Abstract
The incidence of human papillomavirus (HPV)-related oropharyngeal cancer is increasing in some regions. Nevertheless, the epidemiology of this disease has not been extensively investigated in southern Europe. We conducted a retrospective cohort study of patients diagnosed with primary oropharyngeal cancer from 1991 to 2016. Cancer tissues underwent histopathological evaluation, DNA quality control, HPV-DNA detection and p16INK4a immunohistochemistry. Data were collected from medical records. Factors associated with HPV positivity and time trends were evaluated with multivariable Bayesian models. The adjusted prevalence of HPV-related cases in 864 patients with a valid HPV-DNA result was 9.7%, with HPV-DNA/p16INK4a double positivity being considered. HPV-related oropharyngeal cancer was likely to occur in non-smokers and non-drinkers, to be located in the tonsil or diagnosed at advanced stages. Time-trend analysis showed an increasing risk of HPV-related oropharyngeal cancer in the most recent periods (5-year period increase of 30%). This increase was highest and with a clear increasing trend only in the most recent years (2012–2016). The prevalence of HPV-related oropharyngeal cancer started to sharply increase in the most recent years in our setting, as occurred two decades ago in areas where most oropharyngeal cancer cases are currently HPV-related. Our results provide a comprehensive assessment of the epidemiological landscape of HPV-related oropharyngeal cancer in a region of southern Europe.
Similar content being viewed by others
Introduction
The epidemiology of oropharyngeal cancer (OPC) has dramatically changed in the last two decades after the incursion of human papillomavirus (HPV) in the etiological arena of the disease1. Currently, 29,000 new HPV-related OPC cases (representing 30% of the total OPC) are estimated to occur every year worldwide2, with marked differences between geographical regions. HPV-related OPC is increasing in some regions of the world such as North America3 and northern Europe4,5,6,7, where most OPC cases are currently HPV-related. The increasing trends are thought to be related to a decrease in smoking and increased numbers of sexual partners and oral sex practices8, 9. The role of HPV in OPC has important clinical implications given the distinct nature and marked prognostic differences between HPV-related and non-related patients8, 9. Nevertheless, the epidemiology of the disease in southern Europe, where current estimates of HPV prevalence are low (9–24%)2,10, has not been extensively investigated. In Spain, only two studies on HPV involvement in selected OPC cases were available11,12 until our group recently reported the results of a multicenter retrospective cohort of 788 patients with primary OPC consecutively diagnosed between 1991 and 201313. The study identified double positivity for HPV-DNA/p16INK4a as the biomarker with strongest diagnostic accuracy and prognostic value for HPV-related OPC patients and found an HPV prevalence of 7.4% when considering HPV-DNA/p16INK4a double positivity13. A recent Italian study also evaluated the role of HPV in patients with newly diagnosed OPC during the period 2000–2018, reporting a prevalence of HPV-driven OPC of 32.3% and a higher prevalence in the most recent years14.
In this study, we aimed to provide an updated assessment of the time trends of HPV prevalence estimates in OPC, as well as to evaluate further its epidemiological features compared with other high-burden regions.
Methods
Study design and population
We designed a retrospective cohort study of all new patients diagnosed with primary OPC in four hospitals in Catalonia (Spain) from 1991 to 2013 (Catalan Institute of Oncology-Bellvitge Hospital, Hospital del Mar, Hospital Parc Taulí and Hospital de la Santa Creu i Sant Pau). We also included all patients diagnosed with primary OPC in the latter setting through 2016.
The procedures followed have been previously described10. Briefly, cases were identified from medical records/pathology reports of the centers of origin. We included cases fulfilling the following criteria: diagnosis of primary invasive cancer of the oropharynx, any histology, codes from the International Classification of Diseases for Oncology version 3: Tonsil-C09, C02.4, Base of tongue-C01 and Others (soft palate-C05.1, uvula-C05.2, vallecula, glossoepiglottic fold, lateral and posterior wall of the oropharynx, overlapping lesion of the oropharynx and oropharynx unspecified-C10, Waldeyer ring-C14.2); and available access to medical records on demographic, toxic habits and clinical and follow-up information. Formalin-fixed paraffin embedded (FFPE) primary tumour samples from the diagnosis prior to treatment were retrieved when available.
FFPE block processing and histopathological evaluation
All specimens processing was centralized at the Catalan Institute of Oncology (ICO). FFPE blocks were re-embedded whenever necessary. The first and last sections were used for histopathological evaluation after hematoxylin and eosin (H&E) staining. Two in-between sections were used for HPV-DNA testing and genotyping; one additional slide was obtained to assess p16INK4a expression, which was performed at Hospital General de l’Hospitalet (Spain) under the manufacturer’s standards (Roche mtm Laboratories AG IHC. Heidelberg, Germany). A strong and diffuse nuclear and cytoplasmic staining of > 70% of the cancer tissue was considered p16INK4a-positive15. Since our previous study showed high diagnostic accuracy and prognostic value for double positivity for HPV-DNA/p16INK4a13, which is an easier diagnostic algorithm to implement in clinical practice than HPV-mRNA testing, we did not further use HPV-mRNA positivity for the analyses herein presented. A block was classified as adequate for HPV testing if invasive cancer was observed in the two H&E stained sections of the specimen.
Pathology review was based on the WHO pathological criteria for head and neck cancer and was performed blind with respect to the original local diagnosis and HPV-DNA/p16INK4a results, following a pre-established algorithm for diagnostic consensus involving three pathologists, as reported elsewhere10.
HPV-DNA detection and genotyping
We used a PCR with the consensus primers SPF10 PCR and a DNA enzyme immunoassay (DEIA) to test for the presence of HPV-DNA10,13. HPV genotyping was performed using a reverse hybridization line probe assay (LiPA25_v1) on all samples testing positive for viral DNA, targeting 25 HPV types with different oncogenic risk (Laboratory Biomedical Products Rijswijk, The Netherlands). DNA quality was evaluated in all HPV-DNA negative samples by testing for the tubulin-β gene. All DEIA and LiPA25_v1 assays were performed at the ICO.
Statistical analyses
OPC samples testing negative for both viral and human DNA were excluded from the analyses. Descriptive, bivariate and unconditional logistic regression analyses were performed to identify independent factors associated with HPV etiological involvement in OPC according to double positivity for HPV-DNA/p16INK4a and p16INK4a-positivity only. Crude and adjusted odds ratios and their 95% credibility intervals (CI, the Bayesian version of the frequentist confidence intervals but calculated using the posterior distribution of the model’s parameters), were estimated. Moreover, crude and adjusted prevalences were estimated, the latter to account for other covariates that may have an effect on the prevalence estimates. Adjusted prevalences were calculated with R “prediction” function of “prediction” package16 based on the adjusted models. Histological variables were not included in the multivariable analyses as they were considered to be intermediate variables in the carcinogenic process, as previously described17. Bayesian methodology was chosen in all analyses with non-informative prior distribution due to lack of data on the distribution of the parameters18,19 and, moreover, because Bayesian methodology is useful to (1) avoid model fitting problems in parameter estimation due to small counts, and (2) produce robust estimators20.
To more specifically assess time trends in HPV-related OPCs in Spain, a log-binomial regression model was defined for double positivity for HPV-DNA/p16INK4a and prevalence risk ratios (RR) were obtained, with their 95% CI. Patients from 1991 were included in the first 5-year period (1992–1996) in order not to lose sample size. Multivariable models were adjusted for confounders: age, 5-year periods, gender, tobacco/alcohol consumption, subsite and tumour stage.
All analyses were performed using the “bayesglm” function of the “arm” package19 in R. All possible interactions between variables were assessed and stratified by anatomical subsite.
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. The study had formal approval by the ethics committees of the four participating hospitals (i.e. Catalan Institute of Oncology-ICO-Hospital Universitari de Bellvitge, Hospital de la Santa Creu i Sant Pau, Hospital del Mar and Hospital Parc Taulí). Adequate measures to ensure data protection, confidentiality, patients’ privacy and anonymization were taken into account in compliance with European and Spanish current laws and regulations. Informed consent was not available due to the retrospective design of the study and the large proportion of deceased and untraceable patients. Thus, and in accordance to current regulations, informed consent was waved off by the ethics committees for patients diagnosed up to 2013. Informed consent was obtained for patients diagnosed in 2014–2017.
Results
Supplementary Fig. 1 describes the workflow of the OPC targeted cases, samples collected, processed, tested and finally included in the statistical analyses. A total of 76 OPC patients diagnosed in Hospital de la Santa Creu i Sant Pau between 2013 and 2016 were added to the initial retrospective cohort of 788 patients13.
HPV prevalence and factors associated with HPV positivity in OPC
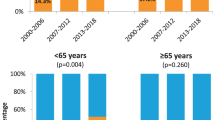
Table 1 shows the demographic and clinical characteristics of the 864 patients included in the analysis, as well as the crude and adjusted prevalences and odds ratios for double positivity for HPV-DNA/p16INK4a. Patients had a mean age at diagnosis of 60 years; they were mostly male (88.9%), heavy smokers (74.4%) and heavy drinkers (49.7%). The most common anatomical subsite was the tonsil (40.5%). Most tumours (63.3%) were squamous cell carcinoma (SCC) with conventional keratinizing morphological features and the most common stage was IVa according to the 7th TNM edition (45.6%). The adjusted prevalence of HPV-related patients was 9.7%.
After adjustment for confounders, HPV-related patients were more likely to be younger than 60 years, non-smokers and non-drinkers, to have tonsillar carcinoma, and to be diagnosed in most recent periods or with stages III–IVa (Table 1). The equivalent results for p16INK4a positivity alone are presented in supplementary Table 1.
HPV prevalence and factors associated with HPV positivity by anatomical subsite
The associations of HPV-positivity with the demographic and clinical characteristics of OPC patients were also examined, stratified by three major anatomical subsites (tonsil, base of tongue and others, Table 2). None of the associations observed in the multivariable analysis for OPC including all sites were observed for subsites other than the tonsil and base of tongue. Crude and adjusted HPV prevalences stratified by the three major anatomical sites are presented for HPV-DNA/p16INK4a double-positivity and p16INK4a-positivity alone in supplementary Tables 3 and 4, respectively.
Time trends of relative risk for HPV positivity in OPC. CI credibility interval; OPC oropharyngeal cancer. Time trends of relative risk for HPV positivity in OPC for each five years period with respect to reference one: 1991–1996. Trends models adjusted by age at diagnosis, gender, period of diagnosis, subsite, stage and tobacco and alcohol consumption. Y axis is represented in logarithmic scale.
Some differences were observed when only p16INK4a-positivity was analysed, for all OPC sites and by subsite (supplementary Tables 1 and 2, respectively). Of note, calendar period and age did not show a clear trend in the multivariable analysis for all OPC sites and for tonsil.
HPV type distribution
Most HPV-DNA positive cases were HPV16 (82.2%), followed by HPV33 (5.9%) and HPV35 (4.0%), (Table 3). When we focused on patients that were double positive for HPV-DNA/p16INK4a, the percentage of HPV16 and HPV35 positive patients increased to 88.5% and 5.1%, respectively, but decreased for other genotypes (Table 3). The same was observed specifically for all anatomical subsites, and the highest proportion of HPV16 was found in sites other than tonsil and base of tongue.
Time trends for HPV positivity
In the time-trend analysis, an increasing RR for HPV positivity was observed when period of diagnosis was analysed as a continuous variable (30% for every 5-year period, supplementary Table 5). When period of diagnosis was considered as a categorical variable, we found the increase to be highest (RR = 2.0, 95% CI 1.1–3.9) and with a clear increasing trend only in the last 5-year period (2012–2016, Fig. 1 and supplementary Table 6). Since the most recent patients came mostly from Sant Pau Hospital, a sensitivity analysis including only Sant Pau patients was performed, and equivalent results were obtained when considering period of diagnosis as a continuous variable (RR = 1.2, 95% CI 1.0–1.4). This trend was not observed when period of diagnosis was considered as a categorical variable (RR = 1.8, 95% CI 0.6–5.8), probably due to the lower number of cases. An increasing trend for risk, of 31% and 66% for every 5-year period when period of diagnosis was considered as a continuous variable, was also detected specifically for tonsillar and base of tongue cancers, respectively (supplementary Tables 6 and 7). When period of diagnosis was considered as a categorical variable, the increase was found only for base of tongue patients in the last 5-year period (Fig. 2). Increasing trends in HPV prevalence seemed to be higher in men than in women (Fig. 2 and supplementary Tables 8 and 9).
Time trends of relative risk for HPV positivity in OPC by anatomical subsite and gender. CI credibility interval, BOT base of tongue. Time trends of relative risk for HPV positivity in OPC for each five years period with respect to reference one: 1991–1996. Trends models adjusted by age at diagnosis, gender, period of diagnosis, subsite, stage and tobacco and alcohol consumption. Y axis is represented in logarithmic scale.
Discussion
To our knowledge, this study is the largest and most comprehensive assessment of the epidemiological landscape of HPV-related and non-related OPC in southern Europe. We had previously hypothesized that differences on the epidemiology of HPV-related OPC between regions may reflect distinct trends in temporal, geographical, and sociodemographic shifts in population exposure to both tobacco smoking and oral HPV infection, leading to a rapidly evolving epidemiology of HPV-related OPC 10.
Observed HPV prevalences were about 10%, as defined by the percentage of cases that were double positive for HPV-DNA/p16INK4a. A recent Italian study also evaluated the role of HPV in patients with newly diagnosed OPC during the period 2000–2018, reporting a prevalence of HPV-driven OPC of 32.3% and a higher prevalence in the most recent years14. The lower prevalence observed in our cohort could be explained by the higher number of patients and the inclusion of patients diagnosed at earlier calendar periods than in the Italian study. Moreover, epidemiological differences between the two populations could also exist and account for such differences. As expected10, the highest HPV prevalences were observed in tonsillar cancers, with 14% of patients estimated to be HPV-related compared with 8% of base of tongue and 5% of other cancer subsites. Also, in accordance with the literature8,9, higher HPV prevalences were observed in more advanced stages based on 7th TNM definition (III and IVa) compared with early stages (I and II).
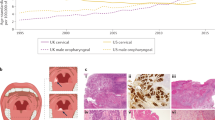
Our results indicate that HPV prevalences in OPC have started to increase sharply in the most recent years in our setting. However, our results reporting HPV prevalence estimates are based on primary OPC tumours from four hospital-based registries and do not necessarily imply an increasing incidence of HPV-associated OPC in our region. Nevertheless, a previous study estimating the incidence trends of OPC in Spain by using data from population-based registries predicted statistically significant increasing trends for OPC over the period 2003–201721. These results, together with our own, support the hypothesis that these increasing trends for OPC are caused by HPV infection. Of note, age-standardized incidence rates for OPC were estimated in 2018 at 1.4 in Spain and 1.3 in southern Europe, respectively22.
If this hypothesis is correct, HPV-related OPC has started to increase in our setting about 20 years later than in other countries such as the US3. This two-decade gap is in agreement with the calendar period differences between smoking drops in the US and Spain (supplementary Fig. 2).
However, it is still unclear whether tobacco and/or alcohol use can act as co-factors and/or effect modifiers in the risk of developing HPV-related OPC23. Therefore, we cannot rule out the possibility that smoking is associated with an increased risk of HPV-positive OPC, as observed in previous studies in the US24.
The increased trends in HPV prevalence were not observed for cancers located at sites other than the tonsil and base of tongue and were more marked in the latter, although the credibility intervals were wider due to the lower number of base of tongue cancers.
After adjustment for confounders, HPV prevalences were found to be higher in younger patients, as observed in other high-burden regions. Of note, most recent publications from the US indicate that a rising proportion of older OPC patients have HPV-positive tumours25,26. HPV prevalences were substantially higher in women than in men, in contrast with what is observed, for instance, in the US3. This was already observed for Europe in an international cross-sectional study conducted by our group10. However, after adjustment for confounders, those gender differences disappeared. A recent Italian study that did not adjust for confounders also observed higher HPV prevalences in women than in men in the univariate analyses14. Our results after adjustment for confounders were supported by the finding of a lower number of ever-smokers among female (62/94, 66.0%) than male (650/709, 91.7%) patients. A systematic review on geographical differences in the proportion of HPV prevalence in OPC between men and women revealed that those differences were mainly a consequence of the vast international variation in male smoking habits27. Notwithstanding, a population-based study describing time trends of cancer incidence and mortality in Catalonia during the period 1993–2007 detected a rising trend in oral cavity and pharyngeal cancers among women but not among men, presumably explained by a higher and earlier rate of smoking cessation among men than among women28. In addition to smoking differences between men and women, HPV transmission appears higher from women to men among heterosexual partners29 and differences in sexual behaviours between geographical regions have not been analysed at this level of detail. Intriguingly, although this study was not powered to precisely evaluate this issue due to the low number of female patients, increasing trends in HPV prevalence were suggested to be higher in women than in men (Fig. 2 and Supplementary Tables 8 and 9).
The gender and calendar differences in OPC HPV prevalence between high-burden regions and our setting could also be partially explained by differences in sexual behaviour, which is a clear risk factor for oral HPV acquisition and HPV-related OPC. Indeed, sexual behaviour greatly varies across regions with proportions of ever having oral sex in the US being higher than 65% compared with less than 20% in countries in southern Europe such as Spain30. Nevertheless, the CLEOPATRE study, which aimed to estimate the prevalence of cervical HPV infection in Spanish women attending cervical cancer screening, found a higher number of sexual partners and earlier ages for sexual debut in younger (18–25 years) versus the oldest women (56–65 years)31. The CLEOPATRE results could thus also partially account for the recent increasing trends in HPV prevalence observed in the present study if it is assumed that changes in oral sex practices have also taken place.
Different trends in tonsillectomy rates could also have contributed to the observed geographical heterogeneity. A previous study from Denmark reported a decrease in tonsillectomy rates concomitant to a simultaneous increase in the risk of OPC32. However, other studies did not find that the observed significant increases in OPC were related to declines in tonsillectomies33,34 reinforcing increased oral HPV exposure as the likely cause.
The differences in the role of HPV in OPC between anatomical subsites observed in our study are consistent with the results of a recent systematic review35 concluding that HPV prevalence differs markedly between OPC subsites and that its role in sites “other” than tonsil or base of tongue is uncertain and needs further evaluation.
A total of 30 out of 108 (27.7%) p16INK4a positive cases were HPV-DNA negative, representing 3.5% of the total sample, despite definition of p16INK4a positivity as staining above > 70%. This percentage is higher than the 10%-20% observed in most studies irrespective of the method of HPV-specific testing applied36, although it is lower than the 47% reported in an Italian study that considered a case to be p16INK4a positive if strong nuclear and cytoplasmic staining was present in > 50% of the tumour cells37. Classification and potential management of p16INK4a-positive—HPV-negative cases is still controversial38,39 although we13 and others37,39 have shown that these cases display similar survival to p16INK4a-negative cases. Moreover, molecular characterization of p16INK4a-positive—HPV-negative OPC cases has shown a similar genetic profile to HPV-negative tumours40. In this regard, we have observed epidemiological differences between OPC cases that were double positive for HPV-DNA/p16INK4a and cases that were p16INK4a-positive only, such as the loss of association with age and calendar period. Age discrepancies could be explained by a higher accumulation of mutations with age in the case of p16INK4a—positive—HPV-negative patients. A powered study aiming to perform an in-depth comparison between p16INK4a-positive—HPV-positive cases and p16INK4a-positive—HPV-negative cases is warranted to elucidate these questions.
Our study has several limitations. Selection of patients was not population-based and thus our results cannot be extrapolated to the general population. Moreover, the modest sample size does not allow firm conclusions to be drawn regarding associations with HPV positivity by subsite or gender, for instance. However, according to preliminary estimates of the OPC incidence in Catalonia using data from population-based registries41, about one third of OPC cases diagnosed each year in Catalonia are seen at the four hospitals of the study, and our sample represents approximately 16% of all incident OPC cases in the region. The retrospective nature of our cohort may have hampered thorough characterization of patients according to risk factors such as tobacco or alcohol use, since this kind of information could only be partially obtained from medical records. The more recent patients were only available for inclusion in the study for Sant Pau Hospital. However, when we addressed this limitation by performing a sensitivity analysis only considering Sant Pau patients, we obtained equivalent results. In addition, paraffin blocks were not available at diagnosis for a substantial number of patients, notably those with base of tongue carcinoma, a location particularly difficult to biopsy, as well as for patients from older periods. Of note, a fraction of these patients (3% from Bellvitge Hospital) had fine needle aspiration samples. However, these were not included according to our exclusion criteria. Our classification of subsites other than tonsil or base of tongue comprised many different locations, including oropharynx unspecified or overlapping lesions that could also include tonsil and base of tongue.
Our findings indicate that HPV prevalence in OPC has started to sharply increase in the most recent years in our setting. These results, together with previous estimates of increasing trends of OPC in Spain, suggest that HPV-related OPC has started to increase as occurred two decades ago in areas where most OPC cases are currently HPV-related. However, there are some differences between the epidemiology of the disease in our setting and other high-burden regions and we do not yet know whether they may change in the near future to approach the epidemiology of HPV-related OPC in high-burden regions.
Importantly, our results strongly suggest that current estimates of HPV prevalence in southern Europe (probably other regions beyond high-burden areas) are outdated and warrant updated studies in selected populations. Continuing surveillance of sexual behaviours, alongside HPV vaccination status, is warranted to predict future HPV-related OPC burden.
References
IARC. IARC Monographs on the evaluation of carcinogenic risks to humans. Monograph89, 223–276 (2007).
De Martel, C., Plummer, M., Vignat, J. & Franceschi, S. Worldwide burden of cancer attributable to HPV by site country and HPV type. Int. J. Cancer141(4), 664–670 (2017).
Chaturvedi, A. K. et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol.29, 4294–4301 (2011).
Nasman, A. et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinoma?. Int. J. Cancer125, 362–366 (2009).
Hannisdal, K., Schjølberg, A., De Angelis, P. M., Boysen, M. & Clausen, O. P. Human papillomavirus (HPV)-positive tonsillar carcinomas are frequent and have a favourable prognosis in males in Norway. Acta Otolaryngol.130, 293–299 (2010).
Garnaes, E. et al. A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000–2010: The largest registry-based study to date. Int. J. Cancer136(9), 2196–2203 (2015).
Rietbergen, M. M. et al. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int. J. Cancer132, 1565–1571 (2013).
Gillison, M. L., Chaturvedi, A. K., Anderson, W. F. & Fakhry, C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol.33(29), 3235–3242 (2015).
Taberna, M. et al. Human papillomavirus-related oropharyngeal cancer. . Ann. Oncol.00, 1–13 (2017).
Castellsagué, X. et al. HPV Involvement in head and neck cancers: Comprehensive assessment of biomarkers in 3680 patients. J. Natl. Cancer Inst.108(6), djv03 (2016).
Rodrigo, J. P. et al. Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990–2009). Int. J. Cancer134(2), 487–492 (2014).
Cerezo, L. et al. OPC related to human papilloma virus: Incidence and prognosis in Madrid,. Clin. Transl. Oncol.16(3), 301–306 (2014).
Mena, M. et al. Double positivity for HPV-DNA/p16INK4a is the biomarker with strongest diagnostic accuracy and prognostic value for human papillomavirus related oropharyngeal cancer patients. Oral. Oncol.78, 137–144 (2018).
Del Mistro, A. et al. Age-independent increasing prevalence of human papillomavirus-driven oropharyngeal carcinomas in North-East Italy. Sci. Rep.10(1), 9320 (2020).
Westra, W. H. Detection of human papillomavirus (HPV) in clinical samples: Evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral. Oncol.50(9), 771–779 (2014).
Leeper, T. J. Prediction: Tidy, Type-Safe 'prediction()' Methods. R package version 0.2.0 (2017).
Alemany, L. et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int. J. Cancer136(1), 98–107 (2015).
Clèries, R. et al. Monitoring the decreasing trend of testicular cancer mortality in Spain during 2005–2019 through a Bayesian approach. Cancer Epidemiol.34(3), 244–256 (2010).
Gelman, A., & Su, Y. Arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. R package version 1.9–3 (2016).
Botta, L. et al. Angelis R, et al on behalf of the RARECAREnet Working group. Bayesian estimates of the incidence of rare cancers in Europe. Cancer Epidemiol.54, 95–100 (2018).
de Souza, D. L., Pérez, M. M. & Curado, M. P. Predicted incidence of oral cavity, oropharyngeal, laryngeal, and hypopharyngeal cancer in Spain and implications for cancer control. Cancer Epidemiol.35(6), 510–514 (2011).
Ferlay, J. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer144(8), 1941–1953 (2019).
Gillison, M. L. et al. Human papillomavirus and diseases of the upper airway: Head and neck cancer and respiratory papillomatosis. Vaccine30(Suppl 5), F34–F54 (2012).
Chaturvedi, A. K., D’Souza, G., Gillison, M. L. & Katki, H. A. Burden of HPV-positive oropharynx cancers among ever and never smokers in the U.S. Population. Oral Oncol60, 61–7 (2016).
Rettig, E. M. et al. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of human papillomavirus is attenuated among older patients: Analysis of the national cancer database. Oral. Oncol.83, 147–153 (2018).
Windon, M. J. et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer124(14), 2993–2999 (2018).
Combes, J. D., Chen, A. A. & Franceschi, S. Prevalence of human papillomavirus in cancer of the oropharynx by gender. Cancer Epidemiol. Biomark. Prev.23(12), 2954–2958 (2014).
Cleries, R. et al. Time trends of cancer incidence and mortality in Catalonia during 1993–2007. Clin. Transl. Oncol.16(1), 18–28 (2014).
Giuliano, A. R. et al. EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int. J. Cancer136(12), 2752–2760 (2015).
Heck, J. E. et al. Sexual behaviours and the risk of head and neck cancers: A pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int. J. Epidemiol.39(1), 166–181 (2010).
Castellsagué, X. et al. Prevalence and genotype distribution of human papillomavirus infection of the cervix in Spain: The CLEOPATRE study. J. Med. Virol.84(6), 947–956 (2012).
Fakhry, C., Andersen, K. K., Christensen, J., Agrawal, N. & Eisele, D. W. The impact of tonsillectomy upon the risk of oropharyngeal carcinoma diagnosis and prognosis in the danish cancer registry. Cancer Prev. Res. (Phila.)8(7), 583–589 (2015).
Chaturvedi, A. et al. Tonsillectomy and incidence of oropharyngeal cancers. Cancer Epidemiol. Biomark. Prev.25(6), 944–950 (2016).
Schache, A. G. et al. HPV-related oropharynx cancer in the United Kingdom: An evolution in the understanding of disease etiology. Cancer Res.76(22), 6598–6606 (2016).
Haeggblom, L., Ramqvist, T., Tommasino, M., Dalianis, T. & Näsman, A. Time to change perspectives on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Res4, 1–11 (2017).
Grønhøj Larsen, C. et al. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: A systematic review. Br. J. Cancer110(6), 1587–1594 (2014).
Perrone, F. et al. Isolating p16 positive/HPV negative oropharyngeal cancer: An effort worth making. Am. J. Surg. Pathol.2011(35), 774–777 (2011).
Jordan, R. C. et al. Validation of methods for OPC HPV status determination in US cooperative group trials. Am. J. Surg. Pathol.36(7), 945–954 (2012).
Coordes, A. et al. Meta-analysis of survival in patients with HNSCC discriminates risk depending on combined HPV and p16 status. Eur. Arch. Otorhinolaryngol.273(8), 2157–2169 (2016).
Rietbergen, M. M. et al. Molecular characterization of p16-immunopositive but HPV DNA-negative oropharyngeal carcinomas. Int. J. Cancer134(10), 2366–2372 (2014).
Galceran, J., Ameijide, A., Alemany, L., Carulla, M., Marcos, R., Vilardell, L., et al. Incidence and mortality of neoplasias related to Human papillomavirus in Catalonia. In XXXV Scientific Meeting of the Spanish Society of Epidemiology (SEE), Oral Presentation, (2017).
Acknowledgements
We thank CERCA Program / Generalitat de Catalunya for institutional support and all members of the study group: Catalan Institute of Oncology/Hospital Universitari de Bellvitge: Laia Alemany, Xavier Bosch, Vanesa Camon, Omar Clavero, Ana Esteban, Yolanda Florencia, Montserrat Gomà, Alicia Lozano, Manel Maños, Antonio Marí, Marisa Mena, Ricard Mesía, Julio Nogués, Oriol Bermejo, Ramon Clèries, Jon Frias-Gomez, Miquel Ángel Pavón, Beatriz Quirós, Silvia de Sanjosé, Miren Taberna, Montserrat Torres, Sara Tous, Griselda Venturas, Marleny Vergara; Hospital General de l’Hospitalet: María Alejo; Hospital de la Santa Creu i Sant Pau: Jacinto García, Xavier León, Montserrat Lopez, Miquel Quer; Hospital del Mar: Marta Guix, Rafael Hijano, Belén Lloveras; Hospital Universitari Parc Taulí: Antón Aguilà, María Rosa Bella, Carmen Blazquez, Teresa Bonfill). This work was supported by grants from the Instituto de Salud Carlos III-ISCIII (Spanish Government) co-funded by FEDER funds / European Regional Development Fund (ERDF)—a way to build Europe (References: PI1102096, PI1401918, PI1500500, PI1501205, RD12/0,036/0,056, CIBERESP CB06/02/0,073 CIBERONC CB16/12/0,040), Agència de Gestió d’Ajuts Universitaris i de Recerca (2017SGR1085), Department of Health of the Generalitat de Catalunya (PERIS-2016–2020 SLT002/16/00,404 and SLT006/17/76), Rio Hortega-SEOM (ISCIII-Spanish Society of Medical Oncology) (personal grant to MT) and from Sanofi Pasteur MSD and Merck & Co, Inc., who had no role in the data collection, analysis or interpretation of the results.
Author information
Authors and Affiliations
Contributions
MM, LA, MT, RC and ST conceptualized the study. MM, LA, MT, RC, ST and JFG designed the study. MM, MT, XL, JG, RM, OB, TB, AA, MG, RH, AB collected the data. BQ, JFG, ST, MM, MT and LA assured the quality control of data and algorithms. OC, MTo, BLL, MA, MAP, MM, BQ, ST, MT and LA analysed and interpreted the data. JFG, SM, ST and RC did the statistical analyses. MM and JFG prepared the manuscript. MM edited the manuscript. All authors reviewed and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
RM has received personal fees and non-financial support from Merck, and personal fees from Astra Zeneca and MSD. MT has received scientific advisory board fees, speaker’s fees, travel grants or non-financial support from Merck, Astra Zeneca, Nanobiotics, MSD and Bristol Meyers. Cancer Epidemiology Research Program (LA, MM, JF, SM, ST, BQ, OC, MT, MTo, MP) has received sponsorship for grants from Merck and co, Roche, Reig-jofre, IDT, Seegene, Hologic and GSK. The rest of authors have declared no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mena, M., Frias-Gomez, J., Taberna, M. et al. Epidemiology of human papillomavirus-related oropharyngeal cancer in a classically low-burden region of southern Europe. Sci Rep 10, 13219 (2020). https://doi.org/10.1038/s41598-020-70118-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70118-7
This article is cited by
-
Global prevalence of human papillomavirus-related oral and oropharyngeal squamous cell carcinomas: a systematic review and meta-analysis
Clinical Oral Investigations (2023)
-
2011–2021 rising prevalence of HPV infection among oropharyngeal carcinoma in France
BMC Cancer (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.