Abstract
The microbiota thriving in the rhizosphere, the thin layer of soil surrounding plant roots, plays a critical role in plant’s adaptation to the environment. Domestication and breeding selection have progressively differentiated the microbiota of modern crops from the ones of their wild ancestors. However, the impact of eco-geographical constraints faced by domesticated plants and crop wild relatives on recruitment and maintenance of the rhizosphere microbiota remains to be fully elucidated. Here we performed a comparative 16S rRNA gene survey of the rhizosphere of 4 domesticated and 20 wild barley (Hordeum vulgare) genotypes grown in an agricultural soil under controlled environmental conditions. We demonstrated the enrichment of individual bacteria mirrored the distinct eco-geographical constraints faced by their host plants. Unexpectedly, Elite varieties exerted a stronger genotype effect on the rhizosphere microbiota when compared with wild barley genotypes adapted to desert environments with a preferential enrichment for members of Actinobacteria. Finally, in wild barley genotypes, we discovered a limited, but significant, correlation between microbiota diversity and host genomic diversity. Our results revealed a footprint of the host’s adaptation to the environment on the assembly of the bacteria thriving at the root–soil interface. In the tested conditions, this recruitment cue layered atop of the distinct evolutionary trajectories of wild and domesticated plants and, at least in part, is encoded by the barley genome. This knowledge will be critical to design experimental approaches aimed at elucidating the recruitment cues of the barley microbiota across a range of soil types.
Similar content being viewed by others
Introduction
By 2050 the world’s population is expected to reach 9.5 billion and, to ensure global food security, crop production has to increase by 60% in the same timeframe1. This target represents an unprecedented challenge for agriculture as it has to be achieved while progressively decoupling yields from non-renewable inputs in the environment2 and amidst climatic modifications which are expected to intensify yield-limiting events, such as water scarcity and drought3.
A promising strategy proposes to achieve this task by capitalising on the microbiota inhabiting the rhizosphere, the thin layer of soil surrounding plant roots4. The rhizosphere microbiota plays a crucial role in plant’s adaptation to the environment by facilitating, for example, plant mineral uptake5 and enhancing plant’s tolerance to both abiotic and biotic stresses6.
Plant domestication and breeding selection, which have progressively differentiated modern cultivated crops from their wild relatives7, have impacted on the composition and functions of the rhizosphere microbiota8. These processes were accompanied by an erosion of the host genetic diversity9 and there are growing concerns that, in turn, these limited the metabolic diversity of the microbiota of cultivated plants10. Thus, to fully unlock the potential of rhizosphere microbes for sustainable crop production, it is necessary to study the microbiota thriving at the root–soil interface in the light of the evolutionary trajectories of its host plants11.
Barley (Hordeum vulgare L.), a global crop12 and a genetically tractable organism13, represents an ideal model to study host-microbiota interactions within a plant domestication framework, due to the fact that wild relatives (H. vulgare ssp. spontaneum) of domesticated varieties (H. vulgare ssp. vulgare) are accessible for experimentation14. We previously demonstrated that domesticated and wild barley genotypes host contrasting bacterial communities15 whose metabolic potential modulates the turn-over of the organic matter in the rhizosphere16. However, the impact of eco-geographical constraints faced by domesticated plants and crop wild relatives on recruitment and maintenance of the rhizosphere microbiota remains to be fully elucidated. Tackling this knowledge gap is a key pre-requisite to capitalise on plant-microbiota interactions to achieve the objectives of climate-smart agriculture, in particular sustainably enhancing crop production17.
Here we investigated whether exposure to different environmental conditions during evolution left a footprint on the barley’s capacity of shaping the rhizosphere bacterial microbiota. We characterised twenty wild barley genotypes from the ‘B1K’ collection sampled in the Southern levant geographic region, one of the centres of domestication of barley18,19. This material represents the three-major barley ‘Ecotypes’ adapted to different habitats in the region20: the Golan Heights and northern Galilee, (‘North Ecotype’), the coastal Mediterranean strip, (‘Coast Ecotype’),and the arid regions along the river Jordan and southern Negev (‘Desert Ecotype’). We further subdivided these ‘Ecotypes’ into 5 groups of sampling locations according to the average rainfall of the areas, as a proxy for plant’s adaptation to limiting growth conditions: ‘Coast 1’, ‘Coast 2’, ‘Desert 1’ and ‘Desert 2’ and ‘North’, respectively. (Table 1; Fig. 1). These wild barley genotypes were grown in a previously characterised soil, representative of barley agricultural areas of Scotland, under controlled environmental conditions, alongside four cultivated ‘Elite’ varieties encompassing the main usage and genetic diversity of the cultivated germplasm (Table 1). We used an Illumina MiSeq 16S rRNA gene amplicon survey to characterise the microbiota inhabiting the rhizosphere and unplanted soil samples. By using ecological indexes, multivariate statistical analyses and barley genome information we elucidated the impact of eco-geographical constraints and host genetics on the composition of the microbial communities thriving at the barley root–soil interface.
Plant growth parameters of the wild and domesticated barley genotypes. (A) Distribution of the twenty wild barley genotypes used in this study in the Israeli geographic region. Individual dots depict the approximate sampling location for a given genotype, colour-coded according to the designated eco-geographic area. (B) Stem dry weight of the barley genotypes at the time of sampling. (C) Ratio between root and shoot dry weight of the indicated samples. In (B) and (C), upper and lower edges of the box plots represent the upper and lower quartiles, respectively. The bold line within the box denotes the median, individual shapes depict measurements of individual biological replicates/genotypes for a given group. Different letters within the individual plots denote statistically significant differences between means by Kruskal–Wallis non-parametric analysis of variance followed by Dunn’s post-hoc test (P < 0.05).
Results
Evolutionary trajectories and eco-geographic adaptation impact on plant growth
Aboveground dry weight from the barley genotypes was measured at early stem elongation as a proxy for plant growth: this allowed us to identify a ‘biomass gradient’ across the tested material. The ‘Elite’ varieties, outperforming wild barley plants, and wild barley genotypes adapted to the more extreme desert environments (i.e., ‘Desert 2’) defined the uppermost and lowermost ranks of this gradient, respectively (P < 0.05, Kruskal–Wallis non-parametric analysis of variance followed by Dunn’s post hoc test; Fig. 1). Conversely, when we inspected the ratio between above- and belowground biomass we noticed an opposite trend: almost invariably wild barley genotypes allocated more resources than ‘Elite’ varieties to root growth compared to stem growth (P < 0.05, Kruskal–Wallis non-parametric analysis of variance followed by Dunn’s post hoc test; Fig. 1). As we sampled plants at a comparable developmental stage (“Methods” section; Figure S1), these observations indicate different growth responses of wild and domesticated genotypes in the tested conditions.
Taxonomic diversification of the barley microbiota across barley genotypes
To study the impact of these differential responses on the composition of the barley microbiota we generated 6,646,864 16S rRNA gene sequencing reads from 76 rhizosphere and unplanted soil specimens. These high-quality sequencing reads yielded 11,212 Operational Taxonomic Units (OTUs) at 97% identity (Supplementary Dataset 1: worksheet 2). A closer inspection of the taxonomic affiliation of the retrieved OTUs revealed that members of five bacterial phyla, namely Acidobacteria, Actinobacteria, Bacteroidetes, Proteobacteria and Verrucomicrobia, accounted for more than 97.8% of the observed reads (Fig. 2, Supplementary Dataset 1: worksheet 3). Among these dominant phyla, Bacteroidetes and Proteobacteria were significantly enriched in rhizosphere compared to bulk soil profiles (ANCOM, cut-off 0.6, alpha 0.05, taxa-based corrected, Supplementary Dataset 1: worksheets 4–5).
The dominant phyla of the bulk soil and rhizosphere microbiota are conserved across barley genotypes. Average relative abundance (% of sequencing reads) of the dominant phyla retrieved from the microbial profiles of indicated samples. Only phyla displaying a minimum average relative abundance of 1% included in the graphical representation. Stars depict phyla enriched in and discriminating between rhizosphere and between bulk soil samples (ANCOM, cut-off 0.6, alpha 0.05, taxa-corrected).
Next, we investigated the lower ranks of the taxonomic assignments (i.e., OTU level) and computed the Observed OTUs, Chao1 and Shannon indexes for each sample type. This analysis further supported the notion of the rhizosphere as a ‘reduced complexity community’, as both the Observed OTUs and Shannon indexes, but not the projected Chao1, identified significantly richer and more even communities in the bulk soil samples compared to plant-associated specimens (P < 0.05, Mann–Whitney U test; Figure S3). Interestingly, when we compared the Chao1 index within rhizosphere samples, we observed that members of the ‘Desert 1’ group assembled a richer community compared with the other genotypes (P < 0.05, Kruskal–Wallis non-parametric analysis of variance followed by Dunn’s post hoc test; Figure S3).
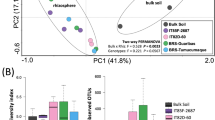
To gain further insights into the impact of the sample type on the barley microbiota we generated a canonical analysis of principal coordinates (CAP) using the weighted Unifrac distance, which is sensitive to OTU relative abundance and phylogenetic relatedness. This analysis revealed a marked effect of the microhabitat, i.e., either bulk soil or rhizosphere, on the composition of the microbiota as evidenced by the spatial separation on the axis accounting for the major variation (Fig. 3). Interestingly, we observed a clustering of bacterial community composition within rhizosphere samples, which was more marked between ‘Desert’ and ‘Elite’ samples (Fig. 3). These observations were corroborated by a permutational analysis of variance which attributed ~ 30% of the observed variation to the microhabitat and, within rhizosphere samples, ~ 17% of the variation to the individual eco-geographic groups (Permanova P < 0.001, 5,000 permutations, Table 2). Strikingly similar results were obtained when we computed a Bray–Curtis dissimilarity matrix, which is sensitive to OTUs relative abundance only (Table 2; Figure S4).
Wild and elite barley genotypes fine-tune the composition of the rhizosphere bacterial microbiota. Canonical analysis of principal coordinates of the Weighted Unifrac dissimilarity matrix of the microbial communities retrieved from the indicated sample types. Individual shape depicts individual biological replicates colour-coded according to the designated eco-geographic area.
Taken together, these data indicate that the composition of the barley microbiota is fine-tuned by plant recruitment cues which progressively differentiate between unplanted soil and rhizosphere samples and, within these latter, wild ecotypes from elite varieties.
A footprint of host eco-geographic adaptation shapes the wild barley rhizosphere microbiota
To gain insights into the bacteria underpinning the observed microbiota diversification we performed a series of pair-wise comparisons between ‘Elite’ genotypes and each group of the wild barley ecotypes. This approach revealed a marked specialisation of the members of the ‘Desert’ ecotype compared to ‘Elite’ varieties as evidenced by the number of OTUs differentially recruited between members of these groups (Wald test, P < 0.05, FDR corrected; Fig. 4; Supplementary Dataset 1: worksheets 7–11). Thus, the wild barley ‘Ecotype’ emerged as an element shaping the recruitment cues of the barley rhizosphere microbiota.
Enrichments of individual bacteria discriminates between elite varieties and wild barley genotypes. Horizontal black bars denote the number of OTUs differentially enriched (Wald test, P value < 0.05, FDR corrected) in the indicated pair-wise comparisons between elite varieties and groups of wild barley genotypes. Vertical bars depict the number of differentially enriched OTUs unique for or shared among two or more comparisons highlighted by the interconnected dots underneath the vertical bars. Coloured vertical bars depict differentially enriched OTUs unique for the pair-wise comparisons between ‘Elite’ and ‘Coast 2’ (C2, dark blue), ‘Coast 1’ (C1, light blue), ‘North’ (N, green), ‘Desert 1’ (D1, yellow) and ‘Desert 2’ (D2, orange), respectively.
A closer inspection of the OTUs differentially recruited between ‘Desert’ wild barley and ‘Elite’ varieties revealed that the domesticated material exerted the greatest selective impact on the soil biota, as the majority of the differentially enriched OTUs were enriched in ‘Elite’ varieties (Wald test, P < 0.05, FDR corrected; Supplementary Dataset 1: worksheets 7 and 8). Next, the taxonomic assignments of these ‘Elite-enriched’ OTUs versus the ‘Desert’ microbiota followed distinct patterns: while the comparison ‘Elite’- ‘Desert 1’ produced a subset of enriched OTUs assigned predominantly to Actinobacteria, Bacteroidetes and Proteobacteria, the comparison ‘Elite’- ‘Desert 2’ displayed a marked bias for members of the Actinobacteria (i.e., 44 out of 104 enriched OTUs, Fig. 5). Consistently, the cumulative abundance of sequencing reads assigned of those Actinobacterial OTUs in ‘Elite’ samples nearly doubled the one recorded for ‘Desert 2’ samples (Figure S5). Within this phylum, we identified a broader taxonomic distribution, as those OTUs were assigned to the families Intrasporangiaceae, Micrococcaceae, Micromonosporaceae, Nocardioidaceae, Pseudonocardiaceae, Streptomycetaceae, as well as members of the order Frankiales. Interestingly, when we inspect intra-ecotype diversification we identified diagnostic OTUs capable of discriminating between ‘Desert 1’ and ‘Desert 2’ (Wald test, P < 0.05, FDR corrected; Supplementary Dataset 1: worksheets 12 and 13), while no such a feature was identified discriminating between ‘Coast 1’ and ‘Coast 2’ at the statistical test imposed. Taken together, our data indicate that wild barley ‘Ecotype’ (i.e., the differential effect of ‘North’, ‘Coast, and ‘Desert’ versus ‘Elite’) acts as a determinant for the rhizosphere barley microbiota whose composition is ultimately fine-tuned by a sub-specialisation within the ‘Ecotype’ itself (i.e., the differential effect of ‘Desert 1’ and ‘Desert 2’).
Actinobacteria are preferentially enriched in and discriminate between elite genotypes and wild barley genotypes adapted to desert environments. Individual external nodes of the tree represent one of the OTUs enriched in the rhizosphere of elite genotypes compared to either (or both) rhizosphere samples desert areas (Wald test, P value < 0.05, FDR corrected). The colours reflect OTUs’ taxonomic affiliation at Phylum level. A black bar in the outer rings depicts whether that given OTU was identified in the comparisons between ‘Elite’ and either ‘Desert 1′ or ‘Desert 2′ genotypes, respectively. Phylogenetic tree constructed using OTUs 16S rRNA gene representative sequences.
These observations prompted us to investigate whether the differential microbiota recruitment between the tested plants was encoded, at least in part, by the barley genome. We therefore generated a dissimilarity matrix using Single Nucleotide Polymorphisms (SNPs) available for the tested genotypes and we inferred their genetic relatedness using a simple matching coefficient (Supplementary Dataset 1: worksheet 14). With few notable exceptions, this analysis revealed three distinct clusters of genetically related plants, represented by and reflecting the ‘Elite’ material, the ‘Desert’ and the ‘Coast’ wild barley genotypes (Figure S6). The genetic diversity between domesticated material exceeded their microbial diversity (compare relatedness of “Elite” samples in Fig. 3 with the ones of Figure S6) as further evidenced by the fact that we failed to identify a significant correlation between these parameters (P value > 0.05). However, when we focused the analysis solely on the pool of wild barley genotypes, we obtained a significant correlation between genetic and microbial distances (Mantel test r = 0.230; P value < 0.05; Fig. 6).
Mantel test between genetic distance and microbial distance in the wild barley rhizosphere. Individual dots depict individual comparison of any given pair of wild barley genotypes between average value of weighted unifrac distance (y-axis) and genetic distance shown as simple matching coefficients (x-axis). The blue line depicts the regression line, the grey shadow the 95% confidence interval, respectively.
Taken together, this revealed a footprint of barley host’s adaptation to the environment on the assembly of the bacteria thriving at the root–soil interface. This recruitment cue interjected the distinct evolutionary trajectories of wild and domesticated plants and, at least in part, is encoded by the barley genome.
Discussion
In this study we investigated how plant genotypes adapted to different eco-geographic niches may recruit a distinct microbiota once exposed to a common environment.
As we performed a ‘common environment experiment’ in a Scottish agricultural soil, we first determined how the chosen experimental conditions related to the ones witnessed by wild barleys in their natural habitats. Strikingly, the aboveground biomass gradient observed in our study, with ‘Elite’ material almost invariably outperforming wild genotypes and material sampled at the locations designated ‘Desert 2’ at the bottom of the ranking, “matched” the phenotypic characterisation of members of the ‘B1K’ collection grown in a ‘common garden experiment’ in a local Israeli soil18. Conversely, belowground resource allocation followed an opposite pattern as evidenced by an increased root:shoot dry weight ratio in wild genotypes compared to ‘Elite’ varieties. As responses to edaphic stress, such as drought tolerance, may modulate the magnitude of above-belowground resource partitioning in plants21 and root traits22, our data might reflect the adaptation of the wild barley exposure to dry areas. Taken together, these results suggest that adaptive responses to eco-geographic constraints in barley have a genetic inheritance component which can be detected and studied in controlled conditions.
As genetically-inherited root traits have been implicated in shaping the rhizosphere microbiota in barley23 and other crops24, these observations motivated us to examine whether these below-ground differences were reflected by changes in microbiota recruitment. The distribution of reads assigned to given phyla appears distinct in plant-associated communities which are dominated in terms of abundance by members of the phyla Acidobacteria, Actinobacteria, Bacteroidetes and Proteobacteria, with these two latter phyla significantly enriched in rhizosphere samples compared to bulk soil controls. This taxonomic affiliation is consistent with previous investigations in barley in either the same23 or in a different soil type15 as well as in other crop plants25. In summary, these data indicate that the higher taxonomic ranks of the barley rhizosphere microbiota are conserved across soil types as well as wild and domesticated genotypes.
The characterisation of the microbiota at lower taxonomic ranks, i.e., the OTU-level, revealed a significant effect of the microhabitat (i.e., either bulk soil or rhizosphere) and, within plant-associated communities, a footprint of eco-geographic adaptation. For instance, alpha diversity indexes clearly pointed at selective processes modulating bacterial composition as the number of Observed OTUs and the Shannon index indicate simplified and reduced-complexity communities inhabiting the rhizosphere compared to unplanted soil. This can be considered a hallmark of the rhizosphere microbiota as it has been observed in multiple plant species and across soils6. Conversely, within rhizosphere samples, alpha-diversity analysis failed to identify a clear pattern, except for the Chao1 index revealing a potential for a richer community associated with plants sampled at the ‘Desert 1’ locations. This motivated us to further explore the between-sample diversity, which is beta-diversity. This analysis revealed a clear host-dependent diversification of the bacteria associated to barley plants manifested by ~ 17% of the variance of the rhizosphere microbiota explained by the eco-geographical location of the sampled material. This value exceeded the host genotype effect on the rhizosphere microbiota we previously observed in wild and domesticated barley plants15, but is aligned with the magnitude of host effect observed in the rhizosphere microbiota of modern and ancestral genotypes of rice26 and common bean27. As these studies were conducted in different soil types, our data suggest that the magnitude of host control on the rhizosphere microbiota is ultimately fine-tuned by and in response to soil characteristics.
The identification of the bacteria underpinning the observed microbiota diversification led to three striking observations. First, the comparison between ‘Elite’ varieties and the material representing the ‘Desert’ ecotype was associated with the largest number of differentially recruited OTUs, while the other wild barley genotypes appeared to share a significant proportion of their microbiota with domesticated plants. A prediction of this observation is that the distinct evolutionary trajectories of wild and domesticated plants per se cannot explain the host-mediated diversification of the barley microbiota. As aridity and temperature played a prominent role in shaping the phenotypic characteristics of barley19,28 it is tempting to speculate that the adaptation to these environmental parameters played a predominant role also in shaping microbiota recruitment.
Second, it is the domesticated material which exerted a stronger effect on microbiota recruitment, manifested by the increased number of host-enriched OTUs compared to wild barley genotypes. This suggests that the capacity of shaping the rhizosphere microbiota has not been “lost” during barley domestication and breeding selection. Our findings are consistent with data gathered for domesticated and ancestral common bean genotypes, which revealed that shifts from native soils to agricultural lands led to a stronger host-dependent effect on rhizosphere microbes29. Due to the intrinsic limitation of 16S rRNA gene profiles of predicting the functional potential of individual bacteria, it will be necessary to complement this investigation with whole-genome surveys30,31 and metabolic analyses16,32 to fully discern the impact of the host genotype on the functions provided by the rhizosphere microbiota to their hosts.
The third observation is the marked quantitative enrichment of OTUs assigned to the phylum Actinobacteria in ‘Elite’ varieties when compared to members of the ‘Desert’ ecotype, in particular plants of the ‘Desert 2’ locations. At first glance, the ‘direction’ of this bacterial enrichment is difficult to reconcile with the eco-geographic adaptation of wild barleys and, in particular, the fact that Actinobacteria are more tolerant to arid conditions33 and, consequently, more abundant in desert versus non-desert soils34. However, the enrichment of Actinobacteria in modern crops compared to ancestral relatives has recently emerged as a distinctive feature of the microbiota of multiple plant species35. Although the ecological significance of this trait of the domesticated microbiota remains to be fully elucidated, studies conducted in rice36 and other grasses, including barley37, indicate a relationship between drought stress and Actinobacteria enrichments. These observations suggest that the wild barley genome has evolved the capacity to recognise microbes specifically adapted to the local conditions and, in turn, to repress the growth of others. For instance, among the bacteria differentially enriched between ‘Desert 1’ and ‘Desert 2’ we identified genera, such as Arthrobacter sp., adapted to extreme environments and long-term nutrient starvation38, possibly reflecting the differential adaptation of ‘Desert 1’ and ‘Desert 2’ plants to soil with limited organic matter39.
Interestingly, we were able to trace the host genotype effect on rhizosphere microbes to the genome of wild barley. This suggests that, similar to other wild species11, microbiota recruitment co-evolved with other adaptive traits. Conversely, the genetic diversity in ‘Elite’ material largely exceeded microbiota diversity. This is reminiscent of studies conducted in maize which failed to identify a significant correlation between polymorphisms in the host genome and alpha- and beta-diversity characteristics of the rhizosphere microbiota40,41. Yet, and again similar to maize42, our data indicate that the recruitment of individual bacterial OTUs in the ‘Elite’ varieties, rather than community composition as a whole, is the feature of the rhizosphere microbiota under host genetic control.
Although these findings were gathered from the individual soil tested and further validation across a range of soil types is required, a prediction from these observations is that the host control of the rhizosphere microbiota is exerted by a limited number of loci in the genome with a relatively large effect. This is congruent with our previous observation that mono-mendelian mutations in a single root trait, root hairs, impact on ~ 18% of the barley rhizosphere microbiota23.
Likewise, this scenario is compatible with a limited number of genes controlling the biosynthesis and rhizodeposition of defensive secondary metabolites which have been implicated in shaping the plant microbiota43. Among these compounds, the indol-alkaloids benzoxazinoids recently gained centre-stage as master regulators of the maize-associated microbial communities44,45,46. Interestingly, H. vulgare has evolved a distinct indol-alkaloid compound, gramine47, which is preferentially accumulated in the tissues of the wild genotypes compared to ‘Elite’ varieites48 and whose physiological properties are comparable to the ones of benzoxazinoids49. Whether gramine or other species-specific secondary metabolites contribute, at least in part, to shape the barley microbiota will be the focus of future investigations.
Since modern varieties have been selected with limited or no knowledge of belowground interactions, how was the capacity of shaping the rhizosphere microbiota retained within the cultivated germplasm? The recent observation that genes controlling reproductive traits display pleiotropic effects on root system architecture50 could provide a direct link between crop selection and microbiota recruitment in modern varieties. These traits, and in particular genes encoding flower developments, show a marked footprint of eco-geographic adaptation and have been selected during plant domestication and breeding28. By manipulating those genes, breeders may have manipulated also belowground traits, and in turn, the microbiota thriving at the root–soil interface. With an increased availability of genetic51 and genomic52 resources for wild and domesticated barleys, this hypothesis can now be experimentally tested and the adaptive significance of the barley rhizosphere microbiota ultimately deciphered. Specifically, intraspecific populations within the wild53 as well as between wild and cultivated51 germplasm, could be deployed in genetic mapping experiments aimed at identifying barley genetic determinants of the rhizosphere microbiota.
Conclusions
Our results revealed a footprint of host’s adaptation to the environment on the assembly of the bacteria thriving at the root–soil interface in barley. This recruitment cue layered atop of the distinct evolutionary trajectories of wild and domesticated plants and, at least in part, is encoded by the barley genome. Although our study was limited to the individual soil investigated, our sequencing survey will provide a reference dataset for the development of indexed bacterial collections of the barley microbiota. These can be used to infer causal relationships between microbiota composition and plant traits, as demonstrated for Arabidopsis thaliana54 and rice55. Furthermore, this knowledge is critical for the establishment of reciprocal transplantation experiments aimed at elucidating the adaptive value of crop-microbiota interactions, similar to what has recently been proposed for the model plant A. thaliana56. However, for crop plants like barley, this will necessarily be conditioned by two elements: identifying the host genetic determinants of the rhizosphere microbiota and inferring microbial metabolic potential in situ. Ultimately, this will help devising strategies aimed at sustainably enhancing crop production for climate-smart agriculture.
Methods
Soil
The soil was sampled from the agricultural research fields of the James Hutton Institute, Invergowrie, Scotland, UK in the Quarryfield site (56° 27′ 5" N 3° 4′ 29" W; Sandy Silt Loam, pH 6.2; Organic Matter 5%; Table S1). This field was left unplanted and unfertilised in the 3 years preceding the investigations and previously used for barley-microbiota interactions investigations23.
Plant genotypes
Twenty wild barley genotypes (H. vulgare ssp. spontaneum) and four ‘Elite’ cultivars (H. vulgare ssp. vulgare) were used and described in Table 1. Wild barley genotypes were selected representing eco-geographical variation of the ‘B1K’ collection18,19. The ‘Elite’ genotypes were selected as a representation of different types of spring barley in plant genetic studies. The cultivar ‘Morex’ is an American six-row malting variety whose genome was the first to be sequenced57. The cultivars ‘Bowman’ and ‘Barke’ are two-row varieties, developed in US for feed and in Germany for malting, respectively, whereas Steptoe is an American six-row type used for animal feed51, 58,59.
Plant growth conditions
Barley seeds were surface sterilized as previously reported60 and germinated on 0.5% agar plates at room temperature. Seedlings displaying comparable rootlet development after 5 days post-plating were sown individually in 12-cm diameter pots containing approximately 500 g of the ‘Quarryfield’ soil, plus unplanted pots filled with bulk soil as controls. Plants were arranged in a randomised design with this number of replicates: ‘Coast1’ number of replicates n = 12; ‘Coast2’ n = 12; ‘Desert1’ n = 11; ‘Desert2’ n = 12; ‘North’ n = 12; ‘Elite’ n = 13 (Supplementary Dataset 1: worksheet 1). Plants were grown for 5 weeks in a glasshouse at 18/14 °C (day/night) temperature regime with 16 h day length and watered every 2 days with 50 ml of deionized water.
Bulk soil and rhizosphere DNA preparation
At early stem elongation, corresponding to Zadoks stages 30–3261, plants were pulled from the soil and the stems and leaves were separated from the roots (Figure S1). Above-ground plant parts were dried at 70 °C for 72 h and the dry weight recorded. The roots were shaken manually to remove excess of loosely attached soil. For each barley plant, the top 6 cm of the seminal root system and the attached soil layer was collected and placed in sterile 50 ml falcon tube containing 15 ml phosphate-buffered saline solution (PBS). Rhizosphere was operationally defined, for these experiments, as the soil attached to this part of the roots and extracted through this procedure. The samples were then vortexed for 30 s and aseptically transferred to a second 50 ml falcon containing 15 ml PBS and vortexed again for 30 s to ensure the dislodging and suspension of the rhizosphere soil. Then, the two falcon tubes with the rhizosphere suspension were mixed and centrifuged at 1,500×g for 20 min, the supernatant was removed, with the rhizosphere soil collected as the pellet, flash frozen with liquid nitrogen and stored at − 80 °C, until further use. After the rhizosphere extraction step, these parts of the roots were combined with the rest of the root system for each plant, thoroughly washed with water removing any attached soil particles and dried at 70 °C for 72 h for root biomass measurement. Bulk soil samples were collected from the 6 cm below the surface of unplanted pots and subjected to the same procedure as above.
DNA was extracted from the rhizosphere samples using FastDNA SPIN Kit for Soil (MP Biomedicals, Solon, USA) according to the manufacturer’s recommendations. The concentration and quality of DNA was checked using a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, USA) spectrophotometer and stored at − 20 °C until further use. DNA concentration was used as a proxy for the proportion of the sampled microbiota and evaluated across sample type (Figure S2).
Preparation of 16 rRNA gene amplicon pools
The hypervariable V4 region of the small subunit rRNA gene was the target of amplification using the PCR primer pair 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR primers had incorporated an Illumina flow cell adapter at their 5′ end and the reverse primers contained 12 bp unique ‘barcode’ for simultaneous sequencing of several samples62. PCR, including No-Template Controls (NTCs) for each barcoded primer, was performed as previously reported with the exception of the BSA at 10 mg/ml concentration per reaction23. Only samples whose NTCs yielded an undetectable PCR amplification were retained for further analysis.
Illumina 16S rRNA gene amplicon sequencing
The pooled amplicon library was submitted to the Genome Technology group, The James Hutton Institute (Invergowrie, UK) for quality control, processing and sequencing as previously described23,63,64. Briefly, samples were sequenced using an Illumina MiSeq platform with the 2 × 150 bp chemistry.
Sequencing reads processing
Sequencing reads were processed and analysed using a custom bioinformatics pipeline. First, Quantitative Insights into Microbial Ecology (QIIME) software, version 1.9.0, was used to process the FASTQ files following default parameters for each step65. The forward and reverse read files from individual libraries were decompressed and merged using the command join_paired_ends.py, with a minimum overlap of 30 bp between reads. Then, the reads were demultiplexed according to the barcode sequences. Quality filtering was performed using the command split_libraries_fastq.py, imposing a minimum acceptable PHRED score ‘-q’ of 20. Next, these high quality reads were truncated at the 250th nucleotide using the function ‘fastq_filter’ implemented in USEARCH66. Only these high-quality PE, length-truncated reads were used for clustering in Operational Taxonomic Units (OTUs) at 97% sequence identity. OTUs were identified using the ‘closed reference’ approach against Silva database (version 132)67. OTU-picking against the Silva database was performed using the SortMeRNA algorithm68, producing in an OTU table containing the abundance of OTUs per sample plus a phylogenetic tree. To control for potential contaminant OTUs amplified during library preparation, we retrieved a list of potential environmental contaminant OTUs previously identified in our laboratory64 and we used this list to filter the results of the aforementioned OTU-enrichment analysis. Additionally, singleton OTUs, (OTUs accounting for only one sequencing read in the whole dataset) and OTUs assigned to chloroplast and mitochondria (taken as plant derived sequences) were removed using the command filter_otus_from_otu_tables.py. Taxonomy matrices, reporting the number of reads assigned to individual phyla, were generated using the command summarize_taxa.py. The OTU table, the phylogenetic tree and the taxonomy matrix, were further used in R for visualizations and statistical analysis.
Statistical analyses I: univariate datasets and 16S rRNA gene alpha and beta-diversity calculations
Analysis of the data was performed in R69 using a custom script with the following packages: Phyloseq70 for processing, Alpha and Beta-diversity metrics, ggplot271 for data visualisations, Vegan72 for statistical analysis of beta-diversity, Ape73 for phylogenetic tree analysis. For any univariate dataset used (e.g., aboveground biomass, DNA concentration) the normality of the data’s distribution was checked using Shapiro–Wilk test. Non-parametric analysis of variance were performed by Kruskal–Wallis Rank Sum Test, followed by Dunn’s post hoc test with the functions kruskal.test and the posthoc.kruskal.dunn.test, respectively, from the package PMCMR.
For Alpha-diversity analysis, the OTU table was rarefied at 11,180 reads per sample and this allowed us to retain 8,744 OTUs for downstream analyses (Supplementary Dataset 1: worksheet 6). The Chao1, Observed OTUs and Shannon indices calculated using the function estimate richness in Phyloseq package. Beta-diversity was analysed using a normalized OTU table (i.e., not rarefied) for comparison. For the construction of the normalized OTU table, low abundance OTUs were further filtered removing those not present at least 5 times in 20% of the samples, to improve reproducibility. Then, to control for the uneven number of reads per specimen, individual OTU counts in each sample were divided over the total number of generated reads for that samples and converted in counts per million. Beta-diversity was analysed using two metrics: Bray–Curtis that considers OTUs relative abundance and Weighted Unifrac that additionally is sensitive to phylogenetic classification74. These dissimilarity matrices were visualized using Canonical Analysis of Principal coordinates (CAP)75 using the ordinate function in the Phyloseq package and its significance was inspected using a permutational ANOVA over 5,000 permutations.
Beta-diversity dissimilarity matrices were assessed by Permutational Multivariate Analysis of Variance (Permanova) using Adonis function in Vegan package over 5,000 permutations to calculate effect size and statistical significance.
Statistical analyses II: analysis of Phyla and OTUs differentially enriched among samples
The analysis of the Phyla whose abundances differentiated among rhizosphere and bulk soil samples was performed with analysis of composition of microbiomes (ANCOM)76 imposing 0.6 cut-off and 0.05 alpha value (taxa-based corrected) as previously described77.
The analysis of the OTUs whose abundances differentiated among samples was performed (a) between individual eco-geographic groups and bulk soil samples to assess the rhizosphere effect and (b) between the rhizosphere samples to assess the eco-geographic effect. The eco-geographic effect was further corrected for a microhabitat effect (i.e., for each group, only OTUs enriched against both unplanted soil and at least another barley genotype were retained for further analysis). The analysis was performed using the DESeq2 method78 with an adjusted P value < 0.05 (False Discovery Rate, FDR corrected). This method was selected since it outperforms other hypothesis-testing approaches when data are not normally distributed and a limited number of individual replicates per condition (i.e., approximately 10) are available79. DESeq2 was performed using the eponymous named package in R with the OTU table filtered for low abundance OTUs as an input.
The number of OTUs differentially recruited in the pair-wise comparisons between ‘Elite’ and wild barley genotypes was visualised using the package UpSetR80.
The phylogenetic tree was constructed using the representative sequences of the OTUs significantly differentiating ‘Elite’ genotypes and either ‘Desert1’ or ‘Desert2’ samples annotated with iTOL81.
Statistical analyses III: correlation plot genetic distance-microbial distance
To assess the genetic variation on the barley germplasm we used the SNP platform ‘BOPA1’82 comprising 1,536 single nucleotide polymorphisms. We used GenAlex 6.583,84 to construct a genetic distance matrix using the simple matching coefficient. Genetic distance for the barley genotypes was visualised by hierarchical clustering using the function hclust in R. Microbial distance was calculated on the average distances for each ecogeographic group using the Weighted Unifrac metric. Correlation between the plant’s genetic and microbial distances was performed using a mantel test with the mantel.rtest of the package ade4 in R. The correlation was visualised using the functions ggscatter of the R packages ggpbur.
Data availability
The sequences generated in the 16S rRNA gene sequencing survey are deposited in the European Nucleotide Archive (ENA) under the accession number PRJEB35359. The version of the individual packages and scripts used to analyse the data and generate the figures of this study are available at https://github.com/BulgarelliD-Lab/Barley_B1K
References
Alexandratos, N. & Bruinsma, J. World agriculture towards 2030/2050: the 2012 revision. (2012).
Tilman, D., Balzer, C., Hill, J. & Befort, B. L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. U.S.A. 108, 20260–20264. https://doi.org/10.1073/pnas.1116437108 (2011).
Porter, J. R. et al. Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Food Security and Food Production Systems, 485–533 (2014).
Schlaeppi, K. & Bulgarelli, D. The plant microbiome at work. Mol. Plant–Microbe Interact. 28, 212–217. https://doi.org/10.1094/MPMI-10-14-0334-FI (2015).
Alegria Terrazas, R. et al. Plant–microbiota interactions as a driver of the mineral turnover in the rhizosphere. Adv. Appl. Microbiol. 95, 1–67. https://doi.org/10.1016/bs.aambs.2016.03.001 (2016).
Bulgarelli, D., Schlaeppi, K., Spaepen, S., Van Themaat, E. V. L. & Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 64, 807–838 (2013).
Purugganan, M. D. & Fuller, D. Q. The nature of selection during plant domestication. Nature 457, 843–848 (2009).
Perez-Jaramillo, J. E., Mendes, R. & Raaijmakers, J. M. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol. Biol. 90, 635–644. https://doi.org/10.1007/s11103-015-0337-7 (2016).
Doebley, J. F., Gaut, B. S. & Smith, B. D. The molecular genetics of crop domestication. Cell 127, 1309–1321 (2006).
Cordovez, V., Dini-Andreote, F., Carrión, V. J. & Raaijmakers, J. M. Ecology and evolution of plant microbiomes. Ann. Rev. Microbiol. 73, 69–88a (2019).
Escudero-Martinez, C. & Bulgarelli, D. Tracing the evolutionary routes of plant–microbiota interactions. Curr. Opin. Microbiol. 49, 34–40 (2019).
Newton, A. C. et al. Crops that feed the world 4. Barley: a resilient crop? Strengths and weaknesses in the context of food security. Food Secur. 3, 141. https://doi.org/10.1007/s12571-011-0126-3 (2011).
Mascher, M. et al. A chromosome conformation capture ordered sequence of the barley genome. Nature 544, 426. https://doi.org/10.1038/nature22043 (2017).
Milner, S. G. et al. Genebank genomics highlights the diversity of a global barley collection. Nat. Genet. 51, 319–326 (2019).
Bulgarelli, D. et al. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17, 392–403. https://doi.org/10.1016/j.chom.2015.01.011 (2015).
Mwafulirwa, L. et al. Barley genotype influences stabilization of rhizodeposition-derived C and soil organic matter mineralization. Soil Biol. Biochem. 95, 60–69. https://doi.org/10.1016/j.soilbio.2015.12.011 (2016).
Lipper, L. et al. Climate-smart agriculture for food security. Nat. Clim. Change 4, 1068–1072 (2014).
Hubner, S. et al. Phenotypic landscapes: phenological patterns in wild and cultivated barley. J. Evol. Biol. 26, 163–174. https://doi.org/10.1111/jeb.12043 (2013).
Hubner, S. et al. Strong correlation of wild barley (Hordeum spontaneum) population structure with temperature and precipitation variation. Mol. Ecol. 18, 1523–1536. https://doi.org/10.1111/j.1365-294X.2009.04106.x (2009).
Hübner, S. et al. Phenotypic landscapes: phenological patterns in wild and cultivated barley. J. Evol. Biol. 26, 163–174 (2013).
Bloom, A. J., Chapin, F. S. III. & Mooney, H. A. Resource limitation in plants-an economic analogy. Annu. Rev. Ecol. Syst. 16, 363–392 (1985).
Comas, L., Becker, S., Cruz, V. M. V., Byrne, P. F. & Dierig, D. A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 4, 442 (2013).
Robertson-Albertyn, S. et al. Root hair mutations displace the barley rhizosphere microbiota. Front. Plant Sci. 8, 1094. https://doi.org/10.3389/fpls.2017.01094 (2017).
Pérez-Jaramillo, J. E. et al. Linking rhizosphere microbiome composition of wild and domesticated Phaseolus vulgaris to genotypic and root phenotypic traits. ISME J. 11, 2244–2257 (2017).
Hacquard, S. et al. Microbiota and host nutrition across plant and animal kingdoms. Cell Host Microbe 17, 603–616 (2015).
Edwards, J. et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. U.S.A. 112, E911-920. https://doi.org/10.1073/pnas.1414592112 (2015).
Perez-Jaramillo, J. E. et al. Linking rhizosphere microbiome composition of wild and domesticated Phaseolus vulgaris to genotypic and root phenotypic traits. ISME J. 11, 2244–2257. https://doi.org/10.1038/ismej.2017.85 (2017).
Russell, J. et al. Exome sequencing of geographically diverse barley landraces and wild relatives gives insights into environmental adaptation. Nat. Genet. 48, 1024 (2016).
Pérez-Jaramillo, J. E. et al. Deciphering rhizosphere microbiome assembly of wild and modern common bean (Phaseolus vulgaris) in native and agricultural soils from Colombia. Microbiome 7, 1–16 (2019).
Garrido-Oter, R. et al. Modular traits of the Rhizobiales root microbiota and their evolutionary relationship with symbiotic rhizobia. Cell Host Microbe 24, 155. https://doi.org/10.1016/j.chom.2018.06.006 (2018).
Karasov, T. L. et al. Arabidopsis thaliana and Pseudomonas pathogens exhibit stable associations over evolutionary timescales. Cell Host Microbe 24, 168–179 (2018).
Paterson, E., Gebbing, T., Abel, C., Sim, A. & Telfer, G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol. 173, 600–610 (2007).
Neilson, J. W. et al. Significant impacts of increasing aridity on the arid soil microbiome. MSystems 2, e00195-0016 (2017).
Fierer, N. et al. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. U.S.A. 109, 21390–21395. https://doi.org/10.1073/pnas.1215210110 (2012).
Pérez-Jaramillo, J. E., Carrión, V. J., de Hollander, M. & Raaijmakers, J. M. The wild side of plant microbiomes. Microbiome 6, 143. https://doi.org/10.1186/s40168-018-0519-z (2018).
Santos-Medellín, C., Edwards, J., Liechty, Z., Nguyen, B. & Sundaresan, V. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. MBio 8, e00764-00717 (2017).
Naylor, D., DeGraaf, S., Purdom, E. & Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 11, 2691–2704 (2017).
Romaniuk, K., Golec, P. & Dziewit, L. Insight into the diversity and possible role of plasmids in the adaptation of psychrotolerant and metalotolerant Arthrobacter spp. to extreme Antarctic environments. Front. Microbiol. 9, 3144 (2018).
Hübner, S. et al. Strong correlation of wild barley (Hordeum spontaneum) population structure with temperature and precipitation variation. Mol. Ecol. 18, 1523–1536 (2009).
Bouffaud, M. L. et al. Is diversification history of maize influencing selection of soil bacteria by roots?. Mol. Ecol. 21, 195–206. https://doi.org/10.1111/j.1365-294X.2011.05359.x (2012).
Peiffer, J. A. et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. 110, 6548–6553. https://doi.org/10.1073/pnas.1302837110 (2013).
Walters, W. A. et al. Large-scale replicated field study of maize rhizosphere identifies heritable microbes. Proc. Natl. Acad. Sci. https://doi.org/10.1073/pnas.1800918115 (2018).
Rolfe, S. A., Griffiths, J. & Ton, J. Crying out for help with root exudates: adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 49, 73–82 (2019).
Cotton, T. A. et al. Metabolic regulation of the maize rhizobiome by benzoxazinoids. ISME J. 13, 1647–1658 (2019).
Hu, L. et al. Root exudate metabolites drive plant–soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 9, 1–13 (2018).
Kudjordjie, E. N., Sapkota, R., Steffensen, S. K., Fomsgaard, I. S. & Nicolaisen, M. Maize synthesized benzoxazinoids affect the host associated microbiome. Microbiome 7, 1–17 (2019).
Grün, S., Frey, M. & Gierl, A. Evolution of the indole alkaloid biosynthesis in the genus Hordeum: distribution of gramine and DIBOA and isolation of the benzoxazinoid biosynthesis genes from Hordeum lechleri. Phytochemistry 66, 1264–1272 (2005).
Larsson, K. A., Zetterlund, I., Delp, G. & Jonsson, L. M. N-Methyltransferase involved in gramine biosynthesis in barley: cloning and characterization. Phytochemistry 67, 2002–2008 (2006).
Matsuo, H. et al. Gramine increase associated with rapid and transient systemic resistance in barley seedlings induced by mechanical and biological stresses. Plant Cell Physiol. 42, 1103–1111 (2001).
Voss-Fels, K. P. et al. VERNALIZATION1 modulates root system architecture in wheat and barley. Mol. Plant 11, 226–229 (2018).
Maurer, A. et al. Modelling the genetic architecture of flowering time control in barley through nested association mapping. BMC Genomics 16, 290 (2015).
Bayer, M. M. et al. Development and evaluation of a barley 50k iSelect SNP array . Front. Plant Sci. 8, 1792. https://doi.org/10.3389/fpls.2017.01792 (2017).
Bdolach, E. et al. Thermal plasticity of the circadian clock is under nuclear and cytoplasmic control in wild barley. Plant Cell Environ. 42, 3105–3120 (2019).
Bai, Y. et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 528, 364–369 (2015).
Zhang, J. et al. NRT1. 1B associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 37, 676–684 (2019).
Thiergart, T. et al. Root microbiota assembly and adaptive differentiation among European Arabidopsis populations. Nat. Ecol. Evol. 4, 122–131 (2020).
International Barley Genome Sequencing Consortium et al. A physical, genetic and functional sequence assembly of the barley genome. Nature 491, 711. https://doi.org/10.1038/nature11543 (2012).
Druka, A. et al. Genetic dissection of barley morphology and development. Plant Physiol. 155, 617–627. https://doi.org/10.1104/pp.110.166249 (2011).
Kleinhofs, A. et al. A molecular, isozyme and morphological map of the barley (Hordeum vulgare) genome. Theor. Appl. Genet. 86, 705–712 (1993).
Bulgarelli, D. et al. The CC-NB-LRR-Type Rdg2a resistance gene confers immunity to the seed-borne barley leaf stripe pathogen in the absence of hypersensitive cell death. PLoS ONE 5, e12599. https://doi.org/10.1371/journal.pone.0012599 (2010).
Tottman, D., Makepeace, R. & Broad, H. An explanation of the decimal code for the growth stages of cereals, with illustrations. Ann. Appl. Biol. 93, 221–234 (1979).
Caporaso, J. G. et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624. https://doi.org/10.1038/ismej.2012.8 (2012).
Caradonia, F. et al. Nitrogen fertilizers shape the composition and predicted functions of the microbiota of field-grown tomato plants. Phytobiomes J. 3, 315–325 (2019).
Pietrangelo, L., Bucci, A., Maiuro, L., Bulgarelli, D. & Naclerio, G. Unraveling the composition of the root-associated bacterial microbiota of Phragmites australis and Typha latifolia. Front. Microbiol. 9, 1650. https://doi.org/10.3389/fmicb.2018.01650 (2018).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. https://doi.org/10.1038/nmeth.f.303 (2010).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. https://doi.org/10.1093/bioinformatics/btq461 (2010).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590-596. https://doi.org/10.1093/nar/gks1219 (2013).
Kopylova, E., Noé, L. & Touzet, H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 28, 3211–3217 (2012).
Team, R. C. R: A Language and Environment for Statistical Computing. (2013).
McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS one 8(4), e61217 (2013).
Wickham, H. ggplot2: elegant graphics for data analysis (Springer, Berlin, 2016).
Oksanen, J.F. et al. vegan: community ecology package. R package version 2.0–7. 2013 (2014).
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004).
Lozupone, C., Lladser, M. E., Knights, D., Stombaugh, J. & Knight, R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 5, 169–172. https://doi.org/10.1038/ismej.2010.133 (2011).
Anderson, M. J. & Willis, T. J. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84, 511–525 (2003).
Mandal, S. et al. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb. Ecol. Health Dis. 26, 27663 (2015).
Morton, J. T. et al. Establishing microbial composition measurement standards with reference frames. Nat. Commun. 10, 1–11 (2019).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. https://doi.org/10.1186/s13059-014-0550-8 (2014).
Weiss, S. et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 5, 27. https://doi.org/10.1186/s40168-017-0237-y (2017).
Conway, J. R., Lex, A. & Gehlenborg, N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics 33, 2938–2940 (2017).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23, 127–128 (2006).
Close, T. J. et al. Development and implementation of high-throughput SNP genotyping in barley. BMC Genomics 10, 582. https://doi.org/10.1186/1471-2164-10-582 (2009).
Peakall, R. O. D. & Smouse, P. E. GenAlEx 6.5: genetic analysis in Excel, Population genetic software for teaching and research—an update. Bioinformatics 28, 2537 (2012).
Peakall, R. & Smouse, P. E. Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6, 288–295 (2006).
Acknowledgements
We are grateful to Prof Andy Flavell (University of Dundee) for providing us with the ‘B1K’ seeds used in this study. We thank Malcolm Macaulay for the technical assistance during the sequencing library preparation. We thank Dr Timothy George (The James Hutton Institute) for the critical comments on the manuscript. This work was supported by a Royal Society of Edinburgh/Scottish Government Personal Research Fellowship co-funded by Marie Curie Actions awarded to DB and a Scottish Food Security Alliance-Crops PhD studentship awarded by the University of Dundee, the University of Aberdeen, and the James Hutton Institute to RAT. RAT and DB are currently supported by the H2020 Innovation Action ‘Circles’ (European Commission, Grant agreement 818290) awarded to the University of Dundee.
Author information
Authors and Affiliations
Contributions
The study was conceived by R.A.T. and D.B. with critical inputs from E.P. and E.B. R.A.T. and K.B.C. performed the experiments. J.M. and P.H. generated the 16S rRNA sequencing reads. J.R. provided access to the molecular marker information of the barley genome. E.F. provided access to the eco-geographical and phenotypic data of the B1K accessions. R.A.T. and D.B. analysed the data. All authors critically reviewed and edited the manuscript and approved its publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alegria Terrazas, R., Balbirnie-Cumming, K., Morris, J. et al. A footprint of plant eco-geographic adaptation on the composition of the barley rhizosphere bacterial microbiota. Sci Rep 10, 12916 (2020). https://doi.org/10.1038/s41598-020-69672-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69672-x
This article is cited by
-
The soil microbiome of Lolium perenne L. depends on host genotype, is modified by nitrogen level and varies across season
Scientific Reports (2024)
-
The plant microbiota signature of the Anthropocene as a challenge for microbiome research
Microbiome (2022)
-
Identifying plant genes shaping microbiota composition in the barley rhizosphere
Nature Communications (2022)
-
A field indicator for rhizosphere effect monitoring in arable soils
Plant and Soil (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.