Abstract
A neurogenic pathway, involving airway TRPV-1, has been implicated in acute cardiovascular events occurring after peaks of air pollution. We tested whether inhaled prostaglandin-E2 (PGE2) and bradykinin (BK) regulate TRPV-1 activity in vivo by changing cough response to capsaicin (CPS) and affecting heart rate variability (HRV), while also taking into account the influence of TRPV-1 polymorphisms (SNPs). Moreover, we assessed the molecular mechanism of TRPV-1 modulation in vitro. Seventeen healthy volunteers inhaled 100 μg PGE2, 200 μg BK or diluent in a randomized double-blind fashion. Subsequently, the response to CPS was assessed by cough challenge and the sympathetic activity by HRV, expressed by low (nLF) and high (nHF) normalized frequency components, as well as nLF/nHF ratio. Intracellular [Ca2+] was measured in HeLa cells, transfected with wild-type TRPV-1, pre-treated with increasing doses of PGE2, BK or diesel exhaust particulate (DEP), after CPS stimulation. Six functional TRPV-1 SNPs were characterized in DNA from each subject. Inhalation of PGE2 and BK was associated with significant increases in cough response induced by 30 μM of CPS (cough number after PGE2 = 4.20 ± 0.42; p < 0.001, and after BK = 3.64 ± 0.37; p < 0.01), compared to diluent (2.77 ± 0.29) and in sympathetic activity (nLF/nHF ratio after PGE2 = 6.1; p < 0.01, and after BK = 4.2; p < 0.05), compared to diluent (2.5–3.3). No influence of SNPs was observed on autonomic regulation and cough sensitivity. Unlike PGE2 and BK, DEP directly activated TRPV-1. Inhalation of PGE2 and BK sensitizes TRPV-1 and is associated with autonomic dysregulation of cardiac rhythm in healthy subjects.
Similar content being viewed by others
Introduction
Increments in air pollutants are associated with increased mortality and morbidity due to cardiopulmonary diseases in the hours or few days following exposure, as shown in several epidemiological studies1,2. These associations are more consistent for particulate matter (PM), and indeed a worsening of cardiac disease is often observed within a few hours after peaks of PM concentrations in urban air, suggesting that very rapid events take place3,4.
The mechanism by which lung exposure to air pollutants is associated with cardiovascular adverse events is currently unclear. Evidence supporting the occurrence of systemic inflammation is inconsistent in the literature and is more compatible with long term rather than acute effects5. Alternatively, a neurohumoral mechanism has been hypothesized6. Vagal bronchopulmonary receptors such as C-fiber endings are primarily responsible for eliciting centrally mediated reflexes (such as coughing) in response to irritants7. There is evidence from animal models and in vitro studies that air pollutants activate sensory Transient Receptor Potential (TRP) channels. Diesel exhaust particulate (DEP) induces cardiovascular adverse effects via activation of TRP Vanilloid 1 (TRPV-1) in rats6. Furthermore, TRPV-1 stimulation in rats by inhalation of concentrated ambient particulates causes changes in cardiac rhythm and ECG morphology8. Interactions between DEP and TRP Ankyrin 1 (TRPA-1), which are found in airway C-fiber afferents, was also observed by Robinson et al. in guinea pig and human sensory nerves9. Therefore, the autonomic control of heart activity seems to be influenced by centrally-mediated reflexes via these afferent unmyelinated C-fibers, which are in turn able to be activated by PM. This may be an explanation for the decrease in heart rate variability (HRV) occurring in susceptible individuals after short-term exposures to PM10. This is further supported by our observation that the association between PM exposure and HRV reduction was not detected in patients taking ß-blockers, which regulated their sympathetic activity11. However, direct evidence that this mechanism is operative in vivo in humans has never been provided. The function of TRPV-1 in vivo can be investigated with the cough challenge after inhalation of specific agonists, such as capsaicin12.
The aim of the present study is to test the hypotheses that the activity of TRPV-1 can be modulated by inhalation of endogenous mediators and that changes in the activity of the TRP channel can interfere with the autonomic regulation of cardiac rhythm. To test these hypotheses in a group of healthy volunteers, we firstly evaluated the cough response to capsaicin before and after inhalation of prostaglandin E2 (PGE2) and bradykinin (BK), in order to demonstrate sensitization of the TRPV-1 channel in vivo. Secondly, we assessed HRV upon modulation of TRPV-1 function with PGE2 and BK. In addition, we assessed whether functional polymorphisms (SNPs) of TRPV-1 can modify the response to PGE2 and BK, since capsaicin cough challenge sensitivity in healthy subjects is dependent on multiple TRPV-1 SNPs13. Finally, we explored the molecular mechanism of TRPV-1 channel modulation in vitro in HeLa cells transfected with wild type TRPV-1. Overall, we provided direct evidence that modulation of TRP channel activity by inhaled stimuli affects autonomic regulation of HRV in vivo in humans.
Results
Cough challenge
Seventeen normal volunteers were recruited: 10 men and 7 women, with a median age of 35 (interquartile range, IQR, 29.5–47). All subjects completed the baseline capsaicin cough challenges without side effects. The Leicester Cough Questionnaire (LCQ) score ranged from 20 to 21, indicating that none of the subjects exhibited cough at baseline, since all the values were nearly at the maximum, i.e. 21. Two subjects were excluded from the study because they felt unwell after inhalation of PGE2 (one due to tachycardia, one due to chest tightness).
The baseline capsaicin dose-cough response curve is displayed in Fig. 1. The median C2 to capsaicin was 23 μM (IQR 12.75–30.0 μM) and the median cough sensitivity, which is expressed as the number of coughs after inhalation of 30 μM of capsaicin, was 2.5 (2.0–3.25). No significant differences in cough response were observed between males and females. The first inhalation of PGE2 and BK caused some coughing in a minority of the subjects, while subsequent doses of test mediators were well tolerated without any coughing. The dose-cough responses to CPS after inhalation of PGE2 and BK were shifted to the left (Fig. 1).
Dose-cough response (mean ± SE) to the cough challenge with capsaicin (CPS). Number of volunteers who completed the tests is 15. Values are displayed as mean ± SE. Black diamonds: full baseline dose-cough response; red circles: cough response to CPS after inhalation of 200 μg bradykinin; green triangles: cough response to CPS after inhalation of 100 μg prostaglandin-E2. The line corresponding to 2 coughs indicates the method to calculate the concentration of the agent evoking the two coughs (C2).
Figure 2 shows the effect of modulation of TRPV-1 with inhaled PGE2 (a) and BK (b) in each subject. A consistent increase in the number of coughs in response to 30 μM capsaicin was observed after inhalation of PGE2 (p = 0.0008) as well as inhalation of BK (p = 0.0058) compared with the diluent. Various combinations of four of the six TRPV-1 SNPs with high capsaicin responsiveness (315M, 585I, 469I and 91S) were detected in our subjects (Table 1). There was evidence that the genotype frequencies of two SNPs (rs8065080 and rs222747), deviated from Hardy–Weinberg equilibrium (chi-square test: Χ2 = 5.0127 and 12.992, respectively) while no deviation was detected for rs224534 and rs22274 (chi-square test: X2 = 0.0194 and 0.4833). Lastly, X2 = Not applicable for the remaining two SNPs (rs17633288 and rs9894618). These genetic polymorphisms were unrelated to the modulation of the TRPV-1 channel by PGE2 (rho = 0.389, p = 0.151) and BK (rho = − 0.017, p = 0.951) as determined by Spearman correlation (see e-Fig. 1a and e-Fig. 1b in additional file 1).
Modulation of the activity of TRPV-1 channels. Number of coughs evoked by 30 μM of capsaicin (n°) after inhalation of prostagliandin-E2 (a; n° = 4.20 ± 0.42; p = 0.0008) and bradykinin (b; n° = 3.64 ± 0.37; p = 0.0058), compared with inhalation of diluent (n° = 2.77 ± 0.29). Horizontal bars represent the mean value. Thicker lines in the plot indicate overlap between two subjects. Wilcoxon Signed Rank was used to assess the changes in cough response before and after inhalation of prostagliandin-E2 and bradykinin.
Heart rate variability
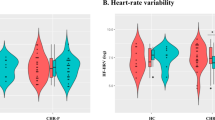
Twelve subjects (4 females and 8 males) completed the HRV experiments after modulation of TRPV-1 with PGE2, and 10 subjects (3 females and 7 males) completed the HRV experiments after modulation of TRPV-1 with BK. The effect of aerosol administration was assessed in 8 subjects in the sessions with PGE2 and in 7 subjects in the sessions with BK, by comparing HRV before and after inhalation of diluent. The selection of these subjects was performed according to the availability of the subjects to participate to the extra session with the aerosol. Therefore there wasn’t any gender orientation for the selection. The normalized low-frequency power (nLF = LF/TP) represents an index of combined sympathetic and vagal modulation14 as well as an index of baroreflex15,16, while the normalized HF (nHF = HF/TP) corresponds to an index of vagal modulation. The low/high-frequency power ratio (LHR = LF/HF) is thus the index of sympathovagal balance. None of the HRV parameters exhibited significant changes after inhalation of diluent or significant differences between males and females (data not shown). Table 2 shows that nHF was significantly reduced after activation of TRP channels by inhaled PGE2, while nLF was significantly increased (compared with inhalation of diluent). Consequently, the sympathovagal balance expressed by the LF/HF ratio was increased in favor of sympathetic tone. The activation of TRP channels with BK shows the same effects on HRV parameters: PGE2 inhalation caused an increase in heart rate (HR), while no significant changes in HR were detected after BK.
Figure 3 shows the individual nHF (a, c) and nLF (b, d) changes compared with diluent. The effect after PGE2 (a, b) and BK (c, d) was determined in n = 12 and n = 10 subjects. Overall, the data demonstrated a consistent behavior among individuals. Genetic polymorphisms of the TRPV-1 channel did not influence the HRV in response to PGE2 and BK (data not shown).
Changes in heart rate variability (HRV) after inhalation of TRP channel modulators,prostagliandin-E2 (a, b) and bradykinin (c,d), compared with inhalation of diluent, were determined in n = 12 and n = 10 subjects. Normalized high frequency power, nHF (a,c) and normalized low frequency power, nLF (b,d) of spectral components in the frequency domain are shown. Horizontal bars represent the mean value. Wilcoxon Signed Rank was used to assess the changes in HRV before and after inhalation of prostagliandin-E2 and bradykinin.
Functionality of TRPV-1 in vitro
We developed a cell-based assay to test the expression and functionality of the TRPV-1 channel. RNA-seq analysis of ENCODE cell lines revealed that most of the members of the TRP family, including TRPV-1, are not expressed in HeLa cells (see e-Table 1 in additional file 1). Therefore, HeLa cells represent the ideal heterologous system to express the TRPV-1 channel.
We wanted to confirm the absence of TRP in HeLa cells at the functional level. As is known, Ca2+ permeable TRP channels generate changes in the intracellular Ca2+ concentration ([Ca2+]) through Ca2+ influx across the plasma membrane17. We took advantage of a widely used genetically-encoded Ca2+ indicator based on the photoprotein aequorin18,19 to dynamically monitor the selective activation and functionality of TRPV-1 in intact cells. We measured intracellular [Ca2+] in response to capsaicin and cinnamaldehyde, which are selective agonists of TRPV-1 and TRPA-1, respectively. As shown in Fig. 4, neither capsaicin nor cinnamaldehyde activate Ca2+ signaling in normal HeLa cells, while histamine stimulation induces a transient increase of intracellular [Ca2+].
Boxplot representing [Ca2+] measurements in control HeLa cells. Where indicated, cells were challenged with 10 μM capsaicin, 10 μM cinnamaldehyde or 100 μM histamine. Variance was calculated by one-way, two-way or three-way ANOVA and multiple comparisons were assessed using the Holm–Sidak post hoc test. In box plots, the boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile.
Indeed, HeLa cells are known to express the histamine H1 receptor which, upon activation, triggers the release of Ca2+ from intracellular stores through the GPCR-PLC-IP3 signaling cascade. Overall, this experiment demonstrates that HeLa cells do not express TRPV-1 and TRPA-1 channels.
Next, we transfected HeLa cells with a plasmid encoding the human TRPV-1 channel with a V5-tag fused at the C-terminal (Fig. 5a). After verifying protein expression by Western blot (Fig. 5b), we measured [Ca2+] in cells expressing heterologous TRPV-1. Figure 5c shows that addition of capsaicin to TRPV-1-expressing HeLa cells triggers an increase in intracellular [Ca2+], as does the positive control histamine. Conversely, cinnamaldehyde is ineffective, causing no significant increase in intracellular [Ca2+]. Finally, we wondered whether TRPV-1 mediates Ca2+ influx from the extracellular milieu in this system as well. To test this, we stimulated TRPV-1-expressing HeLa cells with CPS during the perfusion of a Ca2+-free extracellular buffer. As shown in Fig. 5d, treatment with CPS does not elicit any increase in [Ca2+], nor Ca2+ influx from the extracellular milieu (which would occur if TRPV-1 were present). Conversely, a Ca2+ response is detected with the positive control histamine. Overall, these data confirm that TRPV-1 does not mediate Ca2+ influx from the extracellular milieu, and thus that HeLa cells are an ideal heterologous system to study the function of this channel. Finally, we asked whether the presence of concomitant stimuli could have an impact on TRPV-1 functionality. We thus measured TRPV-1 functionality in cells treated with PGE2, BK or diesel exhaust particulate matter (DEP). For these experiments, we first pre-treated cells with increasing doses of each agent. Neither PGE2 nor BK elicited any increase in [Ca2+] on their own, at any of the concentrations investigated. After 1 h, these pre-treated cells were stimulated with capsaicin, and the [Ca2+] peak was used as readout of TRPV-1 functionality. Treatment with either PGE2 or BK did not modify capsaicin-induced cellular responses (Fig. 6a,b). This suggests that these two inflammatory mediators do not directly sensitize the TRPV-1 channel in our experimental model, possibly because of a lack of a specific transduction pathway. However, treatment with the highest dose of DEP significantly increased TRPV-1-mediated cellular responses (Fig. 6c). These data indicate that TRPV-1 is directly activated by DEP.
(a) Schematic representation of the human TRPV-1 (hsTRPV-1) expressing plasmid. (b) HeLa cells were transfected with control pcDNA3.1 or the TRPV-1-encoding plasmid. Total protein was extracted, separated by SDS-PAGE, and a Western blot was performed by probing the membrane with V5 and GAPDH (loading control) antibodies. (c) Boxplot representing [Ca2+] measurements in HeLa cells expressing TRPV-1. Where indicated, cells were challenged with 10 μM capsaicin, 10 μM cinnamaldehyde or 100 μM histamine. (d) Boxplot representing [Ca2+] measurements in HeLa cells expressing TRPV-1 perfused with a Ca2+-free buffer. Where indicated, cells were challenged with 10 μM capsaicin or 100 μM histamine. Variance was calculated by one-way, two-way or three-way ANOVA and multiple comparisons were assessed using the Holm–Sidak post hoc test. In box plots, the boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile.
(a) Boxplot representing [Ca2+] measurements in HeLa cells expressing TRPV-1 and challenged with 100 nM capsaicin (peak values). Cells were pre-treated with the indicated dose of PGE2 for 1 h. (b) Boxplot representing [Ca2+] measurements in HeLa cells expressing TRPV-1 and challenged with 10 nM capsaicin (peak values). Cells were pre-treated with the indicated dose of bradykinin for 1 h. (c) Boxplot representing [Ca2+] measurements in HeLa cells expressing TRPV-1 and challenged with 10 nM capsaicin (peak values). Cells were pre-treated with the indicated dose of DEP for 1 h. In box plots, the boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 95th and 5th percentiles. Dots represent outlying points. Variance was calculated by one-way, two-way or three-way ANOVA and multiple comparisons were assessed using the Holm–Sidak post hoc test.
Discussion
In this work, we established that TRPV-1 is sensitized by PGE2 and BK inhalation in healthy subjects. The modulation of TRPV-1 activity by these inhaled agents could then influence the autonomic regulation of HRV in our subjects by increasing sympathetic activity and decreasing vagal activity. Based on in vitro experiments, we demonstrated that PGE2 and BK act indirectly on TRPV-1 function. In contrast, TRPV-1 activity is directly increased by stimulation with DEP. These results provide the evidence that airway TRPV-1 is implicated in the autonomic regulation of cardiac rhythm in vivo and suggest that peak pollution may induce acute effects on cardiovascular function by a neurogenic mechanism.
The evidence that airway TRPV-1 is sensitized in vivo was obtained using the cough response to capsaicin, which is a specific ligand of TRPV-1. This was done following pre-treatment with one of two independent inhaled stimuli, PGE2 or BK, administered at doses that were unable to induce cough in normal subjects. The increase in cough response following capsaicin exposure was consistent among individuals and between sensitizing agents. These results build upon those of Choudry et al.20 in which 6 normal volunteers were treated using PGE2 or BK, albeit at does that were 3–fourfold higher than in our study. These doses caused coughing in all 6 subjects on their own, but the authors were able to demonstrate a significant effect of PGE2, but not of BK, on capsaicin-induced coughing.
In addition to TRPV-1, PGE2 and BK were shown to also activate the TRP Ankyrin 1 (TRPA-1) channel in isolated guinea pig sensory nerves21,22. TRPA-1 is a target of a broad group of exogenous stimuli including environmental irritants23. Based on in vitro and animal models, PGE2 and BK are reported to be sensitizers for both TRPV-1 and TRPA-1. The function of TRPA-1 can be assessed in vivo in humans with the cough challenge using inhaled cinnamaldehyde (CNM) as a specific agonist24. However, in our healthy volunteers the cough induced by CNM was milder than that induced by CPS, and the dose-cough response to CNM was almost flat. In addition, TRPA-1 was not consistently sensitized by inhaled PGE2 or BK, as no significant effect was seen after BK and the signal after PGE2 administration was weak (data not shown). Thus, the current study provided evidence of TRPV-1 sensitization after inhalation of these two endogenous mediators in humans in vivo.
Some agonists are thought to be able to both activate and sensitize TRPV-1 channels, but it is difficult to distinguish whether a particular stimulus acts as a direct opener or rather a sensitizer, lowering the activation threshold for another stimulus25. PGE2 and BK bind to specific G Protein-Coupled Receptors (GPCRs) on the cell membrane (EP3 and B2 receptors respectively) and initiate an intracellular signaling cascade. It has been shown that PGE2 activates guinea pig, mouse and human sensory nerves in vitro and causes coughing in animal models via EP3 receptor activation26. In guinea pigs, BK activates sensory nerves and elicits coughing via activation of B2 receptors21. TRPV-1 is also thought to play a role in some GPCR signaling pathways, for example those implicated in BK and PGE2 activity. Indeed, TRPV-1-selective antagonists partially inhibit the tussive response to PGE2 and BK in a guinea pig cough model21. The pathways downstream of GPCR coupling that lead to either sensitization or activation of TRPV-1 have not been fully elucidated, but probably involve the production of diacylglycerol (DAG) and the activation of phosphokinase C (PKC), as DAG and PKC have been found to directly bind the TRPV-1 channel27. It was unknown whether TRPV-1 can also be activated by PGE2 and BK in the absence of the above GPCR-mediated pathways. Our experiments in vitro demonstrated that PGE2 and BK are unable to open functional TRPV-1 channels expressed by HeLa cells, which lack EP3 and B2 receptors. Therefore, these endogenous mediators could be considered to be indirect sensitizers of TRPV-1. This may be one of the reasons why the effect of PGE2 and BK on the channel is irrespective of the presence of TRPV-1 SNPs. In contrast, particulate matter in air pollution, such as DEP, directly interacts with TRPV-1 and causes channel opening. The DEP molecular components responsible for the effect on TRPV-1 have not yet been determined. While the soluble organic material embedded on the particulate carbon core of DEP is a candidate for TRPA-1 activation9, TRPV-1 was also shown to be activated by the solid components of coal fly ash particulate matter28. The size of the DEPs studied were in the range of the respirable fraction which is expected to deposit in the respiratory tract when inhaled in vivo, and which also corresponds to the particle size that is most effective in TRP channel activation in vitro29. It is difficult to establish whether the amount of DEP used for the in vitro experiments is comparable to that which is inhaled in an area with poor air quality due to vehicle traffic. However, the concentrations of DEP used are similar to those used in previous in vitro cell stimulation investigations9,28,30.
Activation of airway sensory nerves by air pollutants in animal models causes an imbalance of autonomic efferent pathways that are mediated centrally at the level of the mid-brain. This produces cardiovascular function changes including an increase in systolic blood pressure, alteration of cardiac rhythm and alteration of electrocardiogram morphology6,8,31. To date there are no studies on the functional changes of TRPV-1 after DEP stimulation in humans. There is in humans, a short term experimental exposure of healthy subjects to diesel exhaust fumes that causes impairment of HRV occurring at the end of the 2 h exposure session32. Although these findings provide some insights into the interaction between inhaled pollutants and acute cardiovascular effects in man, the mechanism remains undetermined. We have observed that an increase in sympathetic activity can be induced by stimuli that are also able to sensitize airway TRPV-1. This provides evidence that signals from airway sensory nerves, once they are integrated at the CNS level, can influence autonomic drive to the heart, as was previously shown in animal models6,8,31. Posture and breathing may also influence HRV, but we can reasonably exclude any confounding effect of these variables. During experiments, ECG recording was performed at rest in a supine position. Respiratory frequency was not controlled but is very unlikely to have been out of the HF band, i.e. slow breathing < 0.15 Hz (9 breath/min) or fast breathing > 0.4 Hz (24 breath/min)33. The normalization of the LF and HF data by TP minimized the effect of changes in heart rate on spectral components of HRV34. In addition, the changes in HRV after TRPV-1 sensitization appear independent on the changes in HR, since they were observed after inhalation of BK which, in contrast to PGE2, does not induce tachycardia. Sensitization of TRPV-1 was also associated with an increase in sympathetic activity and a concomitant reduction of parasympathetic drive. Therefore, these changes in autonomic activity may be a common pathway leading to increased morbidity and mortality from various conditions, including cardiovascular disease. Indeed, substantial evidence exists to support the notion that decreased HRV precedes the development of a number of cardiovascular disease risk factors35.
Our findings identified a mechanism, which is functional in vivo in humans, by which sensitization of airway sensory TRPV-1 channels by inhaled agents induces an increase in sympathetic activity. Since there is evidence that air pollutants can activate TRP channels, we provide a proof of concept that may explain why exposure to peaks of pollutants is associated with short-term cardiovascular adverse events.
Methods
Subjects
Seventeen healthy volunteers were recruited. An approximately equal number of males and females were included. All patients were required to be non-smokers, free of acute or chronic diseases according to medical history and physical examinations, and not taking any regular medication. The study was approved by the local Research Ethics Committee (4057/AO/17) and all subjects gave written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Study design
Leicester Cough Questionnaires (LCQ) were administered before the cough challenges and the scores were evaluated in order to exclude subjects with cough symptoms36. The subjects were required to abstain from ingesting spicy food, garlic, cinnamon and from carrying out outdoor sporting activities within 24 h prior to the tests.
Subjects attended the laboratory on six separate occasions (V1–V6). Baseline cough responses to increasing dilutions of inhaled CPS were assessed (V1). Endogenous modulators of TRPV-1 (PGE2 and BK) or diluent were inhaled in a random order, double-blind fashion, and the cough challenge with CPS was repeated immediately after the last breath of the test modulator (V2-V4). HRV was assessed in each subject after diluent and PGE2 or diluent and BK, which were inhaled at the same concentration and in the same way as for the cough challenge experiments (V5-V6). The experiments were conducted at intervals of at least 5 days.
The presence of six nonsynonymous functional polymorphisms of TRPV-1 (K2N rs9894618, P91S rs222749, I315M rs222747, T469I rs224534, T505A rs17633288 and I585V rs8065080) was assessed in blood DNA samples collected from each subject at V1.
In order to determine whether PGE2 and BK have a direct effect on TRPV-1 activity, functional studies on HeLa cells transfected with the wild type TRPV-1 channel were performed in vitro using capsaicin as an agonist.
CPS cough challenge
The cough challenge was performed according to current guidelines12. Briefly, single vital-capacity breaths of incremental concentrations of CPS (1–1,000 µM; Sigma-Aldrich S.r.l., Milan, IT) were inhaled at 2-min intervals from a nebulizer controlled by a dosimeter (Mefar MB3 CE, Mefar S.p.A, Brescia, Italy). Inhalation flow was limited to approximately 0.5 L/s by applying a critical orifice to the inlet of the nebulizer. Each concentration of the tussive agent was inhaled four times with a 30-s pause between each inhalation. Both the subjects and the investigator were blind to the concentrations of the tussive agent. The number of coughs in the 15-s following each inhalation was recorded using Digital Audio Tape recorder and counted. The average number of coughs after each concentration of the tussive agent was calculated and a concentration response curve for each test was constructed. The concentration of the agent evoking at least two coughs (C2) was determined.
Modulation of TRPV-1
PGE2 and BK were used as modulators of TRPV-1 since there is evidence that they activate the TRP channel expressed by human sensory nerves in vitro21. The PGE2 stock solution was prepared by diluting 10 mg of PGE2 (Tocris Bioscience, Bristol, U.K.) with 1 mL dehydrated alcohol. The resulting 10 mg/mL solution was aliquoted into 0.1 mL single-use vials and stored at − 70 °C. The BK (Sigma-Aldrich S.r.l., Milan, Italy) stock solution was prepared by diluting 20 mg of BK in 1 mL of 10% ethanol in 0.9% NaCl. The resulting 20 mg/mL solution was aliquoted into 0.1 mL single-use vials and stored at − 70 °C. Prior to use the stock solutions were diluted with 0.8 mL 0.9% NaCl solution. The solutions of diluent, PGE2 and BK were inhaled via a De Vilbiss 646 nebulizer connected to a dosimeter (Mefar MB3) with an output of 18 μl per breath. The subjects inhaled five breaths of each solution resulting in a total administration of 100 μg of PGE2 and 200 μg of BK. These doses were chosen since they exhibited biological effects in humans by inhalation and can be safely delivered by aerosol37,38.
Assessment of HRV
HRV was assessed by ECG obtained in a supine position and using a device connected to the patient via two electrodes. HRV data were acquired by a Bluetooth acquisition system (BT16 Plus, FM, Monza, Italy). ECG was recorded at rest for at least 5 min. Then, each subject inhaled 5 breaths of nebulized diluent from a dosimeter and ECG was recorded for at least 5 min. Subsequently, the subject inhaled 5 breaths of PGE2 or BK, in random order and on different days, at the same concentrations used in the modulation of cough response.
HRV was analyzed using Kubios HRV software (ver. 3.3)39. Normal and aberrant complexes were identified and all of the adjacent intervals between normal beats over 5 min intervals were considered. We analyzed the spectral components (HRV frequency domain variables) as the absolute values of power (ms2)18. Power spectral density was analyzed by Fast Fourier Transform. The main spectral components were very low frequency (VLF), low frequency (LF), high-frequency (HF) and the LF/HF ratio. The area under the curve of the spectral peaks within the frequencies 0.01–0.4, 0.01–0.04, 0.04–0.15, and 0.15–0.40 Hz were defined as the total power (TP), very low-frequency power (VLF), low-frequency power (LF), and high-frequency power (HF), respectively. In order to normalize LF and HF, we used the total power within the frequency range of 0.01–0.4 Hz.
Genotyping of TRPV-1 SNPs
Six nonsynonymous functional polymorphisms of TRPV-1 (K2N rs9894618, P91S rs222749, I315M rs222747, T469I rs224534, T505A rs17633288 and I585V rs8065080) were evaluated in DNA extracted from blood samples as previously described13. Briefly, genotyping was performed by commercially available KASP Assay mix (containing the target specific primers) and KASP Master mix. Reactions were set up according to the manufacturer's protocol. As previously described13, samples were run in triplicate on a Steponeplus Real-Time instrument (Applied Biosystems, Foster City, CA) and amplification conditions were equivalent (first step at 94 °C for 15 min, then 10 cycles of 94 °C for 20 s and 61–55° for 60 s (dropping by 0.6 °C per cycle), 26 cycles of 94 °C for 20 s and 55 °C for 60 s). Allelic discrimination was performed using the SDS software v2.3 (Applied Biosystems).
Chemicals, cell culture and transfection
As previously described40, all chemicals were purchased from Sigma-Aldrich, unless otherwise specified. All the experiments were performed in HeLa cells (ATCC Number: CCL-2) cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco #41966052, Thermo Fisher Scientific, Milan, Italy), supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific), containing penicillin (100 U/ml) and streptomycin (100 μg/ml) (Euroclone). Prior to experimentation, cells were seeded onto 13-mm diameter glass coverslips (no. 1.5) and allowed to grow to 50% confluence before transfection. Transfection was performed with a standard Ca2+-phosphate procedure. The TRPV-1-expression plasmid was a kind gift from Christopher A. Reilly40, and the mock vector pcDNA3.1 (Thermo Fisher Scientific) was used as control. Diesel exhaust particulate DEP (Diesel particulate matter, Standard Reference Material 2975) was purchased from the National Institute of Standards and Technology, Boulder, CO, USA. The particle size distribution of DEP suspensions was characterized using a laser analyzer (DIPA 2000, Donner Technologies Ltd, Or Aquiva, Israel) before and after sonication for 10 min. The median diameter of the particles was 0.9 μm (range 0.2–6 μm) and the diameter distribution did not change after sonication, indicating that the particles did not aggregate in the experimental conditions.
[Ca2+]mt measurements
HeLa cells were grown on 13 mm diameter round glass coverslips to 50% confluence and cotransfected with a cytosolic aequorin-based probe (cytAEQ) together with the indicated plasmids. Thirty-six or forty-eight hours after transfection, cells were incubated with 5 μM coelenterazine for 1–2 h in KRB (Krebs–Ringer modified buffer: 125 mM NaCl, 5 mM KCl, 1 mM Na3PO4, 1 mM MgSO4, 5.5 mM glucose, 20 mM HEPES, pH 7.4) at 37 °C supplemented with 1 mM CaCl2, and then transferred to the perfusion chamber. All aequorin measurements were carried out in KRB. For experiments performed in Ca2+-free conditions, KRB was supplemented with 100 μM EGTA. Agonists and other drugs were added as specified in the text. The experiments were terminated by lysing cells with 100 μM digitonin in a hypotonic Ca2+-rich solution (10 mM CaCl2 in H2O), thus discharging the remaining aequorin pool. The light signal was collected and calibrated into [Ca2+] values by an algorithm based on the Ca2+ response curve of aequorin at physiological conditions of pH, [Mg2+], and ionic strength, as previously described41. Alternatively, [Ca2+] measurements were carried out on a PerkinElmer Envision plate reader equipped with a two-injector unit. Cells were transfected as described above in 24-well plates and then re-plated into 96-well plates (1:5 dilution) the day before the experiment. After reconstitution with 5 μM coelenterazine, cells were placed in 70 μl of KRB and the luminescence from each well was measured for 1 min. During the experiment, histamine was first injected at the desired concentration to activate calcium transients, and then a hypotonic, Ca2+-rich, digitonin-containing solution was added to discharge the remaining aequorin pool. Output data were analyzed and calibrated with a custom made macro-enabled Excel workbook. Functional TRPV-1 data were representative of at least three independent transfections.
Statistical analysis
Nonparametric statistics were used to assess the changes in cough response to capsaicin after TRPV-1 stimulation with PGE2 and BK (Wilcoxon Signed Rank). Wilcoxon Signed Rank was also used to test the difference in ECG parameters between diluent, and PGE2 or BK. Variance was calculated by one-way, two-way or three-way ANOVA as indicated in the legends, and multiple comparisons were assessed using the Holm-Sidak post hoc test.
The relationship of the genetic polymorphisms with the modulation of the TRPV-1 channel by PGE2 and BK was determined by Spearman correlation. The statistical analyses were performed using StatView, Minitab and SigmaPlot. A p value of < 0.05 was considered statistically significant.
Ethics approval and consent to participate
The study was approved by the local Research Ethics Committee (4057/AO/17) and all subjects gave written informed consent.
Data availability
All data is available upon request.
Abbreviations
- BK:
-
Bradykinin
- CPS:
-
Capsaicin
- C2:
-
The concentration of capsaicin causing 2 coughs
- DEP:
-
Diesel exhaust particulate
- HRV:
-
Heart rate variability
- LCQ:
-
Leicester Cough Questionnaire
- nHF:
-
High normalized frequency components
- nLF:
-
Low normalized frequency components
- SNPs:
-
Single nucleotide polymorphisms
- PGE2:
-
Prostaglandin-E2
- TRPA-1:
-
Transient receptor potential ankyrin 1
- TRPV-1:
-
Transient receptor potential vanilloid-1
References
Pope, C. A. 3rd. & Dockery, D. W. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manag. Assoc. 56(6), 709–742. https://doi.org/10.1080/10473289.2006.10464485 (2006).
Sun, Q., Hong, X. & Wold, L. E. Cardiovascular effects of ambient particulate air pollution exposure. Circulation 121, 2755–2765. https://doi.org/10.1161/CIRCULATIONAHA.109.893461 (2010).
Peters, A. et al. Exposure to traffic and the onset of myocardial infarction. N. Engl. J. Med. 351(17), 1721–1730. https://doi.org/10.1016/j.accreview.2004.12.026 (2004).
Folino, F. et al. Association between air pollution and ventricular arrhythmias in high-risk patients (ARIA study): a multicentre longitudinal study. Lancet Planet Health 1(2), e58–e64. https://doi.org/10.1016/S2542-5196(17)30020-7 (2017).
Scapellato, M. L. & Lotti, M. Short-term effects of particulate matter: An inflammatory mechanism?. Crit. Rev. Toxicol. 37(6), 461–487. https://doi.org/10.1080/10408440701385622 (2007).
Robertson, S. et al. Pulmonary diesel particulate increases susceptibility to myocardial ischemia/reperfusion injury via activation of sensory TRPV-1 and beta1 adrenoreceptors. Part Fibre Toxicol. 11, 1–12. https://doi.org/10.1186/1743-8977-11-12 (2014).
Lee, L. Y. & Widdicombe, J. G. Modulation of airway sensitivity to inhaled irritants: role of inflammatory mediators. Environ. Health Perspect. 109, 585–589. https://doi.org/10.1289/ehp.01109s4585 (2001).
Ghelfi, E., Rhoden, C. R., Wellenius, G. A., Lawrence, J. & Gonzalez-Flecha, B. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol. Sci. 102(2), 328–336. https://doi.org/10.1093/toxsci/kfn005 (2008).
Robinson, R. K. et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J. Allergy Clin. Immunol. 141(3), 1074–1084. https://doi.org/10.1016/j.jaci.2017.04.038 (2018).
Holguin, F. et al. Air pollution and heart rate variability among the elderly in Mexico City. Epidemiology. 14(5), 521–527. https://doi.org/10.1097/01.ede.0000081999.15060.ae (2003).
Folino, A. F. et al. Individual exposure to particulate matter and the short-term arrhythmic and autonomic profiles in patients with myocardial infarction. Eur. Heart J. 30(13), 1614–1620. https://doi.org/10.1093/eurheartj/ehp136 (2009).
Morice, A. H. et al. ERS guidelines on the assessment of cough. Eur Respir. J. 29(6), 1256–1276. https://doi.org/10.1183/09031936.00101006 (2007).
Liviero, F. et al. Multiple single nucleotide polymorphisms of the transient receptor potential vanilloid 1 (TRPV-1) genes associate with cough sensitivity to capsaicin in healthy subjects. Pulm. Pharmacol. Ther. 61, 1–5. https://doi.org/10.1016/j.pupt.2020.101889 (2020).
Koizumi, K., Terui, N. & Kollai, M. Effect of cardiac vagal and sympathetic nerve activity on heart rate in rhythmic fluctuations. J. Auton. Nerv. Syst. 12(2–3), 251–259. https://doi.org/10.1016/0165-1838(85)90065-7 (1985).
Moak, J. P. et al. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 4(12), 1523–1529. https://doi.org/10.1016/j.hrthm.2007.07.019 (2007).
Rahman, F., Pechnik, S., Gross, D., Sewell, L. & Goldstein, D. S. Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin. Auton. Res. 21(3), 133–141. https://doi.org/10.1007/s10286-010-0098-y (2011).
Gees, M., Colsoul, B. & Nilius, B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2(10), a003962. https://doi.org/10.1101/cshperspect.a003962 (2010).
Rizzuto, R., Simpson, A. W., Brini, M. & Pozzan, T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358(6384), 325–327. https://doi.org/10.1038/358325a0 (1992).
Shimomura, O., Johnson, F. H. & Saiga, Y. Microdetermination of calcium by aequorin luminescence. Science 140(3573), 1339–1340. https://doi.org/10.1126/science.140.3573.1339 (1963).
Choudry, N. B., Fuller, R. W. & Pride, N. B. Sensitivity of the human cough reflex: effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am. Rev. Respir. Dis. 140(1), 137–141. https://doi.org/10.1164/ajrccm/140.1.137 (1989).
Grace, M., Birrell, M. A., Dubuis, E., Maher, S. A. & Belvisi, M. G. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 67(10), 891–900. https://doi.org/10.1136/thoraxjnl-2011-201443 (2012).
Bonvini, S. J. & Belvisi, M. G. Cough and airway disease: the role of ion channels. Pulm. Pharmacol. Ther. 47, 21–28. https://doi.org/10.1016/j.pupt.2017.06.009 (2017).
Geppetti, P., Patacchini, R. & Nassini, R. Transient receptor potential channels and occupational exposure. Curr. Opin. Allergy Clin. Immunol. 14(2), 77–83. https://doi.org/10.1097/aci.0000000000000040 (2014).
Birrell, M. A. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am. J. Respir. Crit. Care Med. 180(11), 1042–1047. https://doi.org/10.1164/rccm.200905-0665OC (2009).
Grace, M. S., Baxter, M., Dubuis, E., Birrell, M. A. & Belvisi, M. G. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br. J. Pharmacol. 171(10), 2593–2607. https://doi.org/10.1111/bph.12538 (2014).
Maher, S. A., Birrell, M. A. & Belvisi, M. G. Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am. J. Respir. Crit. Care Med. 180(10), 923–928. https://doi.org/10.1164/rccm.200903-0388OC (2009).
Grace, M. S., Dubuis, E., Birrell, M. A. & Belvisi, M. G. Pre-clinical studies in cough research: role of transient receptor potential (TRP) channels. Pulm. Pharmacol. Ther. 26(5), 498–507. https://doi.org/10.1016/j.pupt.2013.02.007 (2013).
Deering-Rice, C. E. et al. Transient receptor potential vanilloid-1 (TRPV-1) is a mediator of lung toxicity for coal fly ash particulate material. Mol. Pharmacol. 81(3), 411–419. https://doi.org/10.1124/mol.111.076067 (2012).
Shapiro, D. et al. Activation of transient receptor potential ankyrin-1 (TRPA1) in lung cells by wood smoke particulate material. Chem. Res. Toxicol. 26(5), 750–758. https://doi.org/10.1021/tx400024h (2013).
Li, J. et al. TRPV4-mediated calcium influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environ. Health Perspect. 119(6), 784–793. https://doi.org/10.1289/ehp.1002807 (2011).
Hazari, M. S. et al. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ. Health Perspect. 119(7), 951–957. https://doi.org/10.1289/ehp.1003200 (2011).
Tousoulis, D. et al. Acute exposure to diesel affects inflammation and vascular function. Eur. J. Prev. Cardiol. https://doi.org/10.1177/2047487319898020 (2020).
Hayano, J. & Yuda, E. Pitfalls of assessment of autonomic function by heart rate variability. J. Physiol. Anthropol. 38, 1–8. https://doi.org/10.1186/s40101-019-0193-2 (2020).
Malik, M. et al. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European Society of cardiology and the North American Society of pacing and electrophysiology. Circulation 93(5), 1043–1065 (1996).
Thayer, J. F., Yamamoto, S. S. & Brosschot, J. F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 141(2), 122–131. https://doi.org/10.1016/j.ijcard.2009.09.543 (2010).
Birring, S. S. et al. The Leicester cough monitor: preliminary validation of an automated cough detection system in chronic cough. Eur. Respir. J. 31(5), 1013–1018. https://doi.org/10.1136/thorax.58.4.339 (2008).
Hartert, T. V. et al. Prostaglandin E(2) decreases allergen-stimulated release of prostaglandin D(2) in airways of subjects with asthma. Am. J. Respir. Crit. Care Med. 162, 637–640. https://doi.org/10.1164/ajrccm.162.2.9904038 (2000).
Kharitonov, S. A., Sapienza, M. M., Chung, K. F. & Barnes, P. J. Prostaglandins mediate bradykinin-induced reduction of exhaled nitric oxide in asthma. Eur. Respir. J. 14(5), 1023–1027. https://doi.org/10.1183/09031936.99.14510239 (1999).
Tarvainen, M. P., Niskanen, J. P., Lipponen, J. A., Ranta-Aho, P. O. & Karjalainen, P. A. Kubios HRV-heart rate variability analysis software. Comput. Methods Programs Biomed. 113(1), 210–220. https://doi.org/10.1016/j.cmpb.2013.07.024 (2014).
Reilly, C. A. et al. Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicol. Sci. 73(1), 170–181. https://doi.org/10.1093/toxsci/kfg044 (2003).
Granatiero, V., Patron, M., Tosatto, A., Merli, G. & Rizzuto, R. Using targeted variants of aequorin to measure Ca2+ levels in intracellular organelles. Cold. Spring Harb. Protoc. 1, 86–93. https://doi.org/10.1101/pdb.prot072843 (2014).
Acknowledgements
The authors thank Prof. Marcello Lotti from the University of Padova for his contribution to the conception and design of the study, as well as the delineation of hypotheses. The authors thank also Prof. Massimo Corradi and Dr. Matteo Goldoni, from the University of Parma, Italy, for their help in the characterization of DEP as well as Lauren Emily Wright Ph.D., for English language assistance and Anna Bordin B.Sc., for technical assistance.
Funding
The study was funded by Grant BIRD167128/16 from the Department of Cardiac, Thoracic, Vascular Sciences and Public Health, University of Padova, Italy.
Author information
Authors and Affiliations
Contributions
F.L. contributed to the acquisition, analysis, and/or interpretation of data; wrote the article; and had substantial involvement in its revision prior to submission. M.C.S. contributed to the characterization of the in vitro model; analyzed, and/or interpreted data. D.D.S. contributed to the characterization of the in vitro model; analyzed, and/or interpreted data; wrote the article; and had substantial involvement in its revision prior to submission. F.F. contributed to the conception and design of the study, as well as the delineation of hypotheses; interpreted data, wrote the article and had substantial involvement in its revision prior to submission. M.C. contributed to the genotyping of the subjects; analyzed, and/or interpreted data. P.M. contributed to the acquisition and analysis of data. S.I. contributed to the conception and design of the study, as well as the delineation of hypotheses; to the funding acquisition, and to the supervision of the production. S.P. contributed to the conception and design of the study, as well as the delineation of hypotheses and to the genotyping of the subjects; analyzed and interpreted data; wrote the article and had substantial involvement in its revision prior to submission. P.M. supervised the production of the entire manuscript and contributed to the conception and design of the study, as well as the delineation of hypotheses; acquired, analyzed, and interpreted data; wrote the article; and had substantial involvement in its revision prior to submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liviero, F., Scarpa, M.C., De Stefani, D. et al. Modulation of TRPV-1 by prostaglandin-E2 and bradykinin changes cough sensitivity and autonomic regulation of cardiac rhythm in healthy subjects. Sci Rep 10, 15163 (2020). https://doi.org/10.1038/s41598-020-72062-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72062-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.