Abstract
The purposes of this study were to construct a comprehensive nomogram for providing a simple, precise and personalized prediction of incident multidrug-resistant tuberculosis (MDR-TB) after completing pulmonary tuberculosis treatment (CPTBT). A matched case–control study (1:2 ratios) was performed between 2005 and 2018. A multivariable Cox regression analysis was used to evaluate independent predictors of incident MDR-TB after the CPTBT. A comprehensive nomogram was developed based on the multivariable Cox model. Overall, 1, 836 participants were included in this study. We developed and validated a simple-to-use nomogram that predicted the individualized risk of incident MDR-TB by using 10 parameters after the CPTBT. The concordance index of this nomogram was 0.833 [95% confidence interval (CI) 0.807–0.859] and 0.871 (95% CI 0.773–0.969) for the training and validation sets, respectively, which indicated adequate discriminatory power. The calibration curves for the risk of incident MDR-TB showed an optimal agreement between nomogram prediction and actual observation in the training and validation sets, respectively. The high sensitivity and specificity of nomogram was indicated by using a receiver operating characteristic curve analysis. Through this clinic tool, TB control executives could more precisely monitor, estimate and intervene the risk of incident MDR-TB among individuals with CPTBT.
Similar content being viewed by others
Introduction
The prevalence of multidrug-resistant tuberculosis (MDR-TB) is increasing rapidly in the world1. According to the latest indication given by the World Health Organization (WHO), there are about 500,000 new cases of drug-resistant TB (DR-TB) (of which 78% have the MDR-TB) worldwide in 20182. With 66,000 cases of MDR/rifampicin-resistant TB, China has the second highest number of cases of this disease worldwide2. The MDR-TB remains a serious public health issue globally, causing severely social, familial and economic dysfunctions1.
In recent years, the continuous monitoring indicates that some individuals with completing PTB treatment (CPTBT) evolve into the MDR-TB after a definite period of time. According to our investigation, we find that PTB patients’ surveillance and management are insufficient after the treatment was completed. Although several studies have revealed that a number of clinical and environmental factors (such as acquired infections, prior irregular treatment, and inadequate treatment management of TB) may affect the prevalence of MDR-TB in TB patients3,4,5,6, risk factors of incident MDR-TB are not yet fully understood among individuals with CPTBT.
To reduce the morbidity and mortality of MDR-TB, it is urgent that the government and researchers take measures to explore preventive strategies of MDR-TB risk among individuals with CPTBT. Recently investigators have proved the significance of early prediction and assessment on the MDR-TB risk7,8. A white paper on the predictive, preventive and personalized medicine9 suggests that a central component of preventive strategies is the identification of individuals at risk for development of a disease. Although previous studies have established several models based on predicting the outcome of TB infection and showed certain application value10,11, there is currently no model available for the prediction and assessment of MDR-TB risk in individuals with CPTBT.
To date, in the research field of MDR-TB control, though some variables, such as sociodemographic, clinical, and microbiological predictors12,13,14, have been well recognized as determinants of incident MDR-TB in TB patients, few studies focused on the status of incident MDR-TB among individuals with CPTBT, let alone integrated them so as to comprehensively assess a patient’s specific risk of incident MDR-TB. It is now well established from a variety of studies, that the nomogram model is a graphic algorithm tool aimed at providing an approximate computation of a function15. In clinical practice, the nomogram has been identified as a practical tool of preventive interventions16. In addition, a nomogram can predict and estimate the individualized risk of a disease and quantitatively demonstrate a personalized probability for predicting the incidence of disease outcome15.
In the present study, based on a matched case–control study (1:2 ratios), we selected a population with CPTBT as participants and mainly aimed to (a) identify predictors of incident MDR-TB in individuals with CPTBT, hoping to reduce the morbidity and mortality of MDR-TB; and (b) construct a comprehensive nomogram for providing a simple, precise and personalized prediction of incident MDR-TB among individuals with CPTBT.
Materials and methods
Sample size calculation
To calculate the sample size, we used the following formula17:
where \(N\) = sample size; α = alpha (expected significant level, two-tailed test); β = 1 − power (expected power, two-tailed test); Z statistic (Z)—Z statistic for confidence level; r—number of control subjects matched to each case subject; \({p}_{1}\)—probability of exposure in the case group; \({p}_{0}\)—probability of exposure in the control group (\({p}_{0}\) can be estimated as the population prevalence of exposure); OR = odds ratio (odds ratio of exposures between cases and controls; OR can be estimated as the population OR of exposure).
In this study, the investigators present their results with 95% confidence interval (CI), \({Z}_{0.05}\)= 1.96 (α = 0.05), \({Z}_{0.10}\)= 1.64 (β = 0.10), r = 2, \({p}_{0}\)= 8.0%18, and OR = 2.019. In addition, adopting the 'all-comers' design20 and considering the loss of follow-up, participants’ rejection rate, and sampling error, the final sample size was determined to be 1,900 in the training set. The validation set was chosen by using an ‘all comers’ design.
Workflow
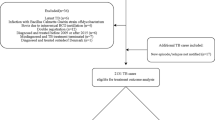
This study workflow was summarized in Fig. 1. Two separate datasets were used to develop and validate a risk-prediction tool based on predictors of incident MDR-TB in individuals with CPTBT. Data of a matched case–control study (1:2 ratios) from January 1, 2005 to December 31, 2018 (n = 1719) were used to derive the risk of MDR-TB among individuals with CPTBT (i.e., a training dataset), while data from the National TB Surveillance System (NTSS) between January 1 and September 30, 2019 (n = 117) was used as an independent dataset to validate the prediction tool (i.e., a validation dataset).
Univariate and multivariate Cox proportional hazards regression models were applied to select optimal risk factors to build a practical instrument for predicting the risk of incident MDR-TB among individuals with CPTBT.
Study design and settings
This study was based on individuals with CPTBT from 2005 to 2019. A matched case–control study (1:2 ratios) was conducted in Hangzhou, China. The subjects with drug resistance detection who were enrolled in the training set between January 1, 2005 and December 31, 2018 constituted the case–control study. Furthermore, participants with drug resistance detection were enrolled in the validation set between January 1 and September 30, 2019 from an ‘all comers’ approach20.
For the present study, the MDR-TB cases were selected from all of TB designated hospitals in Hangzhou City and diagnosed by clinicians through Gene Xpert and traditional drug susceptibility testing (DST)21. The cases were selected in this study using the ‘all comers’ principle20, as long as they met inclusion and exclusion criteria, and the controls were selected by using a random sampling method from the same TB designated hospitals. In this study, the subjects were classified into ‘incident MDR-TB’ (i.e., the case group) and ‘non-incident MDR-TB’ groups (such as the control group) according to the ultimate outcome. The subjects were ultimately selected if they (a) had a history of PTB but did not have MDR-TB confirmed during their previous treatment episodes; (b) were surviving during the study; (c) had a history of TB treatment; (d) had a definite treatment outcome; and (e) could be followed up. The subjects were excluded if (a) they had a history of MDR-TB infection before the present study; (b) no DST results were reported; (c) TB patients were being treated (i.e., patients with an anti-TB drug therapy during the course of study); (d) no treatment outcome could be obtained; (e) subjects who lost or died during the follow-up visit; and (f) the missing data was severe (Fig. 1).
The starting date of previous anti-TB treatment was defined as the starting time of the observation study; while we defined a patient’s observation ending date as the end date of the study, which was the data of incident MDR-TB or December 31, 2018 in the training set or September 30, 2019 in the validation set. Incident MDR-TB for all years were collected between January 1, 2005, and September 30, 2019. In this study, treatment regimens (TRs) were formulated on the basis of patients’ TB history.
Data collection
All data in this retrospective observational study were collected from self-designed standard questionnaires and the NTSS, and were entered in duplicate into an electronic database. A self-designed standard questionnaire was used to collect patients’ sociodemographic data. The NTSS was established in 2005 and used to collect patients’ clinical and laboratory test data in our study. Sociodemographic data included age, gender, areas of residence, a history of direct contact, nationality, family income (FI), occupational risk, education levels, and registered household. Clinical data included mode of TB case finding (MCF), associated with TB at other sites, human immunodeficiency virus (HIV) infection, patients with severe infection, comorbidities, different CPTBT including completing newly diagnosed PTB treatment (CNDT) and completing re-treated PTB treatment (CRT), mode of TB case management (MCM), treatment outcomes of previous PTB, time from illness onset to the first medical visit (TIOFMV) and laboratory confirmation (TIOLC), PTB treatment time, the status of using TRs, and chest radiological findings. Laboratory test data included sputum smear, culture, and DST results at baseline and follow-up visits.
Standard participant reporting included sociodemographic, clinical and microbiological information along with initial and follow-up visits. The sociodemographic, clinical and microbiological data of each participant were collected by trained investigators.
Variables and definitions
The case definitions and classifications used in the present study were consistent with the WHO revised TB definitions and reporting framework22. The main outcome variable was measured as incident MDR-TB or non-incident MDR-TB. Table 1 showed the definitions of this study. The main covariate variables were defined and classified based on the WHO and national guidelines22,23. Sputum smear, culture, and DST results were defined according to the WHO guideline22,24.
Laboratory methods
Traditional laboratory test methods (such as sputum smear and culture) were mainly used for the diagnosis of TB from 2005 to 2014 in the present study25. Moreover, methods of TB diagnosis mainly included the molecular biological detection (e.g., a Gene Xpert method) and traditional laboratory test between 2015 and 201920. A case of MDR-TB was confirmed by using the DST in the TB designated laboratory. The DST was performed on all culture positive isolates against first line [isoniazid (H), rifampicin (R), pyrazinamide (Z), ethambutol (E) and streptomycin (S)] and second line anti-TB drugs (kanamycin and ofloxacin)24. The methods of DST usually include the conventional microbiological DST and Gene Xpert Mycobacterium TB (MTB)/R. The conventional microbiological DST is performed using solid or automated liquid culture media system (BACTEC MGIT 960; Becton Dickinson, Sparks, Maryland, USA) according to standard procedures24. The method of Gene Xpert MTB/R denotes that the resistance to R is detected by using Gene technology24.
The DST detection are performed during the course of follow-up visits. For conventional microbiological DST and Gene Xpert MTB/R detections, samples collected are sent to the TB Program Laboratory of Hangzhou Center for Disease Control and Prevention (a biosafety level-3 laboratory with proficiency testing approved by National Reference Laboratory in China). MDR-TB cases of laboratory cross-contamination are excluded. Drugs with borderline resistance are considered to be resistant.
Statistical analysis
Outcome variable was categorized as a binary variable with incident MDR-TB and non-incident MDR-TB categories. Descriptive analyses were used to examine the distribution of characteristics of participants in the training and validation sets. Continuous variables were described by using mean with standard deviation while categorical data was analyzed by using percent (proportion). A Pearson Chi-square test was used for categorical variables and an independent sample t-test for continuous variables in both training and validation sets.
We used univariable and multivariable Cox regression models to analyze the risk of incident MDR-TB among individuals with CPTBT. Patients who died, loss to follow-up visit and could not be evaluated were excluded from the analysis. Univariable Cox proportional hazard regression analysis was conducted to determine factors associated with incident MDR-TB. Variables were analyzed using hazard ratio (HR) generated by univariable Cox proportional hazards regression.
Subsequently, independent predictors associated with incident MDR-TB were evaluated using HR generated by a multivariable Cox proportional hazard regression model. All variables with P value of ≤ 0.05 were included into a multivariable Cox proportional hazard regression model using backward stepwise method based on the minimum statistics of the Akaike information criterion. Variables with P value of < 0.05 were considered statistically significant in the multivariable Cox proportional hazard regression model and were included in the final predictive model.
Based on the results of multivariate Cox regression analysis in the training set, a nomogram was developed and validated. Nomogram validation included two components. First, the internal validation of clinic nomogram was performed using a concordance index (C-index) by subjecting the nomogram to bootstrapping with 200 resamples26. The predictive accuracy of 1-, 5-, and 10-year probability of incident MDR-TB was evaluated by using the area under receiver operating characteristic (ROC) curve (AUC). Next, the calibration of nomogram was performed by comparing the predicted probability of incident MDR-TB with the observed probability of incident MDR-TB after bias correction (i.e., using a calibration curve). In addition, for external validation, we predicted the risk of incident MDR-TB using data from the other 117 individuals of validation set.
All statistical analyses were performed with R software (version i 386 3.6.1; www.R-project.org, 2019). The multivariable Cox proportional hazard regression model was created using the R software’s ‘survival’ package, while the nomogram and calibration curves were plotted using the ‘rms’ package.
Ethics approval and consent to participate
The study protocol was approved by the Hangzhou Center for Disease Control and Prevention Ethics Committee. Written informed consent was obtained from all participants, or from guardians or parents on behalf of participants under the age of 18 years. In addition, all methods were carried out in accordance with relevant guidelines and regulations.
Results
Characteristics of the subjects
A flow diagram summarizing the identified eligible subjects and the study participants was shown in Fig. 1. Baseline characteristics of the study population were listed in Table 2.
We retrospectively studied 1,836 subjects with CPTBT in Hangzhou from January 1, 2005 to September 30, 2019. Participants in the training set (n = 1719) and the external validation set (n = 117) were analyzed respectively. There was not a significant difference between the two sets (Table 2). The mean age was 48.90 ± 20.95 and 49.41 ± 21.84, and the ratio of males to females was 2.42 to 1 and 3.03 to 1 in the training and validation sets, respectively. Notably, most of the subjects [1, 357 (73.91%)] were with the education level of high school and below (Table 2).
Predictors’ selection
Table 3 summarized the results of the univariate analyses of the association between an individual covariate and the risk of incident MDR-TB among individuals with CPTBT. Twenty of the 44 tested covariates were associated with a high risk of incident MDR-TB from this study population in the training set (P ≤ 0.05). The significant covariates were (a) sociodemographic characteristics, including age < 60 years, a history of direct contact, family income of low level, high-risk occupation, high school and below, and rural areas, (b) clinical characteristics, including passive MCF, HIV infection, CRT, unsuccessful treatment, TIOFMV, FDC-2HRZE/4HR, 2HRZES/6HRE, 3HRZES/6HRE, excellent frequencies of chest X-ray examination (FCXE), duration of pulmonary cavities (DPC), and duration of abnormal X-ray findings, and (c) microbiological characteristics, including frequencies of sputum culture, duration of positive sputum culture (DPSC), and duration of negative sputum culture. The remaining 24 covariates, including gender, nationality, a history of direct contact (e.g., unknown), registered household, associated with TB at other sites, comorbidities, patients with severe infection, MCM, PTB treatment time, TIOLC, 2H3R3Z3/4H3R3, 2H3R3Z3E3/4H3R3, 2HREZ/4H3R3, 2HRZE/4HR, 3HRZE/6HRE, 2H3R3Z3E3S3/6H3R3E3, individualized TRs [i.e., individualized TRs of newly diagnosed PTB patients (NDPPs) and re-treatment PTB patients (RPTPs)], duration of pulmonary miliary tubercles, duration without radiological findings, duration without sputum culture, frequencies of sputum smear, duration of positive sputum smear, duration of negative sputum smear, and duration without sputum smear, were not associated with incident MDR-TB among individuals with CPTBT (P > 0.05).
To further explore independent predictors of incident MDR-TB in individuals with CPTBT, we performed a multivariate Cox proportional hazard regression analysis. Table 4 listed the multivariable Cox regression results for this study population. The analysis showed that less than 60 years, a history of direct contact, passive MCF, HIV infection, CRT, unsuccessful treatment, excellent FCXE, 3HRZES/6HRE, DPC, and DPSC were significantly linked to the MDR-TB risk in the training set (P < 0.05). From this model, we could also see that the unsuccessful treatment (HR 2.72, 95% CI 2.20–3.37, P < 0.001) was one of the strongest predictors for incident MDR-TB in this population (Table 4). These findings were used to create a practical clinical nomogram for predicting the probability of incident MDR-TB among individuals with CPTBT (Fig. 2).
The nomogram for individualized predicting the risk of incident MDR-TB from individuals with CPTBT. MDR-TB: multidrug-resistant tuberculosis; CPTBT: completing pulmonary TB treatment; CNDT: completing newly diagnosed pulmonary TB treatment; CRT: completing re-treated pulmonary TB treatment; HDC: a history of direct contact; MCF: mode of TB case finding; HIVI: human immunodeficiency virus infection; TO: treatment outcome; H: isoniazid; R: rifampicin; Z: pyrazinamide; E: ethambutol; S: streptomycin; FCXE: frequencies of chest X-ray examination; DPC: duration of pulmonary cavities; DPSC: duration of positive sputum culture.
Construction of the nomogram
A nomogram is developed to assess the risk of incident MDR-TB using significant factors from the 1,719 patients’ data in the training set. With 10 independent predictors of training set, it is possible to create a nomogram to predict the probability of incident MDR-TB among individuals with CPTBT (Fig. 2). The top row of the nomogram corresponds to the general score. For each predictor listed on the left (including less than 60 years, a history of direct contact, passive MCF, HIV infection, CRT, unsuccessful treatment, 3HRZES/6HRE, excellent FCXE, DPC, and DPSC), there is a corresponding row on the right indicating possible descriptors. After characterizing the patient for each predictor, a perpendicular line toward the first row should be drawn to identify the value. This action should be performed for all 10 predictors, followed by tallying the final score. This final score should be identified in a total point row and then a perpendicular line is drawn that corresponds to the probability of incident MDR-TB from individuals with CPTBT.
Calibration and validation of the nomogram
After internal validation using the bootstrap technique, the C-index of this nomogram is 0.833 (95% CI 0.807–0.859) and 0.871 (95% CI 0.773–0.969) for the training and validation sets, respectively, which indicates adequate discriminatory power. The calibration plots are also performed separately using the training and external validation sets. As shown in Fig. 3A, the calibration plots show that the predicted 1-, 5-, and 10-year probability of incident MDR-TB corresponded closely with the actual 1-, 5-, and 10-year probability of incident MDR-TB estimated in the training set. Figure 3B illustrates that the nomogram appears well calibrated, and there is a strong correlation between predicted and observed outcomes across the spectrum of predictions in the external validation set.
The calibration curves for predicting the risk of incident MDR-TB from individuals with CPTBT at each time point in the training set (A) and the external validation set (B), respectively. Nomogram predicted the probability of incident MDR-TB from individuals with CPTBT which is plotted on the X-axis and observed the probability of incident MDR-TB from individuals with CPTBT which is plotted on the Y-axis. MDR-TB: multidrug-resistant tuberculosis; CPTBT: completing pulmonary TB treatment.
For the training set, the AUCs of the nomogram predicting the 1-, 5- and 10-year incidence of MDR-TB are 0.904, 0.921, and 0.908, respectively (Fig. 4A). Regarding the external validation set, the AUCs of the nomogram for predicting the 1-, 5- and 10-year incidence of MDR-TB are 0.954, 0.970, and 0.919, respectively (Fig. 4B). As Fig. 4 shows, the nomogram demonstrates the superior prediction ability of incidence of MDR-TB.
Predicting an individual’s MDR-TB risk among individuals with CPTBT
To make it easier to interpret our results, we represented the final reduced model with a nomogram that can be used to calculate a prognostic score and estimate the risk of incident MDR-TB for an individual with CPTBT (Fig. 2). The nomogram produced the following mathematical predictive model for the presence of incident MDR-TB risk in the training set, with h (t, x) denoting the probability of incident MDR-TB among individuals with CPTBT27:
where h (t, x) is the hazard at time t after a defined starting point for an individual with variables x = (x1… xi … xk) is being predicted by h0 (t), the so-called underlying hazard at time t, and the predictor variables x1 to xk (recorded at time zero), each variable xi being multiplied by a corresponding regression coefficient βi. Here, exp stands for exponential function, e.g., exp (βx) = eβx, and the underlying hazard h0 (t) is the hazard at time t of an individual whose xi’s are all zero.
The predicted probabilities associated with each factor are mapped into points on a scale from 0 to 100. The presence or the level of each predictive factor is associated with a point system, allowing summing up the points for all the factors. The total points accumulated by the various covariates correspond to the predicted probability of incident MDR-TB. For example, for an individual with the characteristics of less than 60 years, a history of direct contact, CRT, unsuccessful treatment, excellent FCXE (such as 6 times), 3HRZES/6HRE, DPC (such as 3 months), and DPSC (such as 2 months) among individuals with CPTBT (see Table 5).
Discussion
Up to now, far too little attention has been paid to monitoring and managing the risk of incident MDR-TB among individuals with CPTBT, let alone developed a nomogram so as to comprehensively estimate an individualized risk of incident MDR-TB in individuals with CPTBT. In the present study, we performed a matched case–control study (1: 2 ratios) to explore the predictors of MDR-TB in individuals with CPTBT. According to results of this study, we constructed a comprehensive nomogram for providing a simple, precise and personalized prediction of incident MDR-TB among individuals with CPTBT. Our findings may provide more reliable evidences in developing prevention and control strategies of MDR-TB and guiding TB control executives’ decision-making (e.g., formulate the most effective surveillance, assessment and intervention measures for this population). We anticipate that these results will be useful in reducing the incidence of MDR-TB, the monitoring and management of individuals with CPTBT, and the design of clinical interventions for preventing MDR-TB.
The significance of this study is that it offers a few important features. First, this is the first nomogram for predicting MDR-TB risk in individuals with CPTBT that has collected enough risk factors to allow authentic forecast and assessment analyses. Second, in the validation analyses, whether internal (e.g., the C-index is 0.833 and 0.871 for the training and validation sets, respectively) or external, the comprehensive model outputted both sufficient accuracy and satisfied uniformity in predicting incident MDR-TB. Third, this tool would be easy to use in clinical practice, mainly because the applying of nomogram is very simple, convenient, and economical (i.e., an accurate evaluation is made by just using 10 dominant predictors of incident MDR-TB). Moreover, we observe that the running cost of this model is low, which lies primarily in developing a practice tool (i.e., a risk assessment scale) and incorporating this tool into the treatment information system of TB designated hospital. Fourth, comparing with the logistic regression model (e.g., it may not consider the impact of time effect for predicting the risk of MDR-TB), our study specifically considers estimating the risk of MDR-TB by using a semi-parametric model (i.e., Cox proportional hazard model) to maximize the Wald χ2 statistic.
In the present study, we found that 10 independent predictors were associated with the increased risk of MDR-TB in individuals with CPTBT. Similar results have been described in many previous studies on TB patients3,4,5,6. For example, regarding the unsuccessful treatment, it is still a key predictor for the control of incident MDR-TB. Thus, to monitor and manage the risk of MDR-TB, we not only focused on TB patients, but also concentrated on individuals with CPTBT.
Compared with risk factors of incident MDR-TB in TB cases, there were some different features on predictors of incident MDR-TB among individuals with CPTBT. A notable finding in this study was that a mild association was only found between CRT and incident MDR-TB. This is inconsistent with previous studies3,28, which suggested that re-treatment TB patients was significantly associated with MDR-TB risk. One possible explanation is that the difference is originated from a low susceptibility of drug resistance for re-treatment TB patients after the end of treatment29. To understand the cause further, the causal mechanism needs to be verified. Unlike a study conducted by Zhang et al.30, we observed that gender was not associated with MDR-TB risk. The present study also suggested that older age (≥ 60 years) did not correlate with the risk of MDR-TB. This had important public health implications for younger TB patients. Flora et al.31 reported that HIV infection was not strongly associated with MDR-TB risk. In this study, HIV infection was significantly associated with MDR-TB on the multivariate analysis. According to these data, we can infer that the early prediction and risk assessment of MDR-TB will be crucial among individuals with CPTBT. Resorting to this tool, we can comprehensively predict an individual with CPTBT’s personalized risk of MDR-TB.
In clinical practice, clinicians usually determine whether to treat TB patients according to the clinical, radiological and bacteriological features32,33. The excellent FCXE can early detect the relapse and ensure an immediate treatment in individuals with CPTBT. Thus, the excellent FCXE were advantageous to reducing the risk of MDR-TB among individuals with CPTBT. This finding, while preliminary, suggests that the government should take measures to guarantee the excellent FCXE of individuals with CPTBT in order to contain the epidemic of MDR-TB.
Interestingly, our study identified passive MCF (like physical examination, contact examination, and differential diagnosis of other diseases) as a strong risk factor for incident MDR-TB in individuals with CPTBT. The delayed diagnosis and treatment of TB, as we all know, potentially increased the risk for MDR-TB12. If the TB case finding was delayed, the TB case would develop into a serious TB leading to the course of treatment extended, it might become a risk factor associated with MDR-TB12. Thus, this finding has an important implication that the government should vigorously promote and develop the active finding mode of MDR-TB among individuals with CPTBT. Additionally, a TB control scheme including this nomogram should be formulated by our government.
It is worth mentioning that this study identified the association between 3HRZES/6HRE and the MDR-TB risk among individuals with CPTBT. According to the 2017 WHO guideline34, the category II regimen should no longer be prescribed during the treatment of re-treatment TB patients. Our finding might elucidate a key role of standardized TB treatment against incident MDR-TB and provide a strong evidence for the treatment of RPTPs. From the discussion, one may conclude that the DST should be performed to inform the choice of RPTPs’ TRs. Most notably, this study also observed that RPTPs were treated by using the TRs of 9-month, which were dramatically increased the risk of incident MDR-TB. This association may be attributed to the longer the time of exposure to anti-TB drugs, the greater the chance of occurrence of DR-TB35. To decrease the risk of MDR-TB, it is vital that standardized TRs are implemented by RPTPs.
Some researchers found a highly significant association between the contact with MDR-TB patient and incident MDR-TB6,17,36. Our study also suggested that a history of direct contact was one of the strongest independent predictors for incident MDR-TB in individuals with CPTBT. However, a prospective cohort study in Peru37 found that MDR-TB patients were less able to cause secondary disease in contacts, which might appear to conflict with the result of our study. After considering possible explanations of this discrepancy, our tentative suggestion is that the ethnic characteristic is associated with the risk estimate of MDR-TB38. This result implies that potential intervention measures like early detection of the high-risk population, early isolation and treatment of MDR-TB patient, and personal protective measures of susceptible persons, are urgently needed to curb the epidemic of MDR-TB among individuals with CPTBT.
Besides the novel identified predictors of incident MDR-TB, what the predominant finding in the present study was that we first integrated these existing predictors into an excellent risk prediction tool called nomogram39. According to this practical tool, we can comprehensively predict a personalized risk of incident MDR-TB among individuals with CPTBT.
Most importantly, the best way to interpret and apply these findings is not in terms of how the individual factors contribute to risk but how these parameters can be modified or improved to potentially decrease the incidence of MDR-TB40. Since the pathogenic mechanism of MDR-TB is still unclear, our findings and algorithm should be used to modify identified risk factors of MDR-TB in an effort to minimize morbidity. In terms of our findings, identifying the risk of incident MDR-TB for individuals with CPTBT may have an impact on the treatment, healthcare, surveillance, and management options of TB cases. In addition, the selection of TB patients who need additional treatment, or intensive surveillance and management remains controversial after completing treatment41. This clinic tool may be able to help physicians to solve such problems. Moreover, this nomogram can provide information in the design of clinical intervention, and guiding clinicians’ decision-making regarding the most effective intervention strategies among individuals with CPTBT. For example, according to this algorithm, an individual with CPTBT is found to be high-risk for incident MDR-TB. This finding has an important implication for developing the strategy of early intervention and management in the high-risk population of DR-TB. Overall, our results suggest that this nomogram may display the advanced public health concept of predictive, preventive, and personalized medicine42. This tool deserves to be further explored in future researches of clinic and public health. Considering these advantages of nomogram, our government should guide, support and foster the development of this tool. In addition, a control and prevention proposal including this tool for the risk of MDR-TB should be formulated by the government among individual with CPTBT.
Our study does have some limitations. First, our study is limited by the retrospective nature the data, which could suffer from recall bias and failure to incorporate some recognized prognostic parameters (e.g., the frequency or intensity of exposure). Second, potential confounders such as the mental health status of TB patients, TB drug quality and drug malabsorption could not be controlled. Third, we may not include them if MDR-TB cases did not go to a hospital. However, to reduce enrolment bias, we have retrieved and collected medical records (such as demographic, clinical and bacteriological data) of individuals with CPTBT from the non-local hospitals through the NTSS. Fourth, further efforts regarding prospective data collection and patient follow-up, wider geographic recruitment, and the incorporation of additional factors are encouraged to improve this tool. Despite these limitations, as we know, there are limited numbers of published data on the risk of incident MDR-TB in individuals with CPTBT. Therefore, this study could contribute information about the novel concept of predictive, preventive, and personalized medicine for incident MDR-TB.
Conclusions
So far, unfortunately, we have failed to increase the clinician’s ability to properly predict an individual risk of MDR-TB among individuals with CPTBT. In the present study, we developed and validated a novel tool based on the status of less than 60 years, a history of direct contact, passive MCF, HIV infection, CRT, unsuccessful treatment, 3HRZES/6HRE, excellent FCXE, DPC, and DPSC, which predicted the probability of incident MDR-TB in individuals with CPTBT.
In conclusion, this tool can provide a vital role in counseling individuals with CPTBT and a novel strategy for the prevention and intervention of MDR-TB. In view of the high mortality and medical cost of MDR-TB cases, individuals with CPTBT are in urgent need of the early identifying of at-risk individuals and early intervening before the onset of MDR-TB.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MDR-TB:
-
Multidrug-resistant tuberculosis
- TB:
-
Tuberculosis
- DR-TB:
-
Drug-resistant tuberculosis
- CPTBT:
-
Completing pulmonary TB treatment
- CNDT:
-
Completing newly diagnosed pulmonary TB treatment
- CRT:
-
Completing re-treated pulmonary TB treatment
- NDPPs:
-
Newly diagnosed pulmonary TB patients
- RPTPs:
-
Re-treated pulmonary TB patients
- HDC:
-
A history of direct contact
- FCXE:
-
Frequencies of chest X-ray examination
- DST:
-
Drug susceptibility testing
- TRs:
-
Treatment regimens
- NTSS:
-
National TB surveillance system
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under curve
- HIV:
-
Human immunodeficiency virus
- TO:
-
Treatment outcome
- DPC:
-
Duration of pulmonary cavities
- DPSC:
-
Duration of positive sputum culture
- FDC:
-
Fixed-dose combination
- TIOFMV:
-
Time interval from illness onset to the first medical visit
- TIOLC:
-
Time interval from illness onset to laboratory confirmation
- H:
-
Isoniazid
- R:
-
Rifampicin
- Z:
-
Pyrazinamide
- E:
-
Ethambutol
- S:
-
Streptomycin
- WHO:
-
World Health Organization
- MTB:
-
Mycobacterium tuberculosis
- FMSM:
-
Family members' management or self-management
- CDM:
-
Community doctor management
- MCF:
-
Mode of TB case finding
- DAF:
-
Duration of abnormal X-ray findings
- FSC:
-
Frequencies of sputum culture
- DNSC:
-
Duration of negative sputum culture
- DWSC:
-
Duration without sputum culture
- FSS:
-
Frequencies of sputum smear
- DPSS:
-
Duration of positive sputum smear
- DNSS:
-
Duration of negative sputum smear
- DWSS:
-
Duration without sputum smear
- OR:
-
Odds ratio
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Knight, G. M., McQuaid, C. F., Dodd, P. J. & Houben, R. M. G. J. Global burden of latent multidrug-resistant tuberculosis: Trends and estimates based on mathematical modelling. Lancet Infect. Dis.19, 903–912. https://doi.org/10.1016/S1473-3099(19)30307-X (2019).
World Health Organization. Global tuberculosis report 2019. https://www.who.int/tb/publications/global_report/en/. Accessed 20 October 2019.
Mulisa, G. et al. Multidrug-resistant Mycobacterium tuberculosis and associated risk factors in Oromia Region of Ethiopia. Int. J. Infect. Dis.39, 57–61. https://doi.org/10.1016/j.ijid.2015.08.013 (2015).
Stosic, M. et al. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients in Serbia: A case–control study. BMC Public Health18(1), 1114. https://doi.org/10.1186/s12889-018-6021-5 (2018).
Shariff, N. M., Shah, S. A. & Kamaludin, F. Predictors of death among drug-resistant tuberculosis patients in Kuala Lumpur, Malaysia: A retrospective cohort study from 2009 to 2013. J. Glob. Antimicrob. Resist.6, 102–107. https://doi.org/10.1016/j.jgar.2016.04.005 (2016).
Yang, C. et al. Transmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: A retrospective observational study using whole-genome sequencing and epidemiological investigation. Lancet Infect. Dis.17(3), 275–284. https://doi.org/10.1016/S1473-3099(16)30418-2 (2017).
Xu, C. et al. Attrition and delays before treatment initiation among patients with MDR-TB in China (2006–13): Magnitude and risk factors. PLoS ONE14(4), e0214943 (2019).
Pradipta, I. S. et al. Risk factors of multidrug-resistant tuberculosis: A global systematic review and meta-analysis. J Infect.77(6), 469–478 (2018).
Golubnitschaja, O. & Costigliola, V. General report & recommendations in predictive, preventive and personalised medicine 2012: White paper of the European Association for predictive, preventive and personalised medicine. EPMA J.3(1), 14. https://doi.org/10.1186/1878-5085-3-14 (2012).
Pefura-Yone, E. W. et al. Development and validation of a prognostic score during tuberculosis treatment. BMC Infect. Dis.17(1), 251. https://doi.org/10.1186/s12879-017-2309-9 (2017).
Costa-Veiga, A., Briz, T. & Nunes, C. Unsuccessful treatment in pulmonary tuberculosis: Factors and a consequent predictive mode. Eur. J. Public Health28(2), 352–358. https://doi.org/10.1093/eurpub/ckx136 (2018).
Fregona, G. et al. Risk factors associated with multidrug-resistant tuberculosis in Espírito Santo, Brazil. Rev. Saude Publica51(1), 41. https://doi.org/10.1590/S1518-8787.2017051006688 (2017).
Gebreegziabher, S. B., Bjune, G. A. & Yimer, S. A. Total delay is associated with unfavorable treatment outcome among pulmonary tuberculosis patients in west Gojjam zone, Northwest Ethiopia: A prospective cohort study. PLoS ONE11(7), e0159579. https://doi.org/10.1371/journal.pone.0159579 (2016).
Kumar, K. & Abubakar, I. Clinical implications of the global multidrug-resistant tuberculosis epidemic. Clin. Med. (Lond.)15(Suppl 6), s37–s42. https://doi.org/10.7861/clinmedicine.15-6-s37 (2015).
Mulhall, J. P. et al. Development of nomograms to predict the recovery of erectile function following radical prostatectomy. J. Sex Med.16(11), 1796–1802 (2019).
Kattan, M. W. Comparison of Cox regression with other methods for determining prediction models and nomograms. J. Urol.170(6), S6–S9. https://doi.org/10.1097/01.ju.0000094764.56269.2d (2013).
Kasiulevičius, V., Šapoka, V. & Filipavičiūtė, R. Sample size calculation in epidemiological studies. Gerontologija7(4), 225–231 (2006).
Li, Q. et al. Prevalence and patterns of drug resistance among pulmonary tuberculosis patients in Hangzhou, China. Antimicrob. Resist. Infect. Control7, 61. https://doi.org/10.1186/s13756-018-0348-7 (2018).
Zhao, Y. et al. National survey of drug-resistant tuberculosis in China. N. Engl. J. Med.366(23), 2161–2170. https://doi.org/10.1056/NEJMoa1108789 (2012).
de Boer, S. P. M. et al. Evaluating the ‘all-comers’ design: A comparison of participants in two ‘all-comers’ PCI trials with non-participants. Eur. Heart. J.32(17), 2161–2167. https://doi.org/10.1093/eurheartj/ehr126 (2011).
National Health and Family Planning Commission of the People’s Republic of China. (2017). Diagnostic Criteria for Pulmonary Tuberculosis (WS 288-2017). https://www.cnki.com.cn/Article/CJFDTotal-XFCR201801019.htm. Accessed 21 April 2020.
World Health Organization. Definitions and Reporting Framework for Tuberculosis—2013 Revision. https://www.who.int/tb/publications/definitions/en/. Accessed 10 February 2019.
National Health Commission of the People’s Republic of China. (2020). Technical Specification for Tuberculosis Control and Prevention in China (2020 edition). https://wsjkw.gxzf.gov.cn/uploadfile/20200416/1587004730124090.pdf. Accessed 15 April 2020.
World Health Organization. (2015). Guidelines for Surveillance of Drug Resistance in Tuberculosis—5th Edition. https://www.who.int/tb/publications/2015/drs_guidelines/en/. Accessed 15 February 2019.
Ministry of health of the People’s Republic of China. (2008). Diagnostic Criteria for Pulmonary Tuberculosis (WS 288–2008). https://guide.medlive.cn/guideline/3833. Accessed 21 April 2020.
Brentnall, A. R., Cuzick, J., Field, J. & Duffy, S. W. A concordance index for matched case–control studies with applications in cancer risk. Stat. Med.34(3), 396–405. https://doi.org/10.1002/sim.6335 (2015).
Christensen, E. Multivariate survival analysis using Cox’s regression model. Hepatology7(6), 1346–1358. https://doi.org/10.1002/hep.1840070628 (1987).
Mekonnen, F. et al. Multidrug resistant tuberculosis: Prevalence and risk factors in districts of Metema and West Armachiho, Northwest Ethiopia. BMC Infect. Dis.15, 461–472. https://doi.org/10.1186/s12879-015-1202-7 (2015).
Vicente, D. et al. Low antimicrobial resistance rates of Mycobacterium tuberculosis complex between 2000 and 2015 in Gipuzkoa, Northern Spain. Enferm. Infecc. Microbiol. Clin.37(9), 574–579 (2019).
Zhang, C. et al. Determinants of multidrug-resistant tuberculosis in Henan province in China: A case control study. BMC Public Health16(1), 42. https://doi.org/10.1186/s12889-016-2711-z (2015).
Flora, M. S. et al. Risk factors of multi-drug-resistant tuberculosis in Bangladeshi population: A case control study. Bangladesh. Med. Res. Counc. Bull.39, 34–41 (2013).
Shibabaw, A., Gelaw, B., Wang, S. H. & Tessema, B. Time to sputum smear and culture conversions in multidrug resistant tuberculosis at University of Gondar Hospital, Northwest Ethiopia. PLoS ONE13(6), e0198080 (2018).
Kim, O. H. et al. Association between duration of aminoglycoside treatment and outcome of cavitary Mycobacterium avium complex lung disease. Clin. Infect. Dis.68(11), 1870–1876 (2019).
World Health Organization. Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care, 2017 Update. https://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf. Accessed 23 February 2019.
Espinal, M. A. et al. Determinants of drug-resistant tuberculosis: Analysis of 11 countries. Int. J. Tuberc. Lung Dis.5(10), 887–893 (2001).
Faustini, A., Hall, A. J. & Perucci, C. A. Risk factors for multidrug resistant tuberculosis in Europe: A systematic review. Thorax61(2), 158–163. https://doi.org/10.1136/thx.2005.045963 (2006).
Grandjean, L. et al. Transmission of multidrug-resistant and drug-susceptible tuberculosis within households: A prospective cohort study. PLoS Med.12(6), e1001843. https://doi.org/10.1371/journal.pmed.1001843 (2015).
Gilad, J., Borer, A., Riesenberg, K., Peled, N. & Schlaeffer, F. Epidemiology and ethnic distribution of multidrug-resistant tuberculosis in southern Israel, 1992–1997. Chest117(3), 738–743. https://doi.org/10.1378/chest.117.3.738 (2000).
Collins, G. S. et al. External validation of multivariable prediction models: A systematic review of methodological conduct and reporting. BMC Med. Res. Methodol.14, 40. https://doi.org/10.1186/1471-2288-14-40 (2014).
Verguet, S. et al. Catastrophic costs potentially averted by tuberculosis control in India and South Africa: A modelling study. Lancet Glob. Health5(11), e1123-1132. https://doi.org/10.1016/S2214-109X(17)30341-8 (2017).
Horsburgh, C. R. Jr., Barry, C. E. & Lange, C. Treatment of tuberculosis. N. Engl. J. Med.373(22), 2149–2160. https://doi.org/10.1056/NEJMra1413919 (2015).
Trovato, G. M. Sustainable medical research by effective and comprehensive medical skills: Overcoming the frontiers by predictive, preventive and personalized medicine. EPMA. J.5(1), 14. https://doi.org/10.1186/1878-5085-5-14 (2014).
Acknowledgements
We extend our gratitude to the individuals vital to executing this study. In particular, we thank all designated hospitals of TB treatment in Hangzhou City for supplying clinical data and Hangzhou center for disease control and prevention’s field investigators for supplying epidemiological data. This work is supported by the Medical Science and Technology Project of Zhejiang Province (Grant Number: 2020PY064) and the Health Science and Technology Project of Hangzhou Municipality (Grant Number: OO20190783).
Author information
Authors and Affiliations
Contributions
Q.L.C. contributed to the study conception and design, data analysis, interpretation of the data, and drafting the manuscript. L.X., G.Z. and X.C.W. contributed to the interpretation of the data and critical revision of the manuscript. L.X., G.Z., X.C.W., L.W., Q.C.L., M.L., Y.F.W., Y.Y.H., and Q.J.J. contributed to the collection of the data. All authors gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, Q., Zhao, G., Wang, X. et al. Nomogram for individualized prediction of incident multidrug-resistant tuberculosis after completing pulmonary tuberculosis treatment. Sci Rep 10, 13730 (2020). https://doi.org/10.1038/s41598-020-70748-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70748-x
This article is cited by
-
Can resistance to either isoniazid or rifampicin predict multidrug resistance tuberculosis (MDR-TB)
Bulletin of the National Research Centre (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.