies that reported increased plasma APOE protein levels in pediatric patients with bacterial infections compared with healthy controls51. It has been reported that the administration of exogenous APOE decreased mortality rates by downregulating the inflammatory cascade in APOE-deficient animals23,46,52, suggesting that the APOE protein has anti-inflammatory properties which supported our results.
After further analysis of the association between APOE typing and its expression level, we observed that the protein levels in APOE4+ and APOE4− group, rather than mRNA levels, changed in the cells stimulated by LPS in vitro. This result suggests that the effect of APOE gene polymorphism on sepsis risk is mainly caused by a variation in function of the protein expressed by different APOE genotypes rather than the regulation of APOE at the transcription level. At same time we found that the APOE protein level under LPS stimulation in the APOE4+ group was higher compared with that of no LPS stimulation in the APOE4− group. Furthermore, the presence of the APOEε4 allele was associated with higher levels of the proinflammatory cytokines TNF-α and IL-6 in PBMCs stimulated by LPS. Considering that there are only a relatively small number of APOE4+ individuals in this part of the in vitro experiments, the results may be biased and large samples and multiple centers will be required for verification in the future.
Our results support that APOE gene polymorphism contributed to the prognosis and development of sepsis. Previous evidences suggest that the APOE intervention could relieve the excessive systemic immune response6. In this study we observed that the APOE protein seems to be consumed in the development of sepsis, the decreased mRNA level of APOE may lead to the decrease of protein level in pathological process of sepsis. Interestingly, we observed that APOE4 protein level seems to be consumed more slowly than APOE3 in serum of patients, associated with higher level of inflammatory cytokines and poor prognosis in clinical observation, this may be caused by the different function of APOE 3 and 4 protein. Meanwhile the mechanism on effect of APOE protein in sepsis is still unclear. It has been reported that Human APOE4 could modulate the expression of Sirtuin 1 in brain regions of targeted replacement apoE mice53. In addition, a small molecule APOE4− targeted therapeutic candidate that normalizes sirtuin 1 levels and improves cognition in an Alzheimer’s disease mouse model54, suggesting the potential APOE4 and Sirt1 pathways. It is worth nothing that Sirt1 was recently reported that playing critical role to preventing autoimmunity and participate in sepsis progress55,56. Sirt 1 is a nicotinamide adenine dinucleotide (NAD+) dependent class III histone deacetylase (HDAC) that targets nuclear factor kappa B (NF-kB), a critical transcription factor in the regulation of proinflammatory cytokine production to adapt gene expression to metabolic activity57,58,59. Moreover, SIRT1 could regulated immunometabolic polarity during the hyper-inflammatory and hypoinflammatory phases of sepsis60. The Sirt1 activator could also attenuate multiorgan injury in septic mice model, decrease the production of proinflammatory cytokines and reduce inflammasome activation, suggesting that Sirt1 may play an important role in sepsis through the NF-kb pathway61. So although the potential mechanism of APOE in sepsis is still unclear, these above evidence suggest that APOE4, Sirt1, and NF-kb pathways may be one of the critical pathways for the sepsis progress. Further study on the function of APOE4 may gradually clarify its molecular mechanism in sepsis.
Conclusion
In the present study, we demonstrated that APOE polymorphism was associated with the progression of sepsis in a Chinese Han population. Individuals carrying the APOEε4 allele exhibited an association with the progression of sepsis. A higher APOE4 protein level may contribute to the risk of transitioning from sepsis subtype to septic shock. Our future studies will focus on the mechanisms of APOE4 in the pathogenesis of sepsis and whether the APOEε4 allele can be used as an early warning signals of genetics or the APOE protein could be as a therapy target for the treatment of sepsis in clinical practice.
Methods
Subject enrollment
All patients were from the general intensive care unit (ICU) of the Affiliated Hospital of Guangdong Medical University, the Center Hospital of Wuhan, and Harbin Medical University between August 2016 and December 2018. All of them were ethnic Han Chinese from different families and not blood-related. Patients were screened strictly according to The Third International Consensus Definitions for Sepsis and Septic Shock (2016), sepsis subtype was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, septic shock was defined as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone1, and the final sample included 601 sepsis patients (mean age: 62.1 ± 0.4 years; 47.3% female) and 699 healthy controls (mean age: 62.3 ± 0.5 years; 47.8% female) from three region of China, respectively. The DNA of all subjects was obtained. Patients were not eligible if they were younger than 18 years old, diagnosed with HIV, known to be immunodeficient, taking steroids, receiving radiation therapy, pregnant, or lactating. Those failing to meet the definition and diagnostic criteria in Sepsis 3.0 were also excluded from the patient cohort. The participants in the healthy control group were excluded if they were less than 18 years old or suffered from chronic illness or any recent acute illness (Fig. 1)10. Once a diagnosis of sepsis was confirmed, peripheral blood samples were collected within 12 h. The baseline demographic factors and clinical information were recorded for each patient62.
DNA extraction and genotyping
The TIANamp Blood DNA Kit (TianGen Biotech, Beijing, China) was used according to the manufacturer’s instructions to isolate the genomic DNA from whole blood samples. We used PCR-based restriction fragment length polymorphism (RFLP) analysis to identify the APOE genotype. Finally, we obtained E2/E2 (168 and 50 bp), E3/E3 (145, 50, and 23 bp), and E4/E4 fragments (195 and 23 bp). Different combinations defined the heterozygous genotypes63,64.
Mononuclear cell isolation, plasma collection, and LPS stimulation
Sterile, preservative-free heparin (10 U/mL) was used in the collection of venous blood. Peripheral blood mononuclear cells (PBMCs) were recovered from the interface of a Lymphoprep density gradient, washed twice in RPMI-1640, and resuspended at a concentration of 1 × 106 cells/mL. In vitro, PBMCs from 21 healthy volunteers, who were chosen from the healthy control group at random, were used for LPS stimulation experiments. As soon as possible, plasma was extracted from the blood samples by centrifugation at 3000×g for 10 min and stored at − 80 °C until they were used for cytokine measurements. The supernatants of cells stimulated by 1000 ng/mL LPS for 6 h were collected for the measurement of TNF-α, IL-6, and IL-1β. The supernatants and cells stimulated by 1000 ng/mL LPS for 24 h were used to detect APOE mRNA and protein levels. The control cells were treated with PBS instead of LPS.
Cell culture
The mouse macrophage cell line RAW264.7 was obtained from the Shanghai Cell Bank. Cells were cultured in RPMI-1640 Medium (HyClone, Logan, Utah, USA) in 10% FBS in a humidified incubator containing 5% CO2 at 37 °C. The culture medium for PBMCs consisted of RPMI-1640 Medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% human serum, 20 mM HEPES (pH 7.3), 2 mM l-glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin. LPS (Escherichia coli, 055:B5, Sigma L-2880, Saint Louis, MO, USA) was reconstituted in PBS.
RNA extraction, reverse transcription, and real-time PCR
RNA was extracted from PBMCs of the 21 healthy controls for in vitro LPS stimulation experiments by using the UNIQ-10 Column Trizol Total RNA Extraction Kit (Sangon Biotech, Shanghai, China). The RNA was reverse-transcribed using the First Strand cDNA Synthesis Kit (Thermo) following the manufacturer’s instructions. The integrity of the RNA was verified using 1% agarose gel electrophoresis. The expression levels of APOE were analyzed by quantitative real-time PCR with the SYBR Green method. The APOE primer sequences used in this assay were as follows: human 5′-GTTGCTGGTCACATTCCTGG-3′ (forward) and 5′-GCAGGTAATCCCAAAAGCGAC-3′ (reverse); mouse 5′-CTCCCAAGTCACACAAGAACTG-3′ (forward) and 5′-CCAGCTCCTTTTTGTAAGCCTTT-3′ (reverse). The GAPDH primer sequences used were as follows: human 5′-TGTGGGCATCAATGGAT-TTGG-3′ (forward) and 5′-ACACCATGTATTCCGGGTCAAT-3′ (reverse); mouse 5′-AAGAGGGATGCTGCCCTTAC-3′ (forward) and 5′-TACGGCCAAATCCGTTCACA-3′ (reverse). The relative level of each transcript was normalized to GAPDH. The relative expression levels of APOE mRNA from all participants were determined using the 2−△△Ct method.
Cytokine measurements
The concentrations of APOE, TNF-α, IL-6, and IL-1β in the supernatants of serum and the isolated PBMCs were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions (Beyotime Biotechnology, Shanghai, China).
Western blot analysis
Cells were harvested at the specified times and lysed with RIPA buffer (Beyotime, Shanghai, China) containing 2% PICT and 1% PMSF. The BCA protein assay kit (KeyGen Biotechnologies, Nanjing, China) was used to determine protein concentration. Then, 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was used to separate the proteins. The membrane was blocked with 5% nonfat milk for 60 min at 37 °C and then incubated overnight with specific primary antibodies (anti-APOE, mouse monoclonal antibody, 1:1000 diluted, ab1906, Abcam, Cambridge, UK; anti-β-actin, 1:1000 diluted, Santa Cruz Biotechnology) at 4 °C, followed by horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rat IgG secondary antibodies. The bands were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and normalized to β-actin.
Statistical analyses
Statistical analysis was conducted using GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA) and SPSS version 19.0 (IBM, NY, USA). The association between APOE polymorphisms and sepsis was analyzed using the chi-squared test. The deviation of the allele or genotype frequency was analyzed using Hardy–Weinberg equilibrium (HWE). Student’s t-test and the Mann–Whitney U-test were used for normally distributed and non-parametric data, respectively. ANOVA was performed for all other calculations. The Kaplan–Meier method was used to plot 30-day survival curves for the different APOE genotypes, and the curves were compared using the log-rank test. Our data are expressed as the mean ± standard error of the mean (SEM) or as percentage frequencies. Statistical significance was defined as a p value < 0.05.
Ethic declaration
This study was approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University (Zhanjiang, China) and conducted according to the standards of the Declaration of Helsinki and written informed consents were obtained from all of the participants or their surrogates.
Data availability
The dataset used and analysed during the current study are available from the corresponding author on reasonable request.
References
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810. https://doi.org/10.1001/jama.2016.0287 (2016).
Hotchkiss, R. S. & Sherwood, E. R. Immunology. Getting sepsis therapy right. Science 347, 1201–1202. https://doi.org/10.1126/science.aaa8334 (2015).
Liu, V. et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 312, 90–92. https://doi.org/10.1001/jama.2014.5804 (2014).
Atalan, N. et al. Analysis of Toll-like receptor 9 gene polymorphisms in sepsis. Vivo 30, 639–643 (2016).
Azab, S. F. et al. Interleukin-10-1082 G/A gene polymorphisms in Egyptian children with CAP: A case–control study. Medicine 95, e4013. https://doi.org/10.1097/MD.0000000000004013 (2016).
Angus, D. C. et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 (2001).
Engel, C. et al. Epidemiology of sepsis in Germany: Results from a national prospective multicenter study. Intensive Care Med 33, 606–618. https://doi.org/10.1007/s00134-006-0517-7 (2007).
He, J. et al. The interleukin-27-964A>G polymorphism enhances sepsis-induced inflammatory responses and confers susceptibility to the development of sepsis. Crit. Care 22, 248. https://doi.org/10.1186/s13054-018-2180-0 (2018).
Shao, Y. et al. Association between genetic polymorphisms in the autophagy-related 5 gene promoter and the risk of sepsis. Sci. Rep. 7, 9399. https://doi.org/10.1038/s41598-017-09978-5 (2017).
Cui, L. et al. An ADAM10 promoter polymorphism is a functional variant in severe sepsis patients and confers susceptibility to the development of sepsis. Crit. Care 19, 73. https://doi.org/10.1186/s13054-015-0796-x (2015).
Arcaroli, J., Fessler, M. B. & Abraham, E. Genetic polymorphisms and sepsis. Shock 24, 300–312 (2005).
Holmes, C. L., Russell, J. A. & Walley, K. R. Genetic polymorphisms in sepsis and septic shock: Role in prognosis and potential for therapy. Chest 124, 1103–1115. https://doi.org/10.1378/chest.124.3.1103 (2003).
Basu, S. K. et al. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J. Biol. Chem. 257, 9788–9795 (1982).
Avila, E. M., Holdsworth, G., Sasaki, N., Jackson, R. L. & Harmony, J. A. Apoprotein E suppresses phytohemagglutinin-activated phospholipid turnover in peripheral blood mononuclear cells. J. Biol. Chem. 257, 5900–5909 (1982).
Terkeltaub, R. A., Dyer, C. A., Martin, J. & Curtiss, L. K. Apolipoprotein (apo) E inhibits the capacity of monosodium urate crystals to stimulate neutrophils. Characterization of intraarticular apo E and demonstration of apo E binding to urate crystals in vivo. J. Clin. Invest. 87, 20–26, https://doi.org/10.1172/JCI114971 (1991).
Edgington, T. S. & Curtiss, L. K. Plasma lipoproteins with bioregulatory properties including the capacity to regulate lymphocyte function and the immune response. Cancer Res. 41, 3786–3788 (1981).
Laskowitz, D. T., Lee, D. M., Schmechel, D. & Staats, H. F. Altered immune responses in apolipoprotein E-deficient mice. J. Lipid Res. 41, 613–620 (2000).
Zhang, H., Wu, L. M. & Wu, J. Cross-talk between apolipoprotein E and cytokines. Mediators Inflamm. 2011, 949072. https://doi.org/10.1155/2011/949072 (2011).
Li, K., Ching, D., Luk, F. S. & Raffai, R. L. Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-kappaB-driven inflammation and atherosclerosis. Circ. Res. 117, e1–e11. https://doi.org/10.1161/CIRCRESAHA.117.305844 (2015).
20de Bont, N. et al. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J. Lipid. Res. 40, 680–685 (1999).
Roselaar, S. E. & Daugherty, A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J. Lipid. Res. 39, 1740–1743 (1998).
Rensen, P. C. et al. Human recombinant apolipoprotein E redirects lipopolysaccharide from Kupffer cells to liver parenchymal cells in rats In vivo. J. Clin. Invest. 99, 2438–2445. https://doi.org/10.1172/JCI119427 (1997).
Chuang, K., Elford, E. L., Tseng, J., Leung, B. & Harris, H. W. An expanding role for apolipoprotein E in sepsis and inflammation. Am. J. Surg. 200, 391–397. https://doi.org/10.1016/j.amjsurg.2009.10.017 (2010).
Kattan, O. M., Kasravi, F. B., Elford, E. L., Schell, M. T. & Harris, H. W. Apolipoprotein E-mediated immune regulation in sepsis. J. Immunol. 181, 1399–1408. https://doi.org/10.4049/jimmunol.181.2.1399 (2008).
Haraguchi, G. et al. Pioglitazone reduces systematic inflammation and improves mortality in apolipoprotein E knockout mice with sepsis. Intensive Care Med. 34, 1304–1312. https://doi.org/10.1007/s00134-008-1024-9 (2008).
Weber, C. & Soehnlein, O. ApoE controls the interface linking lipids and inflammation in atherosclerosis. J. Clin. Investig. 121, 3825–3827. https://doi.org/10.1172/JCI60457 (2011).
Paik, Y. K. et al. Nucleotide sequence and structure of the human apolipoprotein E gene. Proc. Natl. Acad. Sci. USA 82, 3445–3449. https://doi.org/10.1073/pnas.82.10.3445 (1985).
Palombo, V. et al. Genome-wide association study of milk fatty acid composition in Italian Simmental and Italian Holstein cows using single nucleotide polymorphism arrays. J. Dairy Sci. 101, 11004–11019. https://doi.org/10.3168/jds.2018-14413 (2018).
Breitner, J. C., Jarvik, G. P., Plassman, B. L., Saunders, A. M. & Welsh, K. A. Risk of Alzheimer disease with the epsilon4 allele for apolipoprotein E in a population-based study of men aged 62–73 years. Alzheimer Dis. Assoc. Disord. 12, 40–44 (1998).
Saunders, A. M. Apolipoprotein E and Alzheimer disease: An update on genetic and functional analyses. J. Neuropathol. Exp. Neurol. 59, 751–758. https://doi.org/10.1093/jnen/59.9.751 (2000).
Raulin, A. C. et al. The molecular basis for apolipoprotein E4 as the major risk factor for late-onset Alzheimer’s disease. J. Mol. Biol. 431, 2248–2265. https://doi.org/10.1016/j.jmb.2019.04.019 (2019).
Stengard, J. H. et al. Apolipoprotein E polymorphism predicts death from coronary heart disease in a longitudinal study of elderly Finnish men. Circulation 91, 265–269. https://doi.org/10.1161/01.cir.91.2.265 (1995).
Schmidt, S. et al. Association of polymorphisms in the apolipoprotein E region with susceptibility to and progression of multiple sclerosis. Am. J. Hum. Genet. 70, 708–717. https://doi.org/10.1086/339269 (2002).
Colton, C. A. et al. APOE and the regulation of microglial nitric oxide production: A link between genetic risk and oxidative stress. Neurobiol. Aging 23, 777–785 (2002).
Bermudez-Mejia, C. et al. Prognostic value of MMP-9-1562 C/T gene polymorphism in patients with sepsis. Biomark Insights 14, 1177271919847951. https://doi.org/10.1177/1177271919847951 (2019).
Fatani, S. H. et al. Assessment of tumor necrosis factor alpha polymorphism TNF-alpha-238 (rs 361525) as a risk factor for development of acute kidney injury in critically ill patients. Mol. Biol. Rep. 45, 839–847. https://doi.org/10.1007/s11033-018-4230-8 (2018).
Mao, Z. R., Zhang, S. L. & Feng, B. Association of IL-10 (-819T/C, -592A/C and -1082A/G) and IL-6-174G/C gene polymorphism and the risk of pneumonia-induced sepsis. Biomarkers 22, 106–112. https://doi.org/10.1080/1354750X.2016.1210677 (2017).
He, J. et al. Association study of MCP-1 promoter polymorphisms with the susceptibility and progression of sepsis. PLoS ONE 12, e0176781. https://doi.org/10.1371/journal.pone.0176781 (2017).
Wang, L. et al. Urinary liver-type fatty acid-binding protein predicts recovery from acute kidney injury. Clin. Nephrol. 84, 255–261. https://doi.org/10.5414/CN108635 (2015).
Shao, Y. et al. Association study between promoter polymorphisms of ADAM17 and progression of sepsis. Cell Physiol. Biochem. 39, 1247–1261. https://doi.org/10.1159/000447830 (2016).
Mahley, R. W. & Rall, S. C. Jr. Apolipoprotein E: Far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1, 507–537. https://doi.org/10.1146/annurev.genom.1.1.507 (2000).
Huang, Y. A., Zhou, B., Nabet, A. M., Wernig, M. & Sudhof, T. C. Differential signaling mediated by ApoE2, ApoE3, and ApoE4 in human neurons parallels Alzheimer’s disease risk. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.2994-18.2019 (2019).
Zhang, H. et al. Patients with apoE3 deficiency (E2/2, E3/2, and E4/2) who manifest with hyperlipidemia have increased frequency of an Asn 291–>Ser mutation in the human LPL gene. Arterioscler Thromb. Vasc. Biol. 15, 1695–1703. https://doi.org/10.1161/01.atv.15.10.1695 (1995).
Takamoto, K., Kawada, M., Ikeda, D. & Yoshida, M. Apolipoprotein E3 (apoE3) safeguards pig proximal tubular LLC-PK1 cells against reduction in SGLT1 activity induced by gentamicin C. Biochim. Biophys. Acta 1722, 247–253. https://doi.org/10.1016/j.bbagen.2004.12.006 (2005).
de-Almada, B. V. et al. Protective effect of the APOE-e3 allele in Alzheimer's disease. Braz. J. Med. Biol. Res. 45, 8–12, https://doi.org/10.1590/s0100-879x2011007500151 (2012).
Lynch, J. R. et al. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J. Biol. Chem. 278, 48529–48533. https://doi.org/10.1074/jbc.M306923200 (2003).
Sullivan, P. M. et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 272, 17972–17980. https://doi.org/10.1074/jbc.272.29.17972 (1997).
Gale, S. C. et al. APOepsilon4 is associated with enhanced in vivo innate immune responses in human subjects. J. Allergy Clin. Immunol. 134, 127–134. https://doi.org/10.1016/j.jaci.2014.01.032 (2014).
Minihane, A. M., Jofre-Monseny, L., Olano-Martin, E. & Rimbach, G. ApoE genotype, cardiovascular risk and responsiveness to dietary fat manipulation. Proc. Nutr. Soc. 66, 183–197. https://doi.org/10.1017/S0029665107005435 (2007).
Zlokovic, B. V. Cerebrovascular effects of apolipoprotein E: Implications for Alzheimer disease. JAMA Neurol. 70, 440–444. https://doi.org/10.1001/jamaneurol.2013.2152 (2013).
Fu, P. et al. Elevated serum ApoE levels are associated with bacterial infections in pediatric patients. J. Microbiol. Immunol. Infect. 47, 122–129. https://doi.org/10.1016/j.jmii.2013.05.010 (2014).
Van Oosten, M. et al. Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. A new therapeutic approach to treat gram-negative sepsis. J. Biol. Chem. 276, 8820–8824, https://doi.org/10.1074/jbc.M009915200 (2001).
Lattanzio, F. et al. Human apolipoprotein E4 modulates the expression of Pin1, Sirtuin 1, and Presenilin 1 in brain regions of targeted replacement apoE mice. Neuroscience 256, 360–369. https://doi.org/10.1016/j.neuroscience.2013.10.017 (2014).
Campagna, J. et al. A small molecule APOE4− targeted therapeutic candidate that normalizes sirtuin 1 levels and improves cognition in an Alzheimer’s disease mouse model. Sci. Rep. 8, 17574. https://doi.org/10.1038/s41598-018-35687-8 (2018).
Ian J M. Genomic medicine and endocrine autoimmunity as key to mitochondrial disease. Glob. J. Endocrinol. Metab. 2, 1–3. https://doi.org/10.31031/GJEM.2018.02.000534(2018).
Martins, I. The future of genomic medicine involves the maintenance of sirtuin 1 in global populations. Mol. Biol. 2, 1–4. https://doi.org/10.15406/ijmboa.2017.02.00013 (2017).
Yeung, F. et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380. https://doi.org/10.1038/sj.emboj.7600244 (2004).
Xie, J., Zhang, X. & Zhang, L. Negative regulation of inflammation by SIRT1. Pharmacol. Res. 67, 60–67. https://doi.org/10.1016/j.phrs.2012.10.010 (2013).
Ishikawa, S. et al. Sirtuin 1 suppresses nuclear factor kappaB induced transactivation and pro-inflammatory cytokine expression in cat fibroblast cells. J. Vet. Med. Sci. 77, 1681–1684. https://doi.org/10.1292/jvms.15-0245 (2016).
Wang, X. et al. Sirtuins and immuno-metabolism of sepsis. Int. J. Mol. Sci. 19, https://doi.org/10.3390/ijms19092738 (2018).
Khader, A. et al. SRT1720, a sirtuin 1 activator, attenuates organ injury and inflammation in sepsis. J. Surg. Res. 219, 288–295. https://doi.org/10.1016/j.jss.2017.06.031 (2017).
Knaus, W. A., Draper, E. A., Wagner, D. P. & Zimmerman, J. E. APACHE II: A severity of disease classification system. Crit. Care Med. 13, 818–829 (1985).
Afroze, D., Yousuf, A., Tramboo, N. A., Shah, Z. A. & Ahmad, A. ApoE gene polymorphism and its relationship with coronary artery disease in ethnic Kashmiri population. Clin. Exp. Med. 16, 551–556. https://doi.org/10.1007/s10238-015-0389-7 (2016).
Hixson, J. E. & Vernier, D. T. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid. Res. 31, 545–548 (1990).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81772048 and No. 81671181); Guangdong Natural Science Foundation (No.2019A1515010933), and the special competitive assignment fiscal funds of Zhanjiang City (No. 2016A01026).The authors thank the staff at the Intensive Care Units of the Affiliated Hospital of Guangdong Medical University, the Central Hospital of Wuhan and the Second Affiliated Hospital of Harbin Medical University, and all of those who were involved in this study.
Author information
Authors and Affiliations
Contributions
L.C. conceived and designed the experiments, and participated in its design and coordination and helped to revise the manuscript. Y.S. performed the statistical analysis and drafted the manuscript. T.Z., W.Z. and J.H. participated in its design and helped to draft and revise the manuscript. F.L. and Y.C. participated in the experiments. Z.L., N.W. and C.L. collected the samples. L.L., Y.H., X.C. and J.L. performed the statistical analysis. P.T., W.F., M.O., J.Y. and Y.L. collected the samples and clinical data and helped to perform the statistical analysis. All authors reviewed the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shao, Y., Zhao, T., Zhang, W. et al. Presence of the apolipoprotein E-ε4 allele is associated with an increased risk of sepsis progression. Sci Rep 10, 15735 (2020). https://doi.org/10.1038/s41598-020-72616-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72616-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.