Abstract
This study aimed to identify prostaglandin synthases (PGS) that mediate bisphenol A (BPA)-induced prostatic hyperplasia and explore their underlying mechanisms. In an in vivo study, male adult Sprague–Dawley rats were treated with different concentrations of BPA (10, 30, 90, or 270 μg/kg, i.g., daily), or with vehicle for 4 weeks. Results revealed that low-dose BPA induced prostatic hyperplasia with increased PCNA/TUNEL ratio. It significantly upregulated the expression of cyclooxygenase-2 (COX-2) and NF-κB in the dorsolateral prostate (P < 0.05) and the expression of lipocalin-type prostaglandin D synthase (L-PGDS) in ventral prostate (P < 0.05). The level of estradiol (E2)/testosterone (T) and expression of androgen receptor (AR) and estrogen receptor α (ERα) were also altered. In vitro studies showed that low-dose BPA (0.1–10 nM) promoted the proliferation of human prostate fibroblasts and epithelial cells, and significantly upregulated the expression of COX-2 and L-PGDS in the cells. The two types of cell proliferation induced by BPA were inhibited by COX-2 inhibitor (NS398) and L-PGDS inhibitor (AT56), with increased apoptosis level. These findings suggested that COX-2 and L-PGDS could mediate low-dose BPA-induced prostatic hyperplasia through pathways involved in cell proliferation and apoptosis, which might be related to the functions of ERα and AR. The role of COX-2/NF-κB pathway in dorsolateral prostate requires further research.
Similar content being viewed by others
Introduction
Bisphenol A (BPA) is a synthetic plasticizer that is widely used to package daily necessities1. Environmental exposure to BPA has potential toxicity to the tissues of male system including those of the testes and prostate2, leading to abnormal prostate development and a trend of hyperplasia3. Prostate epithelial cells and prostate fibroblasts are the two main cell that constitute prostate tissues. Excessive proliferation of epithelial cells and prostate fibroblasts, and the transformation of epithelial cells to mesenchymal cells are involved in the pathogenesis of prostate hyperplasia4,5. Low-dose BPA (0.01-1 nM) has been reported to promote the proliferation of primary prostate epithelial cells in rats6. Similar to estradiol (E2), BPA promotes the proliferation of human prostate epithelial stem cells and increases the possibility of human prostate epithelial carcinoma7. However, the underlying mechanism of its effect on prostate cell proliferation and prostate hyperplasia remains unclear. As a typical environmental endocrine disruptor (EDC), BPA can disturb the endocrine functions by mimicking, enhancing, or inhibiting the endogenous estrogen activity, interfering with the androgen system8, and affecting the expressions of endocrine hormone-related genes and pathways.
Prostaglandin synthases (PGS) catalyze the formation of different active prostaglandins (PGs) in the arachidonic acid metabolic pathway. There are four main PGS that are closely associated with hormonal function. Cyclooxygenase-2 (COX-2) is an inducible prostaglandin H synthase that is less expressed in normal tissues but highly expressed when induced by cell-growth factors, inflammatory factors, and hormones9. In the male reproductive system, the overexpression of COX-2 is related to testosterone-induced hyperplasia, proliferation of seminal vesicle cells, and prostate cancer invasion10. COX-2/PGE signaling pathway is involved in the progression of benign prostatic hyperplasia (BPH)11. PGE2 synthase (PGES) catalyzes the conversion of PGH2 into prostaglandin E2. Similar to COX-2, the membrane-bound prostaglandin E2 synthase1 (mPGES-1) is generally overexpressed in hormone-sensitive diseases. In addition, mPGES-1/PGE2 mediates the development and progression of prostate cancer12,13. As a responsive inflammatory factor, NFκB interacts with the COX-2/PGE pathway to mediate the progressions of prostatitis14 and prostate cancer15. Lipocalin-type prostaglandin D synthase (L-PGDS) is a bifunctional protein that catalyzes the synthesis of prostaglandin D2 and functions as a transporter of lipophilic substances. The production and action of L-PGDS are regulated by the androgen hormone in the male reproductive system. Testosterone and testicular secretion assist in restoration and increase of the L-PGDS level in rat epididymis after castration16. Furthermore, L-PGDS/PGD2 mediates androgen-induced male alopecia (AGA)17, which is subsequently associated with a high incidence of BPH18. Our previous study found that after BPA administration, the PGDS transcription is increased, which might lead to the occurrence of prostate hyperplasia19. Prostaglandin F synthase (PGFS) is a terminal enzyme that catalyzes PGH2 and PGD into prostaglandin F. As a type of PGFS, Aldosterone reductase (AKR1C3) interconverts testosterone with delta(4)-androstene-3,17-dione, inactivates 5alpha-DHT to reduce active androgens in the prostate, and converts delta(4)-androstene-3,17-dione to testosterone (a substrate aromatizable to 17 beta-oestradiol) to enhance estrogenic activity in the mammary gland20. Thus, AKR1C3 is regarded as a target of different hormone-dependent diseases including prostate cancer21.
Our previous study revealed that BPA upregulated the transcriptional level of prostaglandin D2 synthase (PTGDS) gene, inducing prostate hyperplasia in adult rats19. PGS might be involved in the development of prostate, and is closely associated with the progression of prostate diseases. As BPA can interfere with the action of hormones and affect the normal development of prostate during the interaction of PGS with endocrine hormones, we hypothesized that PGS is involved in BPA-induced prostatic hyperplasia.
Materials and methods
Animal treatment
Male Sprague–Dawley (SD) rats were purchased from Sino-British SIPPR/BK Laboratory Animal Co. Ltd (Shanghai, China), housed in standard 80 polypropylene cages with sawdust bedding, and fed a pellet diet (Shanghai Shilin Science & Tech Co., Ltd., China) and water in glass bottles, ad libitum. The feed, water, and cages were all autoclaved, and the litter was changed every two days. The rats were housed in a room maintained at 20–26 °C at a humidity of 40–70% under a 12-h:12-h light/dark cycle. All animals were handled according to the Guidelines for the Care and Use of Laboratory Animals. All experimental procedures were approved by the Laboratory Animal Ethics Committee of the Shanghai Institute of Planned Parenthood Research. Fifty 3-month-old male rats were randomly divided into five groups (n = 10), and treated separately with vehicle and BPA (10, 30, 90, 270 µg/kg, daily gavage) for 4 weeks. All animals were weighed prior to being sacrificed. After the final administration of BPA, the blood from each animal was collected and the animals were sacrificed by decapitation. After the prostate glands were collected, the ventral and dorsolateral lobes were dissected and weighed. A section of the prostate gland was immediately fixed in 10% formalin, while the other was frozen in liquid nitrogen at − 80 °C until further use.
Histological evaluation
The formalin-fixed tissues were embedded in paraffin, and then were cut into 3-μm-thick sections using a microtome. The sections were deparaffinized, rehydrated, and stained using hematoxylin and eosin (H&E). The slides were observed under a microscope (Nikon, Japan).
ELISA detection
Blood was collected from the animals and centrifuged to collect serum. The prostate tissues were cut into pieces, ground with physiological saline (w:v = 1:10), and centrifuged to obtain the supernatant. The serum levels of testosterone (T) and estradiol (E2) and the levels of COX-2, L-PGDS, and PGFS in prostate tissues were measured using enzyme-linked immunosorbent assay kits (Testosterone/Estradiol ELISA Kits, Cayman, USA; PTGS2/ PGD2S/ PGFS ELISA Kits, MyBioSource, USA) and an enzyme-linked immunosorbent analyzer (Zenyth 200st, Austria).
Immunohistochemistry
Immunohistochemical analysis of the paraffin sections of prostate tissues was performed to analyze the expressions of proliferating cell nuclear antigen (PCNA), androgen receptor (AR), estrogen receptor α (ERα), cyclooxygenase-2 (COX-2), L-type prostaglandin synthase (L-PGDS), and transcription factors nuclear factor κB (NF-κB). The sections were incubated with primary antibodies against PCNA (sc-56, Santa Cruz, USA), rabbit anti-AR (BA0004, Wuhan Boster, Wuhan, China), ER polyclonal (212441-1-AP, ProteinTech Group,Inc., USA), rabbit anti-rat COX-2 (sc-166475, Santa Cruz, USA), rabbit anti-rat L-PGDS (ab182141, Abcam, UK), and rabbit anti-rat NF-κB p65 (8242, Cell Signaling, USA), respectively, at a dilution of 1:100 overnight at 4 °C. The sections were then incubated with secondary antibody at 37 °C for 20 min. A negative control was establlished by omitting the use of primary antibodies. The experimental operations were perfomed according to those established in a previous study8. Six tissues sections were selected from each group, and ten microscopic fields for each section were randomly chosen was randomly observed. Semi-quantitative analysis of protein expression and the positive expression rate of PCNA were determined using Image-Pro Plus 6.0 to obtain the optical density of the slice.
TUNEL assay
The rate of apoptosis in prostate tissues was detected using a TUNEL kits (rabbit polyclonal IgG, Abcam, UK). The paraffin sections were treated with proteinase K (20 µg/ml in 10 mM Tris HCl, pH8.0) for 30 min at room temperature. Endogenous peroxidases were blocked with 3% H2O2 for 5 min. Subsequently, the sections were incubated with biotinylated-TdT and enzyme TdT at 37 °C in the dark for 1 h. After the reaction was completed, the biotinylated nucleotides were detected using streptavidin peroxidase conjugate, stained with Harris’s hematoxylin for 5 min, and then stopped via the addition of water. The sections were dehydrated, washed, and mounted. The positive rate of apoptosis was determined using Image-Pro Plus 6.0.
Western blot
Animal tissues were cut into pieces and homogenized with fivefold volumes of ice-cold RIPA lysate buffer (Weiao, China), followed by centrifugation to collect the supernatants. The cells were collected and RIPA lysate buffer (Weiao, China) was added to extract the protein. The total protein concentration was determined using a BCA kit per manufacturer’s protocol. The primary antibodies against COX-2 (sc-166475, Santa Cruz, USA; 1:800), L-PGDS (ab182141, Abcam, UK; 1:1000), NF-κB p65 (8242, Cell Signaling, USA; 1:1000) and GAPDH (WB0197, Weiao, China; 1:2000) were used to detect expressions of the COX-2, L-PGDS and NF-κB p65 proteins, respectively. The secondary antibodies included horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (Jackson, USA; 1:2000). The membrane was stained with ECL reagent (Weiao, China), and the average optical density (IOD) of the bands in the membrane was detected using Image-Pro Plus 6.0. The relative expression of protein was equivalent to the ratio of the IOD of the protein band to that of the GAPDH band.
Cell culture
Human prostate epithelial cells (HPEpiC) and human prostate fibroblasts (HPrF) were purchased from American Sciencell Company, and cultured in prostate epithelial cell medium and fibroblast medium (Sciencell) as described in manufacturer’s protocol.
Cell viability
The cell viability of HPEpiC and HPrF was measured using a CCK-8 kit (DojinDo, Japan). HPEpiC at a density of 5,000 cells per well and HPrF at a density of 2,500 cells per well were seeded in 96-well plates and cultured for 24 h. To determine the effect of BPA on prostate cell proliferation, the cells in 96-well plates were incubated with BPA (0.01 nM–100 nM) diluted in cell medium containing 1‰ DMSO for 72 h. To study the effect of the PGS inhibitors, the cells were treated with 1‰ DMSO, BPA, BPA with COX-2 inhibitor, COX-2 inhibitor (NS398), BPA with L-PGDS inhibitor, and L-PGDS inhibitor (AT56) for 72 h. The CCK-8 reagents were diluted with cell medium (dilution, 1:9). Thereafter, the diluted mixture was added to the wells (100 µl per well). The cells were incubated at 37 °C for 1–4 h. The optical density (OD) was read using a microplate reader (Thermo Fisher Scientific, USA) at a wavelength of 450 nm; the cell viability (% of control) was calculated according to the manufacturer’s instructions.
Apoptosis assay
HPEpiC at a density of 4 × 105 cells per well and HPrF at a density of 2 × 105 cells per well were seeded in 6-well plates and cultured for 24 h. Thereafter, the cells were treated with 1‰ DMSO, BPA, BPA with COX-2 inhibitor, COX-2 inhibitor (NS398), BPA with L-PGDS inhibitor, and L-PGDS inhibitor (AT56) for 72 h. The rate of apoptosis was detected using the FITC Annexin V apoptosis detection kit (BD, USA), and then measured via flow cytometry (Beckman, USA).
Statistical analysis
Statistical comparisons were performed using one-factor analysis of variance (ANOVA). If statistically significant, the differences in the planned comparisons between the control and treatment groups were derived using Dunnett’s least-squares means test. Data were analyzed using SPSS version 23.0 (SPSS, IL, USA) and are presented as mean ± standard deviation.
Results
BPA causes imbalance in cell proliferation and apoptosis and induces prostatic hyperplasia in adult rats
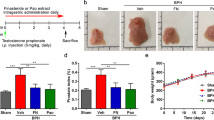
During the 4-week administration period, BPA within the dose range had no significant effect on the weight gain of rats. The organ coefficients of the whole prostate and the ventral prostate were significantly increased in the BPA (30 µg/kg)-treated group compared to the control (P < 0.05) (Fig. 1).
Effect of BPA on body weight and prostate organ coefficient in adult rats. (a) The change in weight of rats during the 4-week period. Effect of BPA on the organ coefficient of prostate (b) and all lobes of the prostate (c). BPA: Bisphenol A. Values are presented as mean ± SD. Organ coefficient of DLP = The weight of DLP/Terminal body weight × 1,000. (n = 10, *P < 0.05 compared to control).
Based on the results of H&E staining, the gland cavity of ventral and dorsolateral prostates in BPA-treated group sufficiently expanded compared to the control group. The glandular secretions were increased in the BPA-treated groups. The epithelial height of the prostate samples in the BPA-treated groups was higher than that in the control group (Fig. 2).
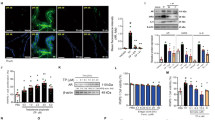
PCNA is a cell-proliferation antigen that is mainly expressed in the nucleus. Herein, a TUNEL assay was performed to detect apoptosis in the tissues. Cell proliferation and apoptosis in tissues were measured using the ratio of PCNA to TUNEL22. Compared to the control, the positive rate of PCNA in the ventral prostates of the BPA group was significantly increased (P < 0.01), and reached a maximum in the BPA (30 µg/kg) group (P < 0.01). The apoptotic rate of ventral prostates was significantly decreased in the low-dose BPA groups (10 and 30 µg/kg) (P < 0.01). Thus, the PCNA/TUNEL ratio in the ventral prostate increased after BPA administration (P < 0.01), and reached a peak in the BPA (30 µg/kg)-treated group (Fig. 3). The changing trends of the PCNA-positive and TUNEL-positive rates after BPA treatment in the dorsolateral prostate were similar to those in the ventral prostate. Compared to the control, the PCNA/TUNEL ratio of dorsolateral prostate was increased in the BPA (30–90 µg/kg)-treated groups (P < 0.01) and decreased in the high-dose BPA-treated group (270 µg/kg) to the level of the control (Fig. 4).
Effect of BPA on cell proliferation and death in the ventral prostate of adult rats. (a) Immunohistochemical images of PCNA and TUNEL in the ventral prostate; (b) Effect of BPA on PCNA-positive ratio and TUNEL-positive ratio in the ventral prostate; (c) Effect of BPA on the ratio of PCNA/TUNEL in the ventral prostate. VP: Ventral prostate. Scale bar = 50 µm, magnification × 200. Values are presented as mean ± SD (n = 4, *P < 0.05, *P < 0.01, compared to the control).
Effect of BPA on cell proliferation and death in the dorsolateral prostate of adult rats. (a) Immunohistochemical images of PCNA and TUNEL in the dorsolateral prostate; (b) Effect of BPA on PCNA-positive ratio and TUNEL-positive ratio in the dorsolateral prostate; (c) Effect of BPA on the ratio of PCNA/TUNEL in the dorsolateral prostate. DLP: dorsolateral prostate. Scale bar = 50 µm, magnification × 200. Values are presented as mean ± SD (n = 4, *P < 0.05, *P < 0.01, compared to the control).
BPA treatment alters the E2 and T levels in the serum and the contents of prostaglandin synthases in the prostate
As shown in Fig. 5a–c, the serum levels of E2 in adult rats were significantly increased in the BPA (30 µg/kg)-treated group due to BPA administration for 4 weeks (P < 0.05) while the testosterone levels in serum were slightly decreased in the BPA-treated group. The ratio of E2 to T was significantly increased in the BPA (30 μg/kg and 270 μg/kg)-treated groups (P < 0.01). As shown in Fig. 5d–f, the COX-2 level in the ventral prostate in the BPA-treated group was lower than that in the control group while that of the dorsolateral prostate was increased in the BPA (30 μg/kg and 90 μg/kg)-treated groups (P < 0.05). The L-PGDS level of the ventral prostate in the BPA-treated groups was significantly higher than that in the control group (P < 0.01) and reached a maximum in the BPA (30 μg/kg)-treated group. The L-PGDS content of the dorsolateral prostate showed no significant change. No significant alteration of the PGFS content in the ventral and dorsolateral prostates was observed upon treatment with BPA.
Effect of BPA on the serum levels of E2 and T and the content of COX-2, L-PGDS, and PGFS in the prostate of adult rats. E2 (a) and T (b) levels in serum, and E2/T ratio (c); (d–f) COX-2, L-PGDS, and PGFS content in the prostate. Values are presented as mean ± SD (n = 6, *P < 0.05, *P < 0.01, compared to the control).
BPA induces a higher expression of AR and ERα.
The results of immunohistochemical analysis (Fig. 6) revealed that ERα expression in the ventral prostate was increased in groups treated with BPA (10 and 30 µg/kg, P < 0.01 and 270 µg/kg, P < 0.05) compared to the control. The ERα expression level in the dorsolateral prostate increased as the BPA dose (30–270 µg/kg) increased (P < 0.05). Compared to the control, AR expression in the ventral prostate was increased in groups treated with BPA (30 µg/kg, P < 0.05 and 90 µg/kg, P < 0.01) while that in the dorsolateral prostate of the BPA-treated groups showed no significant difference.
Immunohistochemical analysis of ERα and AR in the prostate of adult rats. (a) Immunohistochemical images of ERα and AR in the ventral and dorsolateral prostates; Effect of BPA on ERα and AR expression in the ventral prostate (b) and dorsolateral prostate (c). VP: ventral prostate; DLP: dorsal prostate. Scale bar = 50 µm, magnification × 200. Values are presented as mean ± SD (n = 4, *P < 0.05, *P < 0.01, compared to the control).
Low-dose BPA increases the expression of L-PGDS, COX-2 and NF-κB
As shown in Fig. 7, the expression level of COX-2 in the dorsolateral prostate of the BPA-treated group was higher than that in the control group and significantly increased in the BPA (90 µg/kg)-treated group (P < 0.05). In addition, the expression level of L-PGDS was increased in the BPA-treated groups (P < 0.01). The results of western blotting indicated that the relative expression levels of the L-PGDS protein in the ventral prostate and COX-2 protein in the dorsolateral prostate were increased in the BPA (90 µg/kg)-treated groups relative to the control group (P < 0.05, P < 0.01).
Effect of BPA on the expression of COX-2 and L-PGDS in the prostate. (a) Immunohistochemical images of COX-2 in the dorsolateral prostate and L-PGDS in the ventral prostate; Effect of BPA on COX-2 (b) and L-PGDS (c) expression in the prostate. (Scale bar = 50 µm, magnification × 200, n = 4). The protein bands of COX-2 and L-PGDS (d); The expression levels of the COX-2 protein in the dorsolateral prostate (e) and L-PGDS protein in the ventral prostate (f). Blots (d) were cropped from the gel presented in Supplementary Fig. 1. Bands were quantified using densitometry, with the results normalized to GAPDH expression in each sample (n = 3). Values are presented as mean ± SD (*P < 0.05, **P < 0.01, compared to the control).
Low-dose BPA treatment increased the expression of NF-κB p65 in the dorsolateral prostate (Fig. 8). Immunohistochemical staining (Fig. 8a) showed that BPA treatment induced an increase of the NF-κB p65 expression in the dorsolateral prostate. The results of western blotting indicated that the NF-κB p65 protein level was significantly increased in the BPA (90 µg/kg)—treated group (P < 0.05) compared to the control.
Effect of BPA on NF-κB p65 expression in the dorsolateral prostate. (a) Immunohistochemical staining of NF-κB in the prostate (Scale bar = 200 µm, magnification × 100, n = 4); (b) the protein band and the expression level of the NF-κB p65 protein. Bands were quantified using densitometry, with the results normalized to GAPDH expression in each sample (n = 3). Values are presented as mean ± SD (*P < 0.05, compared to the control).
Low-dose BPA promotes prostate cell proliferation and up-regulates the expression of COX-2 and L-PGDS
CCK-8 detection results showed that the viability of human prostate fibroblasts in the BPA-treated group was increased (P < 0.01) relative to the control and reached a maximum in the BPA-treated group (0.1 nM). The viability of human prostate epithelial cells in the BPA-treated groups was also increased (P < 0.01). A similar trend was observed for prostate fibroblasts and reached a maximum with BPA (1 nM) (Fig. 9a). Thus, 0.1 nM and 1 nM were considered as optimal doses of BPA to promote the proliferation of prostate fibroblasts and prostate epithelial cells.
Effect of BPA on the viability of human prostate epithelial cells and human prostate fibroblasts and the expression of COX-2 and L-PGDS proteins in prostate cells. (a) Effect of BPA on the viability of HPEpiC and HPrF. The protein bands of COX-2 and L-PGDS in HPEpiC (b) and HPrF (d). The expression levels of the COX-2 and L-PGDS proteins in HPEpiC (c) and HPrF (e). Blots (b, d) were cropped from the gel presented in Supplementary Figure S2. Bands were quantified using densitometry, with results normalized to GAPDH expression in each sample. HPEpiC: human prostate epithelial cells; HPrF:human prostate fibroblasts. Values are presented as mean ± SD (n = 3, *P < 0.05, **P < 0.01, compared to the control).
Results of western blotting revealed that 0.1–10 nM BPA upregulated the expression of COX-2 and L-PGDS in prostate epithelial cells in a dose-dependent manner. Significant proliferation of prostate fibroblasts and prostate epithelial cells was observed in BPA (10 nM)-treated group (P < 0.05). The expression of COX-2 and L-PGDS in prostate fibroblasts was increased in BPA (0.01 nM)-treated group (P < 0.05, Fig. 9).
Inhibition of COX-2 and L-PGDS inhibits BPA-induced prostate cell proliferation and promotes apoptosis
To determine the effects of the prostate synthase inhibitors on BPA-induced prostate cell proliferation, a CCK-8 assay was used to detect the viability of prostate cells treated with BPA and prostate synthase inhibitors. The apoptotic rate was determined using FITC AV-PI double staining and flow cytometry. As seen in Fig. 10, compared to the BPA-treated group, the COX-2 specific inhibitor (NS398) and L-PGDS specific inhibitor (AT56) significantly inhibited the prostate epithelial cell proliferation induced by BPA (1 nM) (P < 0.01, Fig. 10a). Compared to the BPA (0.1 nM)-treated group, the cell viability of prostate fibroblasts was significantly decreased by NS398 (P < 0.05) and AT56 (P < 0.01) (Fig. 10b). Both NS398 and AT56 inhibited the growth of prostate epithelial cells; however, the inhibitory effects of AT56 were more potent. The apoptotic rate of prostate epithelial cells treated with BPA was lower than that of the control. The administration of BPA combined with NS398 or AT56 increased the apoptotic rate of prostate cells compared to the BPA-treated group.
COX-2 and L-PGDS inhibitors suppressed the proliferation of prostate cells induced by BPA and promoted apoptosis. Effect of the COX-2 and L-PGDS inhibitors on the viability of HPEpiC (a) and HPrF (b); Effect of the COX-2 and L-PGDS inhibitors on HPEpiC (c) and HPrF (d). (HPEpiC: human prostate epithelial cells; HPrF: human prostate fibroblasts. FL1: FITC channel, FL3: PI channel; B2: FITC+/PI+, late stage apoptotic cells; B3: FITC-/PI−, normal cells; B4: FITC+/PI−, viable apoptotic cells; NS398: COX-2 inhibitor, AT56: L-PGD inhibitor).
Discussion
In vivo studies demonstrated that adult SD rats treated with 10–270 μg/kg BPA for 4 weeks could suffer from prostatic hyperplasia, and in vitro studies indicated that low-dose BPA (0.1 nM and 1 nM) significantly promoted the proliferation of prostate fibroblasts and epithelial cells in humans. The PGS COX-2 and L-PGDS showed significant upregulation in both in vivo prostatic hyperplasia and in vitro prostate cell proliferation induced by low-dose BPA. In vitro data further supported that COX-2 and L-PGDS play a critical role in prostate cell proliferation.
Environmental exposure to BPA exerted low-dose effects of promoting proliferation on the prostate. Results from animal experiments indicated that a 1–2 μg/(kg d) exposure level of BPA in the environment1, and a daily safe dose of 50 μg/(kg d)23 could increase the number and volume of the dorsolateral prostate in the mice offsprings3 as well as the weight of the ventral prostate in rats24. This study further confirmed that a daily administration of 10–270 μg/kg BPA in adult rats could induce prostatic hyperplasia, which is characterized by the increased organ coefficient of prostate, PCNA expression and the ratio of proliferation/apoptosis. To study the low-dose effects of BPA on the prostate in vitro, two prostate cell lines representing the prostate fibroblasts and epithelial cells were used. The BPA concentration in vitro is generally lower than 100 nM (10−7 M)25. Further, 1–10 nM BPA disturbs the early morphogenesis of human prostate stem cells in a dose-dependent manner26 and promotes the migration of prostate cancer cells27. This study found that BPA (0.01–100 nM) promoted the proliferation of normal human prostate epithelial cells and fibroblasts, and decreased apoptosis.
An imbalance in cell proliferation and apoptosis is a critical pathogenesis of prostate hyperplasia. In vivo PCNA and TUNEL assays showed that with an increase in the BPA dose, the ratio of proliferation to apoptosis in the ventral and dorsolateral prostates initially increased and then decreased, indicating that low-dose BPA induced prostate hyperplasia by promoting the proliferation of prostate cells. However, the increased apoptosis induced by high-dose BPA treatment might attenuate the trend of hyperplasia28. Prostate is a hormone-sensitive organ, and the interaction between estrogen and androgen is essential to maintain its normal development29,30. As a non-steroidal estrogen, BPA can disturb the expression of hormone receptors in some target tissues and affecte the endogenous hormone activity. By binding to the estrogen receptors ERα and ERβ, BPA regulates estrogen signaling pathways and the expression of target genes. ERα mediates BPA-induced prostate cell proliferation25, while ERβ mediates apoptosis induced by high-dose BPA7, stimulating the ERK-dependent signaling pathway to induce the expression of p53 through a pathway involving the ERβ/EGFR complex31. High-dose BPA has strong anti-androgenic activity32, while low-dose BPA can promote AR expression in prostate cells33. Our study revealed that low-dose BPA could significantly promote the expression of AR in the ventral prostate. When the ratio of estrogen to androgen is increased, the upregulated AR expression might increase the sensitivity of the prostate to androgens, thereby promoting prostate hyperplasia.
COX-2 and L-PGDS play critical roles in maintaining normal physiological function. Abnormal expression is associated with pathological changes in normal systems including the male reproductive system. In this study, we found that low-dose BPA upregulated COX-2 and L-PGDS expression and induced prostatic hyperplasia; however, their inhibition attenuated the effects of BPA. The regulation of COX-2 by BPA might be related to the interaction of COX-2 and estrogen. In various estrogen-dependent tumors including endometrial cancer and ovarian cancer, estrogen promotes the proliferation and invasion of cancer by upregulating COX-2 expression34,35. COX-2 is involved in the positive feedback regulation between estrogen and inflammatory factors, where COX-2/mPGES-1 upregulates the expression of aromatase and increases the levels of estrogen, which in turn upregulates COX-2 expression via combination with the estrogen receptor36. Low-dose BPA also upregulates aromatase, which catalyzes the production of estradiol from testosterone to increase the estradiol levels in adult rats37. In the male reproductive system, COX-2 inhibits the expression of the steroid-derived acute regulatory protein (StAR) gene in Leydig cells and induces an age-related decrease in testosterone levels38. Thus, BPA might directly induce COX-2 expression to increase E2 secretion and decrease T levels, while the increased estrogen in turn upregulates COX-2 expression in the prostate. Previous studies reveal that BPA induces the invasion of prostate cancer and hyperplasia of the dorsolateral prostate via the epithelial-mesenchymal transition (EMT) pathway5,39. The EMT pathway activated by COX-240 is involved in the inflammatory response41. In addition, the upregulation of the NF-κB/COX-2/PGE pathway11 mediates the proliferation of cells. NF-κB activation was triggered by extracellular stimulation to upregulate many intracellular downstream signaling pathways including COX-2/PGE. Previous studies report that DHT treatment, in vivo or ex vivo, induces nuclear NF-κB activation and increases the levels of proinflammatory products of NF-κB activation including COX-242. The dysregulation of NF-κB can induce autoimmune diseases, chronic inflammation, and cancers43. The upregulated NF-κB and its induced signaling pathways are involved in the development of prostate cancer and prostatitis44,45. GHRH stimulates the NFκB p65 pathway, which is involved in inflammation and growth in both BPH-1 and PrEp cells. Conversely, the GHRH antagonist significantly reduces these effects and decreases the inflammatory marker, COX-246. BPA increases the expression of the proinflammatory mediator, PGE2, and its upstream factor, COX-2, with significant induction of phosphorylation and nuclear translocation of NF-κB p6547. The activation of NF-κB by BPA promotes an invasion process in breast cancer cells48. Additionally, low-dose BPA exerts its inhibition on apoptosis via increased anti-apoptotic protein Bcl-2 level49 and upregulates the phosphorylation of NF-κB p6550. Decreased COX-2 levels or COX-2 inhibitors potentiated apoptosis, which is related to the suppression of gene products associated with cell apoptosis (Bcl-2 and Bax)51. Thus, the upregulated NF-κB p65/COX-2 in the prostate might mediate BPA-inhibited apoptosis. The role of the NF-κB/COX-2 pathway in the effect of low-dose BPA on cell proliferation and apoptosis in the prostate deserves further research.
L-PGDS, which is mainly distributed in the Leydig cells of the testis and epithelial cells of the prostate and epididymis52, is associated with the development of male reproductive organs. The function of L-PGDS in reproductive organs is affected by androgen levels. Testosterone and testicular secretion factors in the epididymis regulate the synthesis and secretion of L-PGDS53. High-level androgens in male androgen alopecia (AGA) increase the expression of L-PGDS and PGD2 produced by L-PGDS catalysis54. Low-dose BPA promotes AR expression in prostate cells32. In this study, BPA was found to significantly increase AR and L-PGDS expression in the ventral prostate. Thus, under the conditions of increased estrogen/androgen ratio, low-dose BPA might increase L-PGDS expression by upregulating AR-induced androgen sensitivity of the ventral prostate. Studies have shown that L-PGDS is highly expressed in AGA, which is associated with a high incidence of BPH55. However, the role of L-PGDS in BPH remains unclear. PGDS is a bifunctional protein that catalyzes the synthesis of PGD and transports multiple lipophilic substances52. During PGFS catalysis, the PGD2 produced by L-PGDS catalysis is converted to PGF2, which is bound to the FP receptor to promote cell proliferation by activating the growth factor-dependent MAP kinase pathway21. In vitro studies have indicated that L-PGDS mediates cell proliferation via its lipid-carrying function in different cells. In human epidermal melanocytes, normal L-PGDS levels restrict the growth of epidermal melanocytes by transporting all-trans retinoic acid (RA), while its overexpression dysregulates the proliferation of melanoma cells56. Additionally, the protein L-PGDS accelerates the migration of glial cells and promotes reactive gliosis by interacting with protein kinase C substrate (MARCKS)57. Therefore, we hypothesized that L-PGDS is an apolipoprotein in the prostate that might contribute to the accumulation of the liposoluble substance, BPA, in and around prostate cells. L-PGDS may also assist BPA in promoting proliferation..
Conclusion
This study is the first to reveal the functions of COX-2 and L-PGDS in prostatic tissues. PGS can be upregulated by low-dose BPA treatment to mediate BPA-induced prostatic hyperplasia. COX-2 and ERα, which are regulated by estrogen, might be involved in BPA-induced hyperplasia of the dorsolateral prostate via the activation of signaling pathways, including COX-2/NF-κB pathway, that promote prostate cell proliferation. L-PGDS, the target gene of androgen/AR, mediates BPA-induced hyperplasia of the ventral prostate; however, the underlying mechanism of the modulation of L-PGDS/PGD in cell proliferation warrants further research.
References
Rubin, B. S. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 127, 27–34 (2011).
Hughes, F. S. V. S. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 113, 926–933 (2005).
Timms, B. G., Howdeshell, K. L., Barton, L., Bradley, S. & Richter, C. A. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. PNAS 102, 7014–7019 (2005).
Wang, L. et al. Aberrant transforming growth factor-Β activation recruits mesenchymal stem cells during prostatic hyperplasia. Stem Cell. Transl. Med. 6, 394–404 (2017).
Huang, D. et al. Oral exposure of low-dose bisphenol A promotes proliferation of dorsolateral prostate and induces epithelial-mesenchymal transition in aged rats. Sci. Rep. 8, 1–10 (2018).
Huang, D., Wu, J., Su, X., Yan, H. & Sun, Z. Effects of low dose of bisphenol A on the proliferation and mechanism of primary cultured prostate epithelial cells in rodents. Oncol. Lett. 14, 2635–2642 (2017).
Calderon-Gierszal, E. L. & Prins, G. S. Directed differentiation of human embryonic stem cells into prostate organoids in vitro and its perturbation by low-dose bisphenol A exposure. PLoS ONE 10, e133238 (2015).
Sun, H., Xu, L. C., Chen, J. F., Song, L. & Wang, X. R. Effect of bisphenol A, tetrachlorobisphenol A and pentachlorophenol on the transcriptional activities of androgen receptor-mediated reporter gene. Food Chem. Toxicol. 44, 1916–1921 (2006).
Zhang, S. et al. Hydrogen sulfide promotes cell proliferation of oral cancer through activation of the COX2/AKT/ERK1/2 axis. Oncol. Rep. 35, 2825–2832 (2016).
Narayanan, B. A., Condon, M. S., Bosland, M. C., Narayanan, N. K. & Reddy, B. S. Suppression of N-methyl-N-nitrosourea/testosterone-induced Rat Prostate cancer growth by celecoxib: effects on cyclooxygenase-2, cell cycle regulation, and apoptosis mechanism(S). Clin. Cancer Res. 9, 3503–3513 (2003).
Xie, C. et al. Down-regulated CFTR during aging contributes to benign prostatic hyperplasia. J. Cell. Physiol. 230, 1906–1915 (2015).
Ruan, D. & So, S. Prostaglandin E2 produced by inducible COX-2 and mPGES-1 promoting cancer cell proliferation in vitro and in vivo. Life Sci. 116, 43–50 (2014).
Terzuoli, E. et al. Linking microsomal prostaglandin E synthase-1/PGE-2 pathway with miR-15a and -186 expression: novel mechanism of VEGF modulation in prostate cancer. Oncotarget 7, 44350–44364 (2016).
Jeon, S. H. et al. Extracorporeal shock wave therapy decreases COX-2 by inhibiting TLR4-NFkappaB pathway in a prostatitis rat model. Prostate 79, 1498–1504 (2019).
Lieberman, R. Chemoprevention of prostate cancer: current status and future directions. Cancer Metastasis Rev. 21, 297–309 (2002).
Zhu, H. et al. L-Prostaglandin D synthase expression and regulation in mouse testis and epididymis during sexual maturation and testosterone treatment after castration. Endocrine 24, 39–45 (2004).
Villarreal-Villarreal, C. D. et al. Prostaglandins in Androgenetic Alopecia in 12 Men and 4 Female. J. Eur. Acad. Dermatol. Venereol. 33, e214–e215 (2019).
Jin, T. et al. Association between male pattern baldness and prostate disease: a meta-analysis. Urol. Oncol. 36, 80–87 (2018).
Wu, J., Huang, D., Su, X., Yan, H. & Sun, Z. Oral administration of low-dose bisphenol A promotes proliferation of ventral prostate and upregulates prostaglandin D2 synthase expression in adult rats. Toxicol. Ind. Health. 32, 1848–1858 (2016).
Penning, T. M. et al. Human 3Alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the Aldo-Keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 351, 67–77 (2000).
Penning, T. M. et al. Aldo-keto reductase (AKR) 1C3: role in prostate disease and the development of specific inhibitors. Mol. Cell. Endocrinol. 248, 182–191 (2006).
Colleta, S. J. et al. Acute Exposure to bisphenol A and cadmium causes changes in the morphology of gerbil ventral prostates and promotes alterations in androgen-dependent proliferation and cell death. Environ. Toxicol. 32, 48–61 (2017).
Herath, C. B. et al. Adverse effects of environmental toxicants, octylphenol and bisphenol A, on male reproductive functions in pubertal rats. Endocrine 25, 163–172 (2004).
Campos, M. S. et al. Prepubertal exposure to bisphenol-A induces eralpha upregulation and hyperplasia in adult gerbil female prostate. Int. J. Clin. Exp. Pathol. 96, 188–195 (2015).
Prins, G. S. et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 155, 805–817 (2014).
Derouiche, S. et al. Bisphenol A stimulates human prostate cancer cell migration via remodelling of calcium signalling. Springerplus 2, 54 (2013).
Tarapore, P. et al. Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS ONE 9, e90332 (2014).
Wang, Q. et al. Mitochondrial signaling pathway is also involved in bisphenol A induced germ cell apoptosis in testes. Toxicol. Lett. 199, 129–135 (2010).
Peng, C. C. et al. Action mechanism of ginkgo biloba leaf extract intervened by exercise therapy in treatment of benign prostate hyperplasia. Evid. Based Complement Altern. Med. 2013, 408734 (2013).
Trachtenberg, J., Hicks, L. L. & Walsh, P. C. Androgen- and estrogen-receptor content in spontaneous and experimentally induced canine prostatic hyperplasia. J. Clin. Investig. 65, 1051–1059 (1980).
Bilancio, A. et al. Bisphenol A induces cell cycle arrest in primary and prostate cancer cells through EGFR/ERK/p53 signaling pathway activation. Oncotarget 8, 115620–115631 (2017).
Richter, C. A., Taylor, J. A., Ruhlen, R. L., Welshons, W. V. & Vom, S. F. Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ. Health Perspect. 115, 902–908 (2007).
Wang, K., Kao, A., Chang, C., Lin, T. & Kuo, T. Bisphenol A at environmentally relevant doses induces cyclooxygenase-2 expression and promotes invasion of human mesenchymal stem cells derived from uterine myoma tissue. Taiwan. J. Obstet. Gynecol. 52, 246–252 (2013).
Kim, S., Campbell, J., Yoo, W., Taylor, J. A. & Sandler, D. P. Systemic levels of estrogens and PGE2 synthesis in relation to postmenopausal breast cancer risk. Cancer Epidemiol. Biomark. 26, 383–388 (2017).
Hermenegildo, C., Oviedo, P. & Cano, A. Cyclooxygenases regulation by estradiol on endothelium. Curr. Pharm. Design. 12, 205–215 (2006).
Frasor, J., Weaver, A. E., Pradhan, M. & Mehta, K. Synergistic up-regulation of prostaglandin e synthase expression in breast cancer cells by 17β-estradiol and proinflammatory cytokines. Endocrinology 149, 6272–6279 (2008).
Castro, B., Sanchez, P., Torres, J. M. & Ortega, E. Effects of perinatal exposure to bisphenol a on the intraprostatic levels of aromatase and 5alpha-reductase isozymes in juvenile rats. Food Chem. Toxicol. 115, 20–25 (2018).
Wang, X. et al. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology 146, 4202–4208 (2005).
Oral, D., Erkekoglu, P., Kocer-Gumusel, B. & Chao, M. W. Epithelial-mesenchymal transition: a special focus on phthalates and Bisphenol A. J. Environ. Pathol. Toxicol. Oncol. 35, 43–58 (2016).
Giannoni, E., Bianchini, F., Calorini, L. & Chiarugi, P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid. Redox Signal. 14, 2361–2371 (2011).
Mizoguchi, S. et al. Effects of estrogen receptor beta stimulation in a rat model of non-bacterial prostatic inflammation. Prostate 77, 803–811 (2017).
Gonzales, R. J., Duckles, S. P. & Krause, D. N. Dihydrotestosterone stimulates cerebrovascular inflammation through NFkappaB, modulating contractile function. J. Cereb. Blood Flow Metab. 29, 244–253 (2009).
Trembley, J. H. et al. CK2 pro-survival role in prostate cancer is mediated via maintenance and promotion of androgen receptor and NFkappaB P65 expression. Pharmaceuticals (Basel) 12, 89 (2019).
Fernandez-Martinez, A. B. et al. Vasoactive intestinal peptide induces cyclooxygenase-2 expression through nuclear factor-kappaB in human prostate cell lines differential time-dependent responses in cancer progression. Mol. Cell. Endocrinol. 270, 8–16 (2007).
Locatelli, M. et al. Graminex pollen: phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules 23, 1145 (2018).
Popovics, P., Cai, R., Sha, W., Rick, F. G. & Schally, A. V. Growth hormone-releasing hormone antagonists reduce prostatic enlargement and inflammation in carrageenan-induced chronic prostatitis. Prostate 78, 970–980 (2018).
Huang, F. M., Chang, Y. C., Lee, S. S., Yang, M. L. & Kuan, Y. H. Expression of pro-inflammatory cytokines and mediators induced by bisphenol A via ERK-NFkappaB and JAK1/2-STAT3 pathways in macrophages. Environ. Toxicol. 34, 486–494 (2019).
Castillo, S. R., Gomez, R. & Perez, S. E. Bisphenol a induces migration through a GPER-, FAK-, Src-, and ERK2-dependent pathway in MDA-MB-231 breast cancer cells. Chem. Res. Toxicol. 29, 285–295 (2016).
Zheng, C. M. et al. the Bisphenol A-enhanced activity of thyroid carcinoma cell line B-CPAP is inhibited by icarrin. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 52, 458–462 (2017).
Xiong, S., Wang, Y., Li, H. & Zhang, X. Low dose of bisphenol A activates NF-kappaB/IL-6 signals to increase malignancy of neuroblastoma cells. Cell. Mol. Neurobiol. 37, 1095–1103 (2017).
Fang, Q. et al. Suppression of cyclooxygenase 2 increases chemosensitivity to sesamin through the AktPI3K signaling pathway in lung cancer cells. Int. J. Mol. Med. 43, 507–516 (2019).
Tokugawa, Y. et al. Lipocalin-type prostaglandin D synthase in human male reproductive organs and seminal plasma. Biol. Reprod. 58, 600–607 (1998).
Zhu, H. et al. Expression and regulation of lipocalin-type prostaglandin D synthase in rat testis and epididymis. Biol. Reprod. 70, 1088–1095 (2004).
Fong, P. et al. In silico prediction of prostaglandin D2 synthase inhibitors from herbal constituents for the treatment of hair loss. J. Ethnopharmacol. 175, 470–480 (2015).
Rossi, A. et al. Multi-therapies in androgenetic alopecia: review and clinical experiences. Dermatol. Ther. 29, 424–432 (2016).
Shimanuki, M., Takeda, K., Kawaguchi, M., Suzuki, T. & Shibahara, S. Lipocalin-type prostaglandin D synthase as a marker for the proliferative potential of melanocyte-lineage cells in the human skin. J. Dermatol. 39, 699–704 (2012).
Lee, S. et al. Lipocalin-type prostaglandin D2 synthase protein regulates glial cell migration and morphology through myristoylated alanine-rich C-kinase substrate: prostaglandin D2-independent effects. J. Biol. Chem. 287, 9414–9428 (2012).
Acknowledgements
This work was sponsored by the Natural Science Foundation of Shanghai (grant number 19ZR1444400), the National Natural Science Foundation of China (Grant Number 21007041), Shanghai Project of Public Service Platform for Research & Development (Grant Number 18DZ229100), and the Ministry of Science and Technology of China (Grant Number 2018ZX10301403-005-001).
Author information
Authors and Affiliations
Contributions
S.S.W. and J.H.W. conceived and designed the work. S.S.W., D.Y.H., X.S., H.Y., A.C.M. and L.L. executed the experiments. S.S.W. and D.Y.H. performed the data analysis. S.S.W. wrote the manuscript. J.H.W. and Z.Y.S. theoretically directed the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, S., Huang, D., Su, X. et al. The prostaglandin synthases, COX-2 and L-PGDS, mediate prostate hyperplasia induced by low-dose bisphenol A. Sci Rep 10, 13108 (2020). https://doi.org/10.1038/s41598-020-69809-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69809-y
This article is cited by
-
Comparative RNA-sequencing analysis of the prostate in a mouse model of benign prostatic hyperplasia with bladder outlet obstruction
Molecular and Cellular Biochemistry (2023)
-
Glycoprotein PTGDS promotes tumorigenesis of diffuse large B-cell lymphoma by MYH9-mediated regulation of Wnt–β-catenin–STAT3 signaling
Cell Death & Differentiation (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.