Abstract
Plants of the genus Eucalyptus, cultivated in many countries, have great importance for the world economy. In Brazil, this culture occupies a total of 5.7 million hectares, but native and exotic insect pests can reduce its productivity. Thaumastocoris peregrinus Carpintero & Dellapé (Hemiptera: Thaumastocoridae), an exotic Australian pest, damages Eucalyptus plants. Biological control using the egg parasitoid Cleruchoides noackae Lin & Huber (Hymenoptera: Mymaridae), Heteroptera predators and entomopathogenic fungi, such as Beauveria bassiana and Metarhizium anisopliae, have potential for managing T. peregrinus. Chemical insecticides, including bifenthrin and acetamiprid + bifenthrin, also control this insect. The compatibility of chemical and biological control methods favors integrated pest management. The objective of this study was to evaluate the selectivity of commercial products based on B. bassiana, M. anisopliae and the chemical bifenthrin on the parasitoid C. noackae and its parasitism on T. peregrinus eggs. The selectivity test followed the standards recommended by the International Organization for Biological Control (IOBC). Beauveria bassiana has selectivity to parasitism as well as viability, but was slightly harmful to C. noackae adults; M. anisopliae was innocuous to adults and to the viability of the offspring of this parasitoid, but it reduced the parasitism rate; and bifenthrin did not show selectivity to this parasitoid.
Similar content being viewed by others
Introduction
The area of commercially planted forests in the world increased from 167.5 to 277.9 million hectares from 1990 to 20151. Brazil presently has 5.7 million hectares of Eucalyptus plantations with 24%, 17% and 15% of them in the states of Minas Gerais, São Paulo and Mato Grosso do Sul, respectively. The wood from these plantations is mainly destined for the pulp industry, with a production of 21 million tons in 20182,3.
Insect pests of Australian origin detected in planted forests during the last three decades on a global scale may reduce Eucalyptus productivity4. The bronze bug, Thaumastocoris peregrinus Carpintero & Dellapé (Hemiptera: Thaumastocoridae), was first detected in Brazil in 2008 in the states of São Paulo and Rio Grande do Sul, and has since dispersed to other Eucalyptus-producing states5. This insect develops and produces fertile offspring on most Eucalyptus plantations in Brazil6. Thaumastocoris peregrinus perforates and causes silvering, tanning, drying and defoliation from Eucalyptus plants7.
Biological control is the most widely-used method for managing T. peregrinus8. This method includes the egg parasitoid Cleruchoides noackae Lin & Huber (Hymenoptera: Mymaridae), imported from Australia8,9, the predators Atopozelus opsimus Elkins (Hemiptera: Reduviidae)10 and Supputius cincticeps Stäl (Heteroptera: Pentatomidae)11,12 and entomopathogenic fungi13,14. Beauveria bassiana and Metarhizium anisopliae, registered commercially15 and considered to offer reduced risks, are the most studied entomopathogenic fungi16,17. The chemical insecticides bifenthrin and acetamiprid + bifenthrin are also used to control T. peregrinus in Eucalyptus plantations15.
Natural enemies are important in pest control in planted forests, justifying the search for compatible microbial and chemical products18. The mycoinsecticides and chemical insecticides must have selectivity to the pest natural enemies19,20 in order to maintain the effectiveness of the combined use of these methods.
The objective of this study was to evaluate the effect of commercial products based on B. bassiana and M. anisopliae and of the chemical insecticide bifenthrin on the egg parasitoid C. noackae and on its parasitism on T. peregrinus eggs.
Results
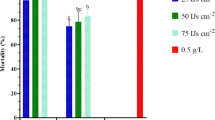
Mortality of Cleruchoides noackae adults
The mortality of C. noackae adults was higher with bifenthrin after the first and tenth hours of exposure to this chemical, with 67% and 90.6%, respectively. Biological products based on B. bassiana and M. anisopliae caused mortality of 40.8% and 22.6%, respectively, of C. noackae adults after 10 h of exposure, higher than the control, distilled and autoclaved water, which was 18% (Fig. 1). Bifenthrin was moderately harmful, B. bassiana was mildly harmful, and M. anisopliae was innocuous to C. noackae adults, presenting a reduction in the beneficial ability of the parasitoid [%E = 100 − (average for each insecticide/average for the percentage in the control treatment) × 100] of 90.60, 40.80 and 22.60, respectively, according to IOBC classification (Table 1).
Parasitism and viability of C. noackae on treated T. peregrinus eggs
Parasitism by C. noackae differed between treatments (ANOVA; F = 4.9259, P = 0.01862), with a lower value for the bifenthrin than in the control, B. bassiana or M. anisopliae. Bifenthrin was moderately harmful (%E = 88.89), B. bassiana innocuous (%E = 2.8), and M. anisopliae slightly deleterious (%E = 36.12) to C. noackae parasitism on treated T. peregrinus eggs. The C. noackae viability ranged from 96 to 100% between treatments, with all the products being classified as harmless (%E = 0 to 5) (Table 2).
Viability of C. noackae in eggs parasitized and treated after one and 10 days of exposure to the insecticides
C. noackae viability in T. peregrinus eggs treated with the insecticides after one day of parasitism differed between treatments (ANOVA; F = 4.301, P = 0.0126), with a lower value for the bifenthrin than in the control and with the product B. bassiana having a value similar to that of M. anisopliae (Table 3). Bifenthrin reduced the viability of this natural enemy on parasitized eggs after 10 days (ANOVA; F = 6.460, P = 0.0018). This chemical was slightly harmful (%E = 30.11 and 34.08), and M. anisopliae (%E = 13.02 and 1.68) and B. bassiana (%E = 4.75 and 4.60) innocuous, after one and 10 days, respectively, for the parasitoid C. noackae (Table 3).
Discussion
The entomopathogenic fungi tested were chosen according to the knowledge and use of these microorganisms in biological control, as well as their reduced environmental impact21. Selectivity tests show the low impact of products on non-target organisms, and allow the recommendation of combinations of mycoinsecticides and chemical insecticides to manage harmful organisms.
The higher mortality of C. noackae adults produced by bifenthrin shows that this chemical is moderately harmful, like most pyrethroids that keep the sodium channels of the neuron membranes open and reach the insect peripheral and central nervous systems22. At the cellular level, these compounds stimulate the neurons to produce repetitive discharges, leading to membrane depolarization and synaptic disorders23. Cyanide pyrethroids cause hypersensitivity, choreoathetosis, tremors, paralysis and insect mortality24,25. The classification of B. bassiana as slightly harmful to C. noackae adults may be related to the production of secondary metabolites, such as organic acids involved in the infection process and linear and cyclic peptidic toxins such as beauvericin from the mycelium of this fungus26,27. The lack of Metarhizium anisopliae toxicity to C. noackae adults is similar to that reported with this fungus on Cotesia flavipes Cameron (Hymenoptera: Braconidae)28 and Trichogramma galloi Zucchi (Hymenoptera: Trichogrammatidae)29. Metarhizium anisopliae is important for biological control because some isolates may be highly specific and others infect a wide host range30. The mortality of C. noackae adults at shorter intervals is due to the reduced longevity of this parasitoid: 0.8 to 1.6 days when they were not fed31 and 3.5 days when they were fed with undiluted honey32, evidencing the importance of the evaluations during the first day of this natural enemy life.
The findings of lower C. noackae parasitism in T. peregrinus eggs treated with bifenthrin agree with reports that this compound is slightly to moderately harmful to Trichogramma chilonis Ishii, Trichogramma ostriniae Pang & Chen, and Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae)33 and harmful to Encarsia formosa Gahan, Encarsia pergandiella Howard (Hymenoptera: Aphelinidae)34, Theocolax elegans Westwood (Hymenoptera: Pteromalidae)35, Eretmocerus mundus Mercet (Hymenoptera: Aphelinidae)36 and Telenomus podisi Ashmead (Hymenoptera: Platygastridae)37, a common impact related to the pyrethroid action mode23. The effect of M. anisopliae, being slightly harmful to C. noackae parasitism on T. peregrinus eggs, differs from that reported for Spalangia cameroni Perkins (Hymenoptera: Pteromalidae), without reduction of its total female reproduction38, and innocuous to Trichogramma pretiosum Riley and T. galloi (Hymenoptera: Trichogrammatidae) parasitism29,39. These differences may be due to the specificity of cyclic peptidic toxins, called destruxins, related to M. anisopliae pathogenicity40. The lack of reduction of parasitism by B. bassiana is similar to that observed with T. pretiosum39, evidencing the selectivity of this fungus to natural enemies. The similar C. noackae viability on T. peregrinus eggs between treatments demonstrates that the products tested are innocuous to the development of this parasitoid in eggs of this host.
The lower C. noackae viability with bifenthrin, sprayed on T. peregrinus eggs at one and 10 days after parasitism, classified as slightly deleterious, differs from the classification of this chemical as having extremely low toxicity for the parasitoids Eretmocerus tejanus Rose & Zolnerowich and E. mundus (Hymenoptera: Aphelinidae), when applied at five and 14 days after parasitism41. This may be related to differences in the host development stage, since C. noackae is protected inside T. peregrinus eggs42, not allowing its direct contact with the insecticide. On the other hand, this differs from the effect on the larval parasitoids E. tejanus and E. mundus41 with a higher exposure to the chemical. The classification of mycoinsecticides as innocuous to C. noackae viability on T. peregrinus eggs parasitized at one and 10 days agrees with that observed for Palmistichus elaeisis Delvare & LaSalle, Tetrastichus howardi Olliff and Trichospilus diatraeae Cherian & Margabandhu (Hymenoptera: Eulophidae)43 and for Telenomus remus Nixon (Hymenoptera: Platygastridae)44, due to the specificity of the entomopathogenic fungi45 without impact on the development of egg parasitoids.
Beauveria bassiana and M. anisopliae, with high selectivity and low impact through contact with adults and in the parasitism and offspring of C. noackae, respectively, have potential for joint use with this parasitoid in pest management programs. However, these mycoinsecticides should be applied 3 days after releasing this parasitoid, avoiding contact with their adults at the time of parasitism. Bifenthrin, the first chemical insecticide registered to control T. peregrinus15, cannot be used with the C. noackae egg parasitoid to manage this pest.
Beauveria bassiana-based mycoinsecticides have selectivity to parasitism and viability and are slightly harmful to C. noackae adults; Metarhizium anisopliae was innocuous to adults and to the viability of this natural enemy offspring, but it reduced C. noackae parasitism on T. peregrinus; bifenthrin did not show selectivity in all bioassays.
Methods
Place of study
The work was carried out at the Laboratory of Biological Control of Forest Pests (LCBPF), Department of Plant Protection, School of Agricultural Sciences, Campus of Botucatu, São Paulo, Brazil, at 25 ± 1 ºC, 70 ± 10% relative humidity and photophase of 12 h.
Rearing Thaumastocoris peregrinus
Thaumastocoris peregrinus adults were collected on two-year-old Eucalyptus grandis × E. urophylla plants at the FCA/UNESP and taken to the laboratory for mass rearing46.
Branches of the hybrid Eucalyptus urophylla var. platyphylla (clone 433) were collected from two-year-old trees, and arranged in bouquets with their bases in 250-ml Erlenmeyer flasks with water on a rectangular plastic tray (40 cm long × 35 cm wide × 8 cm high) to mass rear T. peregrinus in the laboratory. These bouquets were changed every three or four days depending on the need and leaf conditions. On the day of the exchange, the oldest and driest bouquets were placed next to new ones to facilitate the insect migration to the latter46.
Rearing the parasitoid Cleruchoides noackae
Thaumastocoris peregrinus eggs, parasitized by C. noackae, were obtained from the LCBPF. Paper towel strips (1.5 cm wide × 15.0 cm long) were arranged in the upper portion of the leaves of the T. peregrinus breeding bouquets to obtain their eggs. Cleruchoides noackae were multiplied with T. peregrinus eggs with two to three days of age in transparent polystyrene bottles (7.5 cm high × 3.0 cm diameter).
Newly emerged C. noackae adults were transferred with a brush to another transparent polystyrene flask with paper towel strips with two- to three-day-old eggs obtained from the T. peregrinus rearing. Cleruchoides noackae were fed with 50% honey solution in filter paper strips (7.0 cm high × 1.5 cm wide)47.
Selectivity test
The selectivity of mycoinsecticides and the bifenthrin-based insecticide to C. noackae adults and to their parasitism was evaluated in four treatments (Table 4), according to the protocol of the IOBC with the standard test cage48.
One ml per replication of the biological and chemical products was applied in a Potter Tower on the surface of the cages designed according to the standard described by the IOBC and on parasitized or non-parasitized T. peregrinus eggs. Three bioassays were performed.
The first test evaluated the indirect action in the mortality of the parasitoid, exposed by contact to the biological and chemical products, using 100 new individuals per treatment, in five replications of 20 individuals each (per cage). The control had only distilled and autoclaved water. The parasitoids were released in the treated cages and their mortality, after contact with the treated surface, was evaluated.
The second bioassay evaluated the direct action on parasitism and the viability of C. noackae on T. peregrinus eggs treated with the insecticides, with five replications per treatment and 10 eggs, each one offered to a pair of the parasitoids per cage. Paper towel strips with one-day-old T. peregrinus eggs were treated with the insecticides, dried at room temperature and offered to each C. noackae couple for 24 h.
The third bioassay evaluated the C. noackae viability with the products. One-day-old T. peregrinus eggs, exposed to each C. noackae couple for 24 h, were treated in a Potter Tower after one and 10 days post-parasitism, respectively. Five replications with 10 eggs each were used per treatment (Table 4) and age after parasitism (one and 10 days), totaling 400 eggs.
Data evaluation
Mortality, parasitism and viability (%) of C. noackae were evaluated. Mortality of this parasitoid was evaluated after the first hour of contact with the insecticides and then every three hours until completing 10 h, due to its reduced longevity. Parasitism and viability of C. noackae were evaluated after 13 days of parasitism (parasitoid cycle), considering emerged and retained parasitoids and non-parasitized and infertile eggs. The percentage of reduction in parasitoid beneficial ability was calculated for each of the analyzed variables (survival, parasitism and viability; %E) with the equation: %E = [100 − (average for each insecticide/average for the percentage in the control treatment) × 100] to classify the products according to IOBC standards: class 1—innocuous (E < 30%); class 2—slightly deleterious (30 ≤ E ≤ 79%); class 3—moderately harmful (80 ≤ E ≤ 99%); and class 4—harmful (E > 99%)48.
The design was completely randomized, the data submitted to variance analysis and the means compared by the Tukey test at 5% probability using the R Studio software.
References
Payn, T. et al. Changes in planted forests and future global implications. For. Ecol. Manag. 352, 57–67 (2015).
[IBÁ] Industria Brasileira De Árvores. Cenários Ibá Dezembro de 2018. https://www.iba.org/datafiles/e-mail-marketing/cenarios/56-cenarios_2.pdf. Accessed 07 March 2019.
Carvalho, K. H. A., Silva, M. L. & Soares, N. S. Efeito da área e da produtividade na produção de celulose no Brasil. Rev. Árvore 36, 1119–1128. https://doi.org/10.1590/S0100-67622012000600012 (2012).
Paine, T. D., Steinbauer, M. J. & Lawson, S. A. Native and exotic pests of Eucalyptus: A worldwide perspective. Annu. Rev. Entomol. 56, 181–201 (2011).
Wilcken, C. F. et al. Bronze bug Thaumastocoris peregrinus Carpintero & Dellape (Hemiptera: Thaumastocoridae) on Eucalyptus in Brazil and its distribution. J. Plant Prot. Res. 50, 201–205 (2010).
Soliman, E. P. et al. Biology of Thaumastocoris peregrinus in different Eucalyptus species and hybrids. Phytoparasitica 40, 223–230 (2012).
Lima, A. C. V., Wilcken, C. F., Ferreira-Filho, P. J., Serrão, J. E. & Zanuncio, J. C. Intra-plant spatial distribution of Thaumastocoris peregrinus Carpintero & Dellapé (Hemiptera: Thaumastocoridae) on Eucalyptus grandis plants. Phytoparasitica 44, 411–418. https://doi.org/10.1007/s12600-016-0526-1 (2016).
Nadel, R. L. & Noack, A. E. Current understanding of the biology of Thaumastocoris peregrinus in the quest for a management strategy. Int. J. Pest Manag. 58, 257–266 (2012).
Barbosa, L. R. et al. Development of Cleruchoides noackae, an egg-parasitoid of Thaumastocoris peregrinus, in eggs laid on different substrates, with different ages and post-cold storage. Biocontrol 63, 193–202. https://doi.org/10.1007/s10526-017-9863-3 (2018).
Dias, T. K. R. et al. Predation of Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae) by Atopozelus opsimus (Hemiptera: Reduviidae) in Brazil. ISJ-Invert. Surviv. J. 11, 224–227 (2014).
Souza, G. K. et al. First record of a native heteropteran preying on the introduced Eucalyptus pest, Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae), in Brazil. Fla. Entomol. 95, 517–520 (2012).
Zanuncio, J. C., Tavares, W. D. S., Fernandes, B. V., Wilcken, C. F. & Zanuncio, T. V. Production and use of Heteroptera predators for the biological control of Eucalyptus pests in Brazil. Ekoloji 23, 98–104 (2014).
Lorencetti, G.A.T. et al. Eficiência de Beauveria bassiana Vuill. e Isaria sp. para o controle de Thaumastocoris peregrinus Carpintero & Dellapé (Hemiptera: Thaumastocoridae). Cienc. Florest. 28, 403–411 (2018).
Soliman, E. P. et al. Susceptibility of Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae), a Eucalyptus pest, to entomopathogenic fungi. Sci. Agr. 76, 255–260 (2019).
[Agrofit] Ministério da Agricultura, pecuária e abastecimento Brasil Sistema de Agrotóxicos Fitossanitários. https://agrofit.agricultura.gov.br/agrofit_cons/!ap _produto_form_detalhe_cons?p_id_produto_formulado_tecnico=8304&p_tipo_janela=NEW. Accessed 27 Jan 2019.
Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Techn. 17, 553–596 (2007).
Zimmermann, G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci. Techn. 17, 879–920 (2007).
Feltrin-Campos, E., Ringenberg, R., Carvalho, G.A., Glaeser, D.F. & Oliveira, H.N. Selectivity of insecticides against adult Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) on cassava. J. Agri. Sci.-Camb. 11, 546–552 (2019).
Wei, D. A. I. et al. Selectivity and sublethal effects of some frequently-used biopesticides on the predator Cyrtorhinus lividipennis Reuter (Hemiptera: Miridae). J. Integr. Agr. 18, 124–133 (2019).
Maciel, C. G. et al. Trichoderma spp no biocontrole de Cylindrocladium candelabrum em mudas de Eucalyptus saligna. Rev. Árvore 36, 825–832 (2012).
Lacey, L. A. et al. Insect pathogens as biological control agents: back to the future. J. Invertebr. Pathol. 132, 1–41 (2015).
Scott, J. G. Life and death at the voltage-sensitive sodium channel: evolution in response to insecticide use. Annu. Rev. Entomol. 64, 243–257 (2019).
Dong, K. et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Molec. 50, 1–17 (2014).
Soderlund, D. M. & Bloomquist, J. R. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 34, 77–96 (1989).
Narahashi, T. Neuronal ion channels as the target sites of insecticides. Basic Clin. Pharmacol. 79, 1–14 (1996).
Logrieco, A. et al. Beauvericin production by Fusarium species. Appl. Environ. Microbiol. 64, 3084–3088 (1998).
Hugo, M. C. V., Navarro, S. R., Florido, J. E. B., Sierra, R. T. & Tovar, D. C. Metabolitos y conidios de Beauveria bassiana como control de mosco negro fungoso, bajo condiciones de invernadero. Southwest. Entomol. 43, 691–703 (2018).
Rossoni, C. et al. Metarhizium anisopliae and Beauveria bassiana (Hypocreales: Clavicipitaceae) are compatible with Cotesia flavipes (Hymenoptera: Braconidae). Fla Entomol. 97, 1794–1804 (2014).
Oliveira, H. N., Antigo, M. D. R., Carvalho, G. A., Glaeser, D. F. & Pereira, F. F. Selectivity of insecticides used in the sugar-cane on adults of Trichogramma galloi Zucchi (Hymenoptera: Trichogrammatidae). Biosci. J. 29, 1267–1274 (2013).
Clarkson, J. M. & Charnley, A. K. New insights into the mechanisms of fungal pathogenesis in insects. Trends Microbiol. 4, 197–203 (1996).
Mutitu, E. K. et al. Biology and rearing of Cleruchoides noackae (Hymenoptera: Mymaridae), an egg parasitoid for the biological control of Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae). J. Econ. Entomol. 106, 1979–1985 (2013).
Souza, A. R. D. et al. Longevity of Cleruchoides noackae (Hymenoptera: Mymaridae), an egg parasitoid of Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae), with various honey concentrations and at several temperatures. Fla. Entomol. 99, 33–37 (2016).
Cheng, S. et al. Comparative susceptibility of thirteen selected pesticides to three different insect egg parasitoid Trichogramma species. Ecotox. Environ. Safe. 166, 86–91 (2018).
Jones, W.A., Wolfenbarger, D.A. & Kirk, A.A. Response of adult parasitoids of Bemisia tabaci (Hom.: Aleyrodidae) to leaf residues of selected cotton insecticides. Entomophaga 40, 153–162 (1995).
Oliveira, E. E., Aguiar, R. W. S., Sarmento, R. A., Tuelher, E. S. & Guedes, R. N. C. Seletividade de inseticidas a Theocolax elegans parasitoide de Sitophilus zeamais. Biosci. J. 18, 11–16 (2002).
Fernández, M. D. M. et al. Efficacy of a long-lasting bifenthrin-treated net against horticultural pests and its compatibility with the predatory mite Amblyseius swirskii and the parasitic wasp Eretmocerus mundus. Pest Manag. Sci. 73, 1689–1697 (2017).
Stecca, C. S. et al. Impact of insecticides used in soybean crops to the egg parasitoid Telenomus podisi (Hymenoptera: Platygastridae). Neotrop. Entomol. 47, 281–291 (2018).
Nielsen, C., Skovgård, H. & Steenberg, T. Effect of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) on survival and reproduction of the filth fly parasitoid, Spalangia cameroni (Hymenoptera: Pteromalidae). Environ. Entomol. 34, 133–139 (2005).
Potrich, M. et al. Seletividade de Beauveria bassiana e Metarhizium anisopliae a Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Neotrop. Entomol. 38, 822–826 (2009).
Kershaw, M. J., Moorhouse, E. R., Bateman, R., Reynolds, S. E. & Charnley, A. K. The role of destruxins in the pathogenicity of Metarhizium anisopliae for three species of insect. J. Invertebr. Pathol. 74, 213–223 (1999).
Jones, W. A., Ciomperlik, M. A. & Wolfenbarger, D. A. Lethal and sublethal effects of insecticides on two parasitoids attacking Bemisia argentifolii (Homoptera: Aleyrodidae). Biol. Control 11, 70–76 (1998).
Souza, G. K. et al. Reproductive tract histology of Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae). Ann. Entomol. Soc. Am. 107, 853–857 (2014).
Rossoni, C. et al. Development of Eulophidae (Hymenoptera) parasitoids in Diatraea saccharalis (Lepidoptera: Crambidae) pupae exposed to entomopathogenic fungi. Can. Entomol. 148, 716–723 (2016).
Amaro, J. T. et al. Selectivity of different biological products to the egg parasitoid Telenomus remus (Hymenoptera: Platygastridae). Rev. Bras. Entomol. 62, 195–197 (2018).
Sarwar, M. Biopesticides: an effective and environmental friendly insect-pests inhibitor line of action. Int. J. Eng. Adv. Res. Technol. 1, 10–15 (2015).
Barbosa, L.R. et al. Criação massal do percevejo bronzeado, Thaumastocoris peregrinus: Carpinteiro and Dellapé, 2006 (Hemiptera, Thaumastocoridae). https://ainfo.cnptia.embrapa.br/digital/bitstream/item/145907/1/Criacao-massal-do-percevejo-bronzeado.pdf (2016). Accessed 02 Nov 2018.
Barbosa, L.R. et al. Orientações para a criação massal e liberação em campo de Cleruchoides noackae para controle biológico do percevejo bronzeado do eucalipto. https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1081194/1/LivroTA1393completo.pdf (2017). Accessed 02 Nov 2018.
Hassan, S. A. Guidelines for testing the effects of pesticides on beneficial organisms: Description of test methods. Bull. OILB SROP/IOBC WPRS Bull. 15, 186p (1992).
Acknowledegments
To the Brazilian agencies “Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq)”, “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Finance Code 001)”, “Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG)” and “Programa Cooperativo sobre Proteção Florestal (PROTEF) do Instituto de Pesquisas e Estudos Florestais (IPEF)”. David Michael Miller, a professional editor and proofreader and native English speaking, has reviewed and edited this article for structure, grammar, punctuation, spelling, word choice, and readability.
Author information
Authors and Affiliations
Contributions
M.M.D., L.K.B., S.G.M.V., A.R.S., L.R.B. and C.F.W. designed the research; M.M.D., L.K.B., S.G.M.V. and A.R.S. performed the experiments; M.M.D., L.K.B., S.G.M.V., A.R.S., L.R.B., M.A.S., J.E.S. and J.C.Z. analyzed the data, participated in writing and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Domingues, M.M., Becchi, L.K., Velozo, S.G.M. et al. Selectivity of mycoinsecticides and a pyrethroid to the egg parasitoid Cleruchoides noackae (Hymenoptera: Mymaridae). Sci Rep 10, 14617 (2020). https://doi.org/10.1038/s41598-020-71151-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71151-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.