Abstract
The Indian black clam Villorita cyprinoides (Family: Cyrenidae), an extractive commercially exploited species with aquaculture importance contributing more than 70% of clam fishery in India, is endemic to the Indian peninsula. Currently, there is very sparse information, especially on the molecular data of Villorita. The present study aims to provide a comprehensive knowledge of mitogenome architecture and assess the phylogenetic status of Cyrenidae. This has resulted in reporting the first complete mitogenome of V. cyprinoides using next-generation sequencing technology. The A+T circular mitogenome was 15,880 bp long, exhibiting 13 protein-coding genes (PCGs) including ATP8 (absent in several bivalves), 22 transfer RNA, and two ribosomal RNA genes residing in the heavy strand in a clockwise orientation and a gene order akin to Corbicula fluminea. The molecular phylogeny inferred from a concatenated multi-gene sequence [14 mitochondrial (12 PCGs, rrnS and rrnL) and two nuclear genes (Histone H3, 18S rRNA)] from 47 representative species of superorder Imparidentia, clustered V. cyprinoides and Cyrenid clams to a single clade supporting the monophyly of Cyrenidae. The subsequent mitochondrial gene order analysis substantiates the close relationship of V. cyprinoides and C. fluminea, analogous to phylogenetic output. The multilocus tree topology calibrated with verified fossil data deciphered the origin and diversification of Cyrenid clams during late Triassic-early Jurassic. The data derived from this study shall contribute remarkably for further insights on cryptic species identification, molecular characterization of bivalve mitogenomes and mitochondrial evolutionary history of genus Villorita. Moreover, complete mitogenome can aid in potential marker development for assessing the genetic health of black clam populations.
Similar content being viewed by others
Introduction
In 2018, global molluscan aquaculture production was 17.7 million tons1, elucidating its astounding potential as a food resource of international importance. The practice of inclusion of bivalves in the diet has had an exquisite role in human ethos for ages2, 3. The dependence on bivalves for food, industries including jewellery, medical, and ecosystem services challenged the scientific community to enrich the information on various aspects including phylogeny, evolutionary history, genetic improvement and breeding of superior strains for enhancing aquaculture production4,5,6.
Mitochondrial DNA (mt-DNA), the lone double-stranded circular extranuclear genome in eukaryotes prevailed by endosymbiosis, is of crucial importance because of its ATP synthesis in the cell through oxidative phosphorylation7, 8. Besides energy production, small size, high copy number, conserved genes, high rate of evolution, low recombination and maternal inheritance enables mitochondria as a potential marker in molecular studies8, 9. Bivalve mitogenome as metazoans typically consists of 13 protein-coding genes for OXPHOS, 2 rRNA, 22 tRNAs and an A+T rich region controlling replication and transcription10. However, peculiar mitochondrial features like extensive genome reorganisation and occurrence of Doubly Uniparental Inheritance (DUI) in certain bivalves extended supplementary information on evolutionary patterns across species and among conspecifics11,12,13. The popularity of mitogenome as a marker of choice for phylogenetic studies is on arise due to a surge in published mitogenomic data which provides improved phylogenetic resolution14. Nevertheless, molecular studies employing combined mitochondrial and nuclear markers played a pivotal role in imparting accurate and refined knowledge on bivalve molecular systematics, phylogenetics, population health analysis and comparative phylogenomics15, 16.
Villorita cyprinoides (Gray,1825) (Indian black clam), a bivalve belonging to family Cyrenidae inhabiting on or even below the surface of bottom sediment in the brackish water ecosystem, is endemic to the peninsular India17. Cyrenidae constitutes a diverse range of species with differing attributes (e.g. economically important along with invasive taxa) is known for retention of brackish water genera and parallel radiation of species to freshwater and brackish water environments18. Even though the brackish water biome is the natural habitat of V. cyprinoides, wide salinity tolerance of this resilient species enables its range expansion to the freshwater environment19, as exemplified in Vembanad Lake (Kerala, India) seasonally divided by the Thanneermukkom Barrage (Fig. 1a). The genus Villorita embracing V. cyprinoides is traditionally considered to be monotypic20 and acclaimed as prime species in Indian clam fisheries, prominently cherished for both meat and shell21. Despite the tedious collection and processing exercise for the marketing of black clam (Fig. 1b–g), the species always maintains a supreme demand in the market due to its nutritive value22. The use of the species for local consumption, export and protein supplement in shrimp and poultry feed, makes the natives depend mainly on the clam for their sustenance and livelihood23. The calcium-rich shell, a by-product in clam fishery is a potential raw material for the manufacture of lime and cement21 (Fig. 1h). The recent study which revealed the presence of antioxidative and anti-inflammatory metabolites from V. cyprinoides24 opens up new avenues of research in nutraceuticals. Apart from the top-notch economic significance, bioremediation, biocontrol of phytoplankton as well as biomonitoring of estuarine heavy metal pollution is feasible through Villorita25, 26.
(a) The Thanneermukkom Barrage dividing the Vembanad Lake to Northern and Southern regions. (b) Clam collection by diving and handpicking. (c) Collection using kolli, an indigenous hand rake net. (d) Transportation of collected clams to shore. (e) Meat shucking by cooking the clam. (f) Separation of clam meat by sieving. (g) Marketing of clam meat. (h) Shells of black clam burn in a specially built kiln for manufacture of cement. Photo courtesy to Jeena N. S (a, g), Vidya R (1b–d) and Asokan P. K (e, f, h) of CMFRI, Kochi, Kerala. The images were processed manually in Adobe Photoshop CC, 2020.
Villorita cyprinoides distributed in Vembanad Lake, a Ramsar site and longest lake in India, contribute 88.2% to the black clam fishery of the country27. The lake is crossed by the Thanneermukkom Barrage constructed (in 1974, ~ 2 km in length) to prevent the intrusion of saltwater, a potential threat to the rice production in Kuttanad paddy fields (~ 50,000 acres lying south to Vembanad Lake) known for the unique practice of rice cultivation below sea level (Fig. 1a). The barrage divides the lake to a northern side with flourishing beds of black clam and a southern part with a diminishing clam population. The reproductive success of the black clam depends on salinity and the decline in the clam population on the southern side may be correlated with the drop in salinity associated with closure (in December–May) and opening of the barrage during monsoon creating a freshwater environment. Further, flooding results in the burial of clam under silt and clay carried by floodwater and washing off the natural beds creating ecological changes28. The imbalanced ecosystem created through the construction of barrages results in restricted gene flow within conspecifics29. The detrimental effect of the decline in black clam population in the southern side of the Vembanad Lake affecting the livelihood of people depending on clam fishery urged the Govt. of Kerala to take immediate actions for rehabilitation through clam relaying (G.O(Rt)No. 460/16/F&PD of Fisheries and Ports (C) Department, Thiruvananthapuram, dated 01.08.16) to replenish the stock so as to ensure a sustainable supply of food, as implemented in many developed countries30, 31. Also, the dredging for the sub-fossil deposit of clamshell for industrial use32, pollution due to effluents from shrimp processing plants, eco-tourism, urban and agricultural runoff and infestation of the invasive Eichhornia crassipes engender threats to the clam33 in its natural habitat.
The economic and ecological eminence of Villorita cyprinoides results in continuous demand leading to overexploitation of the species including juveniles. Extensive harvesting of clam from the wild, niche alterations and exploitation of juveniles could be a potential threat to the existence of the species34. The selective pressure applied in stock by overfishing cannot be reversed through genetic selection on phenotypic traits35. In-depth studies on the exploitation of juvenile black clams suggested implementation of Minimum Legal Size (MLS) in clam fishery to reduce economic loss and preserve genetic variance36. The ecosystem degradation as well as overexploitation, if continued at the present level, may result in the reduction of effective population size leading to increased homozygosity, reduction in fitness due to faster erosion in genetic variation of related traits and ultimate genetic drift in far or near future. Along with genetic information, the need for breeding standardisation and proper farming technique is inevitable for a sustainable black clam fishery.
Villorita cyprinoides, a resource of paramount importance in meeting the country’s need for a cheap and balanced diet, has received less attention at the genetic level. The present status of black clam fishery demands genetic investigation and analysis for the proper implication of management and conservation. The present work is a genuine attempt to determine the mitogenome architecture of V. cyprinoides thereby delivering ample molecular data for genus Villorita. The multi-gene phylogeny from mitochondrial and nuclear genes was attempted to allocate V. cyprinoides in the phylogenetic network and resolving the phylogenetic status of Cyrenidae. Additionally, mitochondrial gene order (GO) analysis and estimation of divergence time were also inferred using multiple genes and fossil data.
Results and discussion
Mitogenome characteristics
The female mt-genome of Villorita cyprinoides is 15,880 bp in length, comparable to previously reported bivalve mitogenomes. Gene content of V. cyprinoides displayed 37 typical sets of genes found in metazoan mitogenome10 constituting 13 protein-coding genes (PCGs); encoding three electron transport chain (ETC) complexes (nd1-nd6, nd4l, cytb and cox1-cox3) and F0F1 ATP synthase complex (ATP6 and ATP8) in OXPHOS. Besides PCGs two ribosomal RNA (rRNA) and 22 transfer RNA (tRNA) genes were observed with the putative control region (CR). All the 37 genes are located on major (H-strand) strand with forward orientation like in other Heterodont bivalves37. Figure 2 plots the circular map of black clam mitogenome.
Mitochondrial genome organisation of Villorita cyprinoides visualised with DNA Plotter. The outer black circle represents the complete mitogenome of V. cyprinoides (scale denoted in base pair). The second ring represents mitochondrial proteins encoding genes, and the third circle denotes both rRNAs and tRNAs wherein all genes predicted in a positive frame. Colour in the box codes for specific categories of mitochondrial genes that are abbreviated according to standard usage. Fourth circle represents the distribution of GC % following yellow colour as below average and green colour, above-average values. The inner-circle represents the distribution of GC skew %, displaying blue colour as below average and red colour, above-average values.
The base composition of the mt-genome holds A = 25.1%, T = 42.9%, G = 22.8%, C = 9.2% with A+T content representing 68% of the total genome. A positive GC skew (0.424) and negative AT skew (− 0.262) were noticed indicating a bias towards Ts and Gs. Comprehensive analysis of mitogenomes, PCGs and rRNAs amongst 47 species from orders Veneroida, Myida, and Cardiida disclosed rich A+T content and low C content along with positive GC and negative AT skew (Supplementary Table S1).
Protein coding genes
All the 13 PCGs reported in V. cyprinoides mitogenome were identified within 11,835 bp length (74.5% of mitogenome), and A+T content predicted as 67.5% ranging from 63.8% (nad1) to 74.4% (nad4l). PCGs together encode 3,932 codons including nad5 (558aa) as the longest protein-coding gene and ATP8 (37aa) as the shortest. Nucleotide bias between all PCGs was estimated through base skewness (Table 1).
Most of the PCGs employ ATN (ATG, ATA and ATT) as an initiation codon. In addition to the classical metazoan start codon, GTG (Valine) initiated translation38,39,40 of ATP6 and nad2 gene in Cyrenid clams41 including V. cyprinoides. Ten PCGs were terminated by TAG, and TAA codons whilst remaining were terminated by truncated codons T– and TA- which presumably functions as complete termination codon after post-transcriptional polyadenylation42, 43 (Table 2).
ATP8 gene, which lacks in most of the heterodont bivalves was identified in V. cyprinoides, encoding a protein of 37 amino acids. No putative conserved domains were detected by pBLAST, whereas conserved amino acid block (WW**KR*Y*F) were identified as in certain species in family Veneridae44 and Cardiidae45 (Supplementary Figure S2).
Following the Relative Synonymous Codon Usage (RSCU) value of V. cyprinoides, all the 62 codons (excluding stop codon, TAA and TAG) were utilised in unusual frequencies (Table 3). In V. cyprinoides third codon position (75.1) contains high A+T content compared to first (62.6) and second (64.6) position. Bias towards A+T in the third codon position might be interconnected with degeneracy46 or natural selection on Wobble nucleotide47. Codon usage bias indicates TTT (Phe) is the most likely used codon after GTT (Val) whereas, CTC (Leu), CAC (His), TGC (Cys) were least used. It is evident that frequently used codons are A+T rich; while the lowest used were abundant in G+C content. The commonly used amino acid is Leucine than Phenylalanine, while Glutamine is less used.
Ribosomal and transfer RNAs
Twenty canonical tRNAs along with single gene duplication of trnS and trnL were bestrewed within the genome (8.9% of mitogenome). tRNAscan-SE identified only eleven tRNA gene whereas ARWEN detected all tRNA genes except trnH, predicted by position and sequence homology to trnH gene of Corbicula fluminea and Arctica islandica. Individual tRNA gene length varies from 62 bp (trnH, trnW) to 69 bp (trnS1, trnM) with anticodons identical to other heterodont bivalves. Separate anticodons encode for duplicated Serine (UCN, AGN) and Leucine (UUR, CUN) genes. All tRNAs can be folded to typical clover-leaf secondary structure except for trnS genes. The secondary structure of 22 predicted tRNA genes were summarised in Fig. 3.
Cloverleaf secondary structure of 22 tRNA genes in Villorita cyprinoides mitogenome generated using PseudoViewer v.3.0 (https://pseudoviewer.inha.ac.kr/).
The cloverleaf structure of tRNAs consists of 6–8 nucleotide pairs in aminoacyl acceptor stem, 5–6 pairs in anticodon and 4–6 pairs in DHU (dihydrouridine) and TψC stem with mismatched Watson–Crick base pairing. Anticodon of all tRNA genes was succeeded by Uracil and preceded by Guanine or Adenine in the anticodon loop of V. cyprinoides. The DHU stem of least conserved trnS1 and trnS2 genes48 were replaced by a large DHU loop, an accepted feature in molluscs44, 49. DHU stem of trnY gene is reduced to one nucleotide pair.
BLAST searches confirmed MITOS annotated ribosomal RNA genes, rrnL and rrnS in V. cyprionoides with length 1183 bp and 861 bp respectively (12.8% of mitogenome). Large ribosomal RNA, considered as the largest rRNA locus (rrnL) in bivalves50 flanked between the genes cytb and ATP8 as in genus Corbicula, Calyptogena, Cyclina, Dosinia and Meretrix (except Meretrix meretrix). Also, the position of small ribosomal RNA (rrnS), between trnT and trnM gene was consistent with genus Corbicula, Calyptogena, Cyclina, and Dosinia (Fig. 2). The exact boundaries of rRNA genes can only be determined by transcript mapping. Therefore, the indefinite length of rrnS and rrnL were resolved by comparison of their sequence similarity with species in family Cyrenidae and Veneridae.
Non-coding regions
A total of 590 bp intergenic spacer (IGS) sequences, sizes varying from 1 to 147 bp spreading across 23 regions were determined. All non-coding regions above 50 bp fold to stem and loop structure and the longest non-coding region flanked by trnP and trnF having 147 bp length fragment can be considered as control region (CR). The fragment between trnP and trnF form the most stable secondary structure with palindrome sequence, and AT content slightly above Villorita mitogenome, which are the general characters of control region46, 51 controlling replication and transcription of the genome (Supplementary Figure S3).
Five gene overlappings comprising 1–3 bp were noticed between trnH-trnE, trnL2-nad1, trnL1-trnV, trnY-trnS1, and, trnE-trnS2.
Gene rearrangement and synteny
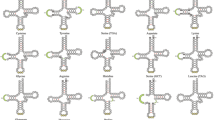
Gene rearrangement, accepted as a marker of common ancestry52, varies unpredictably owing to single strand gene distribution in bivalves50. The gene rearrangement explained through TDRL (Tandem Duplication Random Loss) model is believed to transpire in bivalves as in vertebrates53. Though overall gene order is not well conserved within bivalve superfamilies, gene arrangement is substantially similar after excluding tRNA genes. Conversely, gene arrangements in Imparidentian bivalve families are nearly identical except for tRNA duplication, and translocation of genes, but variable between families. The conserved gene order or synteny blocks displayed in superfamilies are shown in Fig. 4.
The mitogenome order of bivalves in genus level alters dramatically54 albeit, comparative mt-genome analysis of Imparidentian clams revealed conserved gene order within the genus level. V. cyprinoides presented highly conserved synteny block with Corbicula fluminea for all 37 genes, but variable in gene length. Alternatively, species belonging to family Vesicomyidae (Archivesica sp. and Calyptogena magnifica) displays exact gene order as V. cyprinoides after removing tRNA genes though they represent a different family. These results reflect Vesicomid clams are related to family Cyrenidae than other families. Also, V. cyprinoides and Corbicula fluminea may be evolved recently and are closely related since gene rearrangement in closely related species is less than in distant55. The gene orders conserved in Cyrenid clams were identified as R-cytb-rrnL-ATP8-nad4, ATP6-nad3, and L-nad1-L-V. Species within genus Donax, Solen, Dosinia, shared identical gene order with less rearrangement like Tridacna, Paphia, and Lutraria (purging ATP8). The gene order within Cardioidea is highly rearranged and least conserved compared to other superfamilies.
Phylogenetic analysis
Amplification of Histone H3 and small ribosomal rRNA (18S rRNA) nuclear genes resulted in a generation of 350 bp and 1725 bp amplicons respectively. The phylogenetic analysis was assessed after the exclusion of ambiguous loci using Gblock upon alignment. DNA sequences of mitochondrial (12 PCGs except for ATP8, rrnS and rrnL) and nuclear protein-coding (Histone H3), and non-coding genes (18S rDNA) after Gblock analysis were concatenated bestowing 11,767 base sequences for further phylogenetic reconstruction. The best partitioning scheme and best-fit substitution models were generated for concatenated multi-gene data providing 17 best subset partitions (Supplementary Table S4). Phylogenetic estimation incorporating the subset partitions and evolutionary models for both Maximum likelihood (ML) and Bayesian Inference (BI) analysis generated a well-resolved tree with similar topology. All major clades were well supported with posterior probability > 95 and bootstrap > 85. The combined tree with node label showing posterior probability (PP) values and bootstrap (BS) replicates was displayed in Fig. 5.
The Bayesian Phylogram evaluated based on nucleotide sequences of multilocus data (mitochondrial and nuclear) from 47 species of superorder Imparidentia. The ML tree exhibited identical topology. Node labels correspond to Bayesian posterior probability and ML bootstrap values and scale bar specify substitutions per site. Vertical coloured bar designate superfamilies.
The phylogeny of Venerida, the most debated order in monophyletic subclass Heterodonta, is still undergoing extensive revision56,57,58,59. The present study considered 47 species, including 30 Venerida, 16 Cardiida, and one Myida confirmed the monophyly of the Venerid family, Cyrenidae60. Family Cyrenidae (Superfamily: Cyrenoidea) form a well-supported clade corroborating monophyly, placing G. coaxans towards the stem, and V. cyprinoides and C. fluminea to its crown. Cyrenidae co-clusters with Arcticoidae establishing Arcticoidae as immediate sister taxon, whereas Vesicomyidae (Glossoidea) lineage form a separate clade.
D. polymorpha (family: Dreissenidae), freshwater bivalve belonging to order Myida, found nested within Venerid taxa, questioning the phylogenetic status of the orders, and subdivision of Henterodonta to Venerida and Myida61. In contrast, this result substantiates the separate radiation of Cyrenidae and Dreissenidae to the freshwater lineage from marine ancestors18. The monophyletic status of Mactridae (Superfamily: Mactroidea) was confirmed, which appeared as a sister group to Dreissenidae62. Mactridae clustered with major clade [(Veneridae + Vesicomyidae) + (Cyrenidae + Arcticoidae)].
Phylogenetic analysis of Veneridae, the most diverse and economically important taxon in Venerida recovered the monophyletic status63 forming Vesicomyidae as a sister group. Although phylogenetic analysis suggests Vesicomyidae as a sister group, Veneridae shares close kin with Cyrenidae and Arcticoidae. The close relation of Veneridae with Vesicomyidae, Arcticoidae and Cyrenidae was supported by the presence of hinge characters, a morphological synapomorphy64. The major clade of Veneridae split separately, forming two distinct subclades retaining Cyclininae, Meretricinae, and Pitarinae in one clade, and Dosiniinae and Tapetinae subfamilies in another, agreeable with the previous studies65.
Furthermore, Pharidae and Solenidae supported as the sister group to each other, converged to form a well-resolved clade pointing to the monophyly of superfamily Solenoidea as expected66.
Being sister taxon to each other, Tellinoidea and Cardioidea validities the monophyly of order Cardiida. Five Tellinoidean families aggregated to a single clade with high PP and bootstrap value indicating monophyly of Tellinoidea but failed to resolve familial relationship62. However, species from Donacidae clustered to a branch, forming a sister group with other Tellinoid families9. Conversely, the monophyletic status of Carridae and Tridacnidae were evident in our phylogenetic analysis67, wherein Cardioidea itself is monophyletic.
The monophyletic status of Cardiida and Solenoidea were well supported, but we could not ratify the exact phylogenetic status of Tellinoidean families. Like Cardioidea and Solenoidea, the monophyly of Mactridae was validated, but recent studies support paraphyletic evolution66. This disparity might be due to limited taxon sampling because of less available mitogenome and nuclear gene data, exemplifying the importance of using multiple genes in phylogenetics.
Divergence time estimation
The multi-gene tree topology after phylogenetic analysis were employed for molecular dating. Evolutionary ages and divergence were estimated through calibration of the molecular clock using multiple fossil data (Table 4). The time-calibrated phylogenetic tree is shown in Fig. 6.
Early Jurrasic fossil record of Corbicula sp.(201–170 mya) was used to establish the primary appearance of Cyrenid clam68. According to the dated tree, diversification of Cyrenid clams from genus Geloina occurred roughly around early Jurassic, 179 mya (HPD, Highest Posterior Density: 169–198 mya) whereas, the evolutionary split between Corbicula and Villorita lineage appeared during early Cretaceous, 124 mya (HPD: 56–173 mya).
The splitting of vesicomyid clams is dated near to the early Cenozoic era i.e., 47 mya (HPD: 14–102 mya) corroborating previous investigation69. Also, the evolutionary radiation of Imparidentian bivalves is found to be in the Mesozoic Era in tune with preceding researches57, 58. The evolutionary radiations of major superfamilies from our study were found to be Solenoidea − 239 mya-, Tellinoidea − 328 mya-, Mactroidea − 233 mya- and Veneroidea − 268 mya.
Conclusion
Even though enormous mitogenomes of clams have been characterised, there is dearth in molecular data for economically and ecologically significant clam, Villorita cyprinoides. The present study determined the first complete mitochondrial genome of multivalent endemic black clam, Villorita cyprinoides, which can be considered as keystone species that can perform top-down and bottom-up control70. Further, characterisation of mitogenome of V. cyprinoides resembles typical metazoan mitogenome architecture including the ATP8 gene, which is absent in several heterodont bivalves. Besides, conserved gene order (GO) analysis states the similarity of V. cyprinoides to C. fluminea, which is acceptable with detected phylogeny, revealing that both are closely associated and only recently diverged from an immediate common ancestor. The comparative GO analysis also revealed the conserved gene order of protein-coding and rRNA genes of V. cyprinoides and Vesicomid clams like Archivesica sp. and C. magnifica.
The present phylogenetic analysis using both mitochondrial and nuclear genes resolved the monophyletic status of family Cyrenidae confirming a sister relationship with the Arcticoid family. This study also estimated the diversification of Cyrenid clams during the early Jurassic-Cretaceous period. Resolving phylogenetic uncertainty and estimation of divergence time in family Cyrenidae is important, as it is a potential taxon to trace bivalve radiation to the inland water system because it retains both brackish water and freshwater genera18. Though we verified monophyly of Cyrenidae and enriched the transparency of phylogenetic status, integrated investigation of other Cyrenid clams with dense species sampling is required to conserve our conclusion and to provide evolutionary divergence among and between taxon.
Mitogenome sequences can be utilised for comparative phylogenomic studies within family Cyrenidae and development of genetic markers for identifying cryptic species if any. Apart from this, the genetic markers developed from this genome sequence can be used to study the population structure of V. cyprinoides since structure analysis is mandatory before the effective implementation of clam relaying programmes71. Even though no report on family Cyrenidae for the presence of an alternative mitochondrial inheritance pattern, Doubly Uniparental Inheritance (DUI)72, needs to be determined in the genus Villorita. At present, a reliable fishery-dependant data is available for stock assessment of black clam18 and the mitogenome characterised here has scope to provide genetic markers to have fishery-independent estimates of stock biomass. The findings shall provide a better base to evaluate if stock biomasses are at sustainable levels.
Methods
Specimen collection
Samples of V. cyprinoides were collected from Muhamma region in Vembanad Lake (approximate GPS coordinates: 9°36′18.6″N 76°22′01.2″E), Kerala, India at June 2017, transported and maintained alive in Central Marine Fisheries Research Institute (CMFRI, Kochi, India) hatchery. Specimens used in this study were identified by taxonomical literature73, 74 and COI barcoding75. Sex was determined by microscopic examination of the gonads. The report of heteroplasmy in male clams from several bivalve families76 prompted us to select a female specimen with the average size for tissue extraction. Tissue from adductor muscles was extracted and stored in absolute alcohol and RNA later (− 20 °C) in duplicate. After a day of tissue extraction, the samples stored in RNA later were transported in dry ice to Xcelris Genomics (Gujarat, India).
Genomic DNA extraction, PCR amplification and sequencing
Total genomic DNA was extracted from ethanol fixed adductor muscle tissue using the Qiagen DNeasy Blood and Tissue Kit (QIAGEN, Valencia, CA, USA) and stored in − 80 °C. NANODROP ONE (Thermo Scientific) assessed the quantity and purity of the extracted DNA. Isolated genomic DNA served as a template for the amplification of nuclear genes (Histone H3, 18S rRNA). Both the genes were amplified in 25 µl reaction volume constituting 12.5 µl TaKaRa EmeraldAmp GT PCR Master Mix, 0.5 µl of 10 µM of each forward primer (18S rRNA: 5´ AACCTGGTTGATCCTGCCAGT 3´, H3: 5′ ATGGCTCGTACCAAGCAGACVGC 3′) and reverse primer (18S rRNA: 5′ GATCCTTCTGCAGGTTCACCTAC 3´, H3: 5′ ATATCCTTRGGCATRATRGTGAC 3′)77, 78 and 1 µl of 100 ng/µl DNA. PCR reaction was performed on BIORAD T100 TM thermal cycler (Biorad, USA) following the standard thermal regime with an annealing temperature of 55 °C for 18S rRNA and 52 °C for Histone H3 gene. The amplified product was purified and sequenced bidirectionally. Complementary sequences were visualised, edited and assembled into consensus sequence using BioEdit v7.1.9. The edited and assembled nucleotide sequences were deposited in GenBank.
Mt-DNA isolation, library preparation, and sequencing
Mt-DNA was isolated and validated with mt-DNA specific genes for qualitative analysis. The concentration of the mt-DNA was measured using Qubit Fluorometer (Life Technologies, USA).
The paired-end sequencing library was prepared using QlAseq FX DNA Library Kit. 100 ng DNA was enzymatically sheared into smaller fragments. DNA fragments with an average length of 700 bp were end-repaired, 3′ adenylated and ligated to Illumina adapters. Adapter-ligated libraries were PCR amplified, quantification and size evaluation of the library was performed in Bioanalyzer 2100 using High Sensitivity DNA chip (Agilent Technologies) and sequenced on Illumina sequencing platform (Illumina HiSeq 2500, USA).
Mitogenome assembly
Raw sequences generated after sequencing (2 × 150 PE) were retrieved in the FASTQ format. The quality of the reads was evaluated using FastQC software79. Illumina adapters, sequences less than 60 bp and low-quality sequences (Phred scores < 30) were removed using Trimmomatic80 leaving 17 million (PE) high quality reads. High-quality reads were subjected to De novo assembly using CLC workbench 6 (Qiagen, Germany) with a read map back algorithm. Similarity search of high coverage scaffolds (> 15kbps) was carried out against NCBI’s Nucleotide (NT) database using the BLASTN81 algorithm.
Sequence annotation and analysis
The scaffold identified as mitogenome of Villorita cyprinoides was annotated using MITOS282 web server substituting translation table 5. Besides MITOS2, Protein-Coding Genes (PCGs) were identified using the online ORF Finder Tool (https://www.ncbi.nlm.nih.gov/orffinder/). Ribosomal subunit (rrnS and rrnL) genes were confirmed using NCBI BLAST (https://www.nchi.nlm.nih.gov/BLAST/ ) searches. Validation of tRNA gene, structure prediction, and visualisation was accomplished with tRNAScan-SE v2.083 and ARWEN v1.284 under invertebrate mitochondrial settings. The cloverleaf structures of predicted tRNA genes were drawn using PseudoViewer v.3.0 (https://pseudoviewer.inha.ac.kr/)85 by sequence and structure input format and off numbering draw option. Control Region (CR) was annotated using DNA folding form from the Mfold web server (https://unafold.rna.albany.edu/?q=mfold/DNA-Folding-Form)86 retaining all the options as default except folding temperature (20 °C) and exterior loop-type (flat). Boundaries of MITOS2 annotated genes were refined based on the above software results using MEGA 787. DNAPlotter88 was used to generate a circular visual overview of annotated mitogenome. The curated mitogenome sequence (Accession No: MK481950) was submitted to the NCBI database through the BankIt sequence submission tool (https://www.ncbi.nlm.nih.gov/BankIt/). Conserved gene order analyses of mt-genomes in superfamily level were conducted in superorder Imparidentia.
Nucleotide composition statistics of PCGs excluding stop codon and mitochondrial ribosomal RNAs were calculated separately by Geneious v11.1.589. Codon usage bias and Relative Synonymous Codon Usage (RSCU) of protein-coding genes were inferred using the codon usage table computed through DnaSP v5.10.0190. AT and GC skew were analysed by the formula AT skew = (A + T)/(A-T) and GC Skew = (G + C)/(G−C)91.
Phylogenetic reconstruction and divergence time estimation
The phylogenetic position of V. cyprinoides concerning superorder Imparidentia was appraised. For better-resolved molecular phylogeny, 47 bivalve mitogenomes belonging to 15 families from superorder Imparidentia and their available nuclear (18S rRNA and Histone H3) genes from GenBank database were used (Supplementary Table S5). The nucleotide sequence of all the 12 protein-coding genes excluding stop codons was used whereas the ATP8 gene was omitted from analysis owing to its lack in most of the bivalves. Besides coding genes, sequences of the small and large ribosomal subunit of mitogenome coupled with nuclear small ribosomal subunit and Histone H3 were incorporated. Seven species belonging to class Polyplacophora, Gastropoda and from order Pectenoida served as outgroups.
The amino acid sequence of 12 protein-coding genes and nucleotide sequences of rrnL, rrnS and nuclear 18S rRNA, Histone H3 genes were separately aligned using Clustal W92 implemented in MEGA7 with a default setting for PCGs. The aligned amino acid sequences of protein-coding genes were manually corrected and back-translated to nucleotide sequences. On the contrary, gap opening and extension costs for ribosomal gene alignments were set to 20/4 due to the high sequence variability in analysed taxa. After alignment, Gblocks v0.91b93 under default condition, changing the type of sequence, t = dna, identified and eliminated the ambiguous loci from mitochondrial and nuclear (18S) ribosomal RNA gene.
Appropriate subset partitions and evolutionary models for genes (Supplementary Table S4) were selected by PartitionFinder v.1.1.194 under the greedy algorithm, unlinked branch length, and Bayesian information criterion (BIC) parameters. Protein coding genes and non-coding genes are considered as separate partitions. The best-fit model for partitions was chosen to perform Maximum likelihood (ML) and Bayesian Inference (BI). Phylogenetic analysis using the Maximum likelihood method was inferred using IQ-tree with 1,000 ultrafast bootstrapping replicates95. Bayesian analysis was implemented using MrBayes v.3.296. The programme was run for 2 × 107 generations comprising two simultaneous analysis with four chains, sampling every 100 generations. The average standard deviation of split frequency beneath 0.01 was considered as the stationarity phase, which is evaluated in Tracer v.1.6 (https://tree.bio.ed.ac.uk/software/tracer/), and the 50% majority rule summarised trees after discarding the first 25% as burn-in. Remaining trees were used to construct a consensus tree, edited and visualised in FigTree v.1.4.3 (https://tree.bio.ed.ac.uk/software/figtree/).
Estimation of divergence time between species and clade were determined using Bayesian relaxed clock models and MCMC algorithms97, 98 implemented in the mcmctree programme of PAML software v4.999. While the rooted tree from the previous analysis was used to calibrate evolutionary rates by incorporating multiple fossil ages spanning from the earliest bivalve fossil to fossil from family Cyrenidae (Table 4) and parameters model = 4, alpha = 0.5, clock = 2 and rgene gamma = 2.2. Besides, a loose upper bound (maximal age constraint of 1,000 mya) was specified due to non-calibration at the root. The analysis was conducted with auto finetune for 20,000 iterations and the first 2000 iterations were discarded as burnin. The consensus tree was edited and visualised in FigTree v.1.4.3 (https://tree.bio.ed.ac.uk/software/figtree/ ).
Ethics statement
No permission is required to collect and study the black clam, Villorita cyprinoides. They are not under an endangered or protected list and thus no control over the collection of samples.
Consent for publication
The authors hereby declare that consent of the concerned individuals have been obtained to the publication of images Fig. 1b–h.
References
FAO. The State of World Fisheries and Aquaculture (2020).
Aru, V., Khakimov, B., Sørensen, K. M. & Engelsen, S. B. Journal of food composition and analysis the foodome of bivalve molluscs: from hedonic eating to healthy diet. J. Food Compos. Anal. 69, 13–19 (2018).
Butler, P. G., Freitas, P. S., Burchell, M. & Chauvaud, L. Archaeology and sclerochronology of marine bivalves. In Goods and Services of Marine Bivalves 413–444 (Springer International Publishing, 2018). https://doi.org/10.1007/978-3-319-96776-9_21
Gosling, E. Bivalve Molluscs Biology, Ecology and Culture (Blackwell Publishing, New York, 2003).
National Research Council (U.S.). Committee on Best Practices for Shellfish Mariculture and the Effects of Commercial Activities in Drakes Estero, P. R. N. S. Ecosystem Concepts for Sustainable Bivalve Mariculture. (National Academies Press, 2010).
Vaughn, C. C. & Hoellein, T. J. Bivalve impacts in freshwater and marine ecosystems. Annu. Rev. Ecol. Evol. Syst. 49, 183–208 (2018).
Martijn, J., Vosseberg, J., Guy, L., Offre, P. & Ettema, T. J. G. Deep mitochondrial origin outside the sampled alphaproteobacteria. Nature 557, 101–105 (2018).
Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144–148 (1961).
Fernández-Pérez, J., Nantón, A., Ruiz-Ruano, F. J., Camacho, J. P. M. & Méndez, J. First complete female mitochondrial genome in four bivalve species genus Donax and their phylogenetic relationships within the Veneroida order. PLoS ONE 12, 1–18 (2017).
Boore, J. L. Animal mitochondrial genomes. Nucleic Acids Res. 27, 1767–1780 (1999).
Boore, J. L. & Brown, W. M. Big trees from little genomes: mitochondrial gene order as a phylogenetic tool. Curr. Opin. Genet. Dev. 8, 668–674 (1998).
Froufe, E. et al. Mesozoic mitogenome rearrangements and freshwater mussel (Bivalvia: Unionoidea) macroevolution. Heredity (Edinb) 124, 182–196 (2020).
Guerra, D. et al. Evolution of sex-dependent mtDNA transmission in freshwater mussels (Bivalvia: Unionida). Sci. Rep. 7, 1–13 (2017).
Lee, Y. et al. A mitochondrial genome phylogeny of Mytilidae (Bivalvia: Mytilida). Mol. Phylogenet. Evol. 139, 106533 (2019).
Fernández-Pérez, J., Froufe, E., Nantón, A., Gaspar, M. B. & Méndez, J. Genetic diversity and population genetic analysis of Donax vittatus (Mollusca: Bivalvia) and phylogeny of the genus with mitochondrial and nuclear markers. Estuar. Coast. Shelf Sci. 197, 126–135 (2017).
Sharma, P. P. et al. Into the deep: a phylogenetic approach to the bivalve subclass Protobranchia. Mol. Phylogenet. Evol. 69, 188–204 (2013).
Ansari, Z. A., Parulekar, A. H. & Matondkar, S. G. P. seasonal changes in meat weight and biochemical composition in the black clam Villorita cyprinoides. Indian J. Mar. Sci. 10, 128–131 (1981).
Graf, D. L. Patterns of freshwater bivalve global diversity and the state of phylogenetic studies on the unionoida, sphaeriidae, and cyrenidae. Am. Malacol. Bull. 31, 135–153 (2013).
Arun, A. U. Gametogenic cycle in Villorita cyprinoides and the influence of salinity. Aquacul. Aquar. Conserv. Legis. 2, 433–447 (2009).
Lydeard, C. & Cummings, K. S. Freshwater Mollusc of the World: A Distribution Atlas (JHU Press, Baltimore, 2019).
Vijaya Krishna, A. & Ammini, P. Population characteristics of Villorita cyprinoides in Vembanad Lake India. Lake Reserv. Manag. 34, 130–140 (2018).
Joy, M. & Chakraborty, K. Nutritional qualities of the low-value bivalve mollusks Paphia malabarica and Villorita cyprinoides at the estuarine waters of the Southwestern Coast of India. J. Aquat. Food Prod. Technol. 26, 54–70 (2017).
Vipinkumar, V. P. et al. Impact of self-help groups in gender mainstreaming: a case study on clam processing units in Pookaitha village, Kottayam, Kerala m. J. Mar. Biol. Assoc. India. 59, 102–108 (2017).
Joy, M. & Chakraborty, K. Antioxidative and anti-inflammatory pyranoids and isochromenyl analogues from Corbiculid bivalve clam Villorita cyprinoides. Food Chem. 251, 125–134 (2018).
George, R., Martin, G. D., Nair, S. M. & Chandramohanakumar, N. Biomonitoring of trace metal pollution using the bivalve molluscs, Villorita cyprinoides, from the Cochin backwaters. Environ. Monit. Assess. 185, 10317–10331 (2013).
Paul, T. T., Salim, S. S., Manoharan, V. S. & Unnithan, U. Identification and evaluation of ecosystem services provided by clam (Villorita cyprinoides) fisheries in wetland. Indian J. Trop. Biodivers. 23, 21–29 (2015).
CMFRI. Annual Report 2017–18. (2018).
Suja, N. & Mohamed, K. S. The black clam, Villorita cyprinoides, fishery in the State of Kerala, India. Mar. Fish. Rev. 72, 48–61 (2010).
Tiemann, J. S., Gillette, D. P., Wildhaber, M. L. & Edds, D. R. Effects of lowhead dams on riffle-dwelling fishes and macroinvertebrates in a midwestern river. Trans. Am. Fish. Soc. 133, 705–717 (2004).
Muehlbauer, F. et al. Ocean & Coastal Management Bivalve aquaculture transfers in Atlantic Europe. Part A : Transfer activities and legal framework. Ocean Coast. Manag. 89, 127–138 (2014).
Muehlbauer, F. et al. Ocean & coastal management bivalve aquaculture transfers in Atlantic Europe. Part B: Environmental impacts of transfer activities. Ocean Coast. Manag. 89, 139–146 (2014).
Kripa, V. et al. Clam fisheries of Vembanad Lake, Kerala with observations on the socio-economic conditions of the clam fisheries. Mar. Fish. Inf. Serv. Tech. Ext. Ser. 179, 14–16 (2004).
Laxmilatha, P. & Appukuttan, K. K. A review of the black clam (Villorita cyprinoides) fishery of the Vembanad Lake. Fish. Res. 49, 85–91 (2002).
Vidya, R. et al. Exploitation of Juvenile Clam, Villorita cyprinoides from Vembanad lake. IFAF (2017)
Law, R. Fishing, selection, and phenotypic evolution. ICES J. Mar. Sci. 57, 659–668 (2000).
Suja, N. & Mohamed, K. S. Use of minimum legal size in managing black clam (Villorita cyprinoides) fishery in India. Int. J. Aquat. Biol. 1, 306–315 (2014).
Yuan, Y., Li, Q., Yu, H. & Kong, L. The complete mitochondrial genomes of six heterodont bivalves (Tellinoidea and Solenoidea): variable gene arrangements and phylogenetic implications. PLoS ONE 7, e32353 (2012).
Wolstenholme, D. R. Animal mitochondrial DNA: structure and evolution. Int. Rev. Cytol. 141, 173–216 (1992).
Dreyer, H. & Steiner, G. The complete sequences and gene organisation of the mitochondrial genomes of the heterodont bivalves Acanthocardia tuberculata and Hiatella arctica—And the first record for a putative Atpase subunit 8 gene in marine bivalves. Front. Zool. 3, 1–14 (2006).
Yuan, Y., Li, Q., Kong, L. & Yu, H. The complete mitochondrial genome of the grand jackknife clam, Solen grandis (Bivalvia: Solenidae): a novel gene order and unusual non-coding region. Mol. Biol. Rep. 39, 1287–1292 (2012).
Zhang, T. et al. The complete mitogenome of clam Corbicula fluminea determined using next-generation and PacBio sequencing. Mitochondrial DNA Part B Resour. 4, 1660–1661 (2019).
Ojala, D., Montoya, J. & Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 290, 470–474 (1981).
Boore, J. L. & Brown, W. M. Mitochondrial genomes of Galathealinum, Helobdella, and Platynereis: Sequence and gene arrangement comparisons indicate that Pogonophora is not a phylum and Annelida and Arthropoda are not sister taxa. Mol. Biol. Evol. 17, 87–106 (2000).
Lv, C., Li, Q. & Kong, L. Comparative analyses of the complete mitochondrial genomes of Dosinia clams and their phylogenetic position within Veneridae. PLoS ONE 13, 1–18 (2018).
Imanishi, Y., Tanaka, M. & Fujiwara, M. Complete mitochondrial genome sequence of Japanese cockle Fulvia mutica (Cardiidae). Fish. Sci. 79, 949–957 (2013).
Plazzi, F., Ribani, A. & Passamonti, M. The complete mitochondrial genome of Solemya velum (Mollusca: Bivalvia) and its relationships with Conchifera. BMC Genomics 14, 409 (2013).
Yu, H. & Li, Q. Mutation and selection on the wobble nucleotide in tRNA anticodons in marine bivalve mitochondrial genomes. PLoS ONE 6, e16147 (2011).
Xu, X., Wu, X. & Yu, Z. The mitogenome of Paphia euglypta (Bivalvia: Veneridae) and comparative mitogenomic analyses of three venerids. Genome. 53, 1041–1052 (2010).
Sun, S., Kong, L., Yu, H. & Li, Q. The complete mitochondrial DNA of Tegillarca granosa and comparative mitogenomic analyses of three Arcidae species. Gene 557, 61–70 (2015).
He, C. B. et al. The complete mitochondrial genome of the hard clam Meretrix meretrix. Mol. Biol. Rep. 38, 3401–3409 (2011).
Wang, H., Zhang, S., Xiao, G. & Liu, B. Complete mtDNA of the Meretrix lamarckii (Bivalvia: Veneridae) and molecular identification of suspected M. lamarckii based on the whole mitochondrial genome. Mar. Genomics 4, 263–271 (2011).
Boore, J. L., Lavrov, D. V. & Brown, W. M. Gene translocation links insects and crustaceans. Nature 392, 667–668 (1998).
Yu, Z., Wei, Z., Kong, X. & Shi, W. Complete mitochondrial DNA sequence of oyster Crassostrea hongkongensis-a case of "Tandem duplication-random loss" for genome rearrangement in Crassostrea?. BMC Genomics. 9, 477 (2008).
Ren, J., Liu, X., Jiang, F., Guo, X. & Liu, B. Unusual conservation of mitochondrial gene order in Crassostrea oysters: evidence for recent speciation in Asia. BMC Evol. Biol. 10, 394 (2010).
Belda, E., Moya, A. & Silva, F. J. Genome rearrangement distances and gene order phylogeny in γ-proteobacteria. Mol. Biol. Evol. 22, 1456–1467 (2005).
Giribet, G. & Wheeler, W. On bivalve phylogeny: a high-level analysis of the Bivalvia (Mollusca) based on combined morphology and DNA sequence data. Invertebr. Biol. 121, 271–324 (2005).
Plazzi, F. & Passamonti, M. Towards a molecular phylogeny of Mollusks: Bivalves’ early evolution as revealed by mitochondrial genes. Mol. Phylogenet. Evol. 57, 641–657 (2010).
Bieler, R. et al. Investigating the bivalve tree of life—an exemplar-based approach combining molecular and novel morphological characters. Invertebr. Syst. 28, 32 (2014).
González, V. L. et al. A phylogenetic backbone for Bivalvia: an RNA-seq approach. Proc. R. Soc. B Biol. Sci. 282, 20142332 (2015).
Park, J. & Foighil, O. D. Sphaeriid and corbiculid clams represent separate heterodont. Mol. Phylogenet. Evol. 14, 75–88 (2000).
Canapa, A., Barucca, M., Marinelli, A. & Olmo, E. A molecular phylogeny of heterodonta (Bivalvia) based on small ribosomal subunit RNA sequences. Mol. Phylogenet. Evol. 21, 156–161 (2001).
Taylor, J. D., Williams, S. T., Glover, E. A. & Dyal, P. A molecular phylogeny of heterodont bivalves (Mollusca: Bivalvia: Heterodonta): new analyses of 18S and 28S rRNA genes. Zool. Scr. 36, 587–606 (2007).
Mikkelsen, P. M., Bieler, R., Kappner, I. & Rawlings, T. A. Phylogeny of Veneroidea (Mollusca: Bivalvia) based on morphology and molecules. Zool. J. Linn. Soc. 148, 439–521 (2006).
Gardner, R. N. Middle-Late Jurassic bivalves of the superfamily Veneroidea from New Zealand and New Caledonia. New Zeal. J. Geol. Geophys. 48, 325–376 (2005).
Canapa, A., Schiaparelli, S., Marota, I. & Barucca, M. Molecular data from the 16S rRNA gene for the phylogeny of Veneridae (Mollusca: Bivalvia). Mar. Biol. 142, 1125–1130 (2003).
Combosch, D. J. et al. A family-level tree of life for bivalves based on a Sanger-sequencing approach. Mol. Phylogenet. Evol. 107, 191–208 (2017).
Campbell, D. C. Molecular evidence on the evolution of the Bivalvia. Geol Soc. Lond. Spec. Publ. 177, 31–46 (2000).
Poulton, T. P. Hettangian through Aalenian (Jurassic) guide fossils and biostratigraphy, Northern Yukon and adjacent Northwest Territories. Bull. Geol. Sur. Can.410, 1–95 (1991).
Johnson, S. B., Krylova, E. M., Audzijonyte, A., Sahling, H. & Vrijenhoek, R. C. Phylogeny and origins of chemosynthetic vesicomyid clams. Syst. Biodivers. 15, 346–360 (2017).
Gallardi, S. B. Effects of bivalve aquaculture on the environment and their possible mitigation: a review. Syst. Biodivers. https://doi.org/10.4172/2150-3508.1000105 (2014).
Beaumont, A. Genetic considerations in transfers and introductions of scallops. Aquac. Int. 8, 493–512 (2000).
Breton, S., Beaupré, H. D., Stewart, D. T., Hoeh, W. R. & Blier, P. U. The unusual system of doubly uniparental inheritance of mtDNA: isn’t one enough?. Trends Genet. 23, 465–474 (2007).
Rao, N. V. S. Handbook Freshwater Molluscs of India (Zoological Survey of India, Kolkatta, 1989).
Rao, N. V. S. Indian Seashells: Part-2 Bivalvia (Zoological Survey of India, Kolkatta, 2017).
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299 (1994).
Capt, C. et al. Unorthodox features in two venerid bivalves with doubly uniparental inheritance of mitochondria. Sci. Rep. 10, 1–13 (2020).
Medlin, L., Elwood, H. J., Stickel, S. & Sogin, M. L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71, 491–499 (1988).
Colgan, D. J. et al. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust. J. Zool. 46, 419–437 (1998).
Andrews, S. FastQC: a quality control tool for high throughput sequence data. Available online at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc. (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Bernt, M. et al. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 69, 313–319 (2013).
Lowe, T. M. & Eddy, S. R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25, 955–964 (1997).
Laslett, D. & Canback, B. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 24, 172–175 (2008).
Byun, Y. & Han, K. PseudoViewer: web application and web service for visualizing RNA pseudoknots and secondary structures. Nucleic Acids Res. 34, W416–W422 (2006).
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Carver, T., Thomson, N., Bleasby, A., Berriman, M. & Parkhill, J. DNAPlotter : circular and linear interactive genome visualization. Bioinformatics 25, 119–120 (2009).
Kearse, M. et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 (2012).
Librado, P. & Rozas, J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009).
Perna, N. T. & Kocher, T. D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 41, 353–358 (1995).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Talavera, G. & Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56, 564–577 (2007).
Lanfear, R., Calcott, B., Ho, S. Y. & Guindon, S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701 (2012).
Nguyen, L., Schmidt, H. A., Haeseler, A. V. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2014).
Ronquist, F. et al. MrBayes 3.2 efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Yang, Z. & Rannala, B. Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Mol. Biol. Evol. 23, 212–226 (2006).
Rannala, B. & Yang, Z. Inferring speciation tunes under an episodic molecular clock. Syst. Biol. 56, 453–466 (2007).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Landing, E. D. Upper precambrian through lower cambrian of Cape Breton Island: Faunas, paleoenvironments and stratigraphic revision. J. Paleontol. 65, 570–595 (1991).
Brasier, M. D., Hewitt, R. A. & Brasier, C. J. On the late precambrian-early cambrian hartshill formation of warwickshire. Geol. Mag. 115, 21–36 (1978).
Devaere, L, Clausen, S, Sosa-Leon, J. P, Palafox-Reyes, J. J, Buitron-Sanchez, B .E and Vachard, D. Early cambrian small shelly fossils from northwest Mexico: Biostratigraphic implications for Laurentia. Palaeontol. Electron. 22, 1–60 (2019).
Fang, Z. J. et al. Supraspecific Taxa of the Bivalvia first named, described, and published in China (1927–2007). Univ. Kansas Paleontol. Contrib. 17, 1–157 (2009).
Hayami, I. A systematic survey of the Mesozoic Bivalvia from Japan. Univ. Mus. Univ. Tokyo Bull. 10, http://umdb.um.u-tokyo.ac.jp/DKankoub/Bulletin/no10/no10000.html (1975).
Elias, M. Late Mississippian fauna from the redoak hollow formation of southern Oklahom Part I. J. Paleontol. 31, 370–342 (1957).
Paleobiology Database. The Paleobiology Database. (2018). Available at: https://paleobiodb.org/classic/checkTaxonInfo?taxon_no=281202.
Acknowledgements
The research was completed with additional financial support from the Indian Council of Agricultural Research – Central Marine Fisheries Research Institute (ICAR-CMFRI) Project No. MFD/MOL/17. The authors express sincere gratitude to Dr. A. Gopalakrishnan, Director, CMFRI for the support and facilities provided for the research. We are indebted to Dr. K. Sunil Mohamed, Head, Molluscan Fisheries Division, CMFRI for his valuable suggestions. This work is a part of the Ph.D. thesis of S.R and the author acknowledges the Council of Scientific and Industrial Research (CSIR), India for the fellowship received for this research.
Author information
Authors and Affiliations
Contributions
J.N.S conceptualised the experiment. S.R and J.N.S collected the data, performed experiments, analysed and wrote the paper. V.R helped in compiling fishery data from Kerala, A.P.K critically reviewed the manuscript and P.V coordinated the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahuman, S., Jeena, N.S., Asokan, P.K. et al. Mitogenomic architecture of the multivalent endemic black clam (Villorita cyprinoides) and its phylogenetic implications. Sci Rep 10, 15438 (2020). https://doi.org/10.1038/s41598-020-72194-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72194-1
This article is cited by
-
Mitochondrial genomes of Tapes dorsatus and Cardita variegata: insights into Heteroconchia phylogeny
Journal of Oceanology and Limnology (2024)
-
The first complete mitogenome of Acharax sp. (Protobranchia, Solemyida, Solemyidae): comparisons with other Solemyidae bivalves and deep-sea adaptive characteristics
Journal of Oceanology and Limnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.