Abstract
Plasmodium falciparum causes the most severe form of malaria in humans. The adhesion of the infected erythrocytes (IEs) to endothelial receptors (sequestration) and to uninfected erythrocytes (rosetting) are considered major elements in the pathogenesis of the disease. Both sequestration and rosetting appear to involve particular members of several IE variant surface antigens (VSAs) as ligands, interacting with multiple vascular host receptors, including the ABO blood group antigens. In this study, we subjected genetically distinct P. falciparum parasites to in vitro selection for increased IE adhesion to ABO antigens in the absence of potentially confounding receptors. The selection resulted in IEs that adhered stronger to pure ABO antigens, to erythrocytes, and to various human cell lines than their unselected counterparts. However, selection did not result in marked qualitative changes in transcript levels of the genes encoding the best-described VSA families, PfEMP1 and RIFIN. Rather, overall transcription of both gene families tended to decline following selection. Furthermore, selection-induced increases in the adhesion to ABO occurred in the absence of marked changes in immune IgG recognition of IE surface antigens, generally assumed to target mainly VSAs. Our study sheds new light on our understanding of the processes and molecules involved in IE sequestration and rosetting.
Similar content being viewed by others
Introduction
Malaria remains a major cause of infectious disease mortality and morbidity in many parts of the World1. It is caused by haemo-protozoan parasites of the genus Plasmodium, which infect and multiply within erythrocytes. The formation of rosettes, which is the binding of multiple uninfected erythrocytes to a central infected erythrocyte (IE), was first reported in the simian malaria parasite P. fragile2, but it is also common among human malaria parasites3,4,5,6,7. In two of these, P. falciparum and P. vivax, rosetting has repeatedly been associated with severe disease8,9,10,11, although the functional significance of rosetting is not fully understood12.
Rosetting involves multiple parasite-encoded ligands and host erythrocyte receptors13. Particular members of the three major parasite multi-gene families var (P. falciparum only)14,15,16, rif17,18, and stevor19 have been implicated as ligands, while a range of glycoproteins, proteoglycans, and carbohydrate moieties on the erythrocyte surface have been proposed as host receptors. These latter molecules include the blood group determinants A and B20, CD3621,22, heparan sulphate23, complement receptor 1 (CR1)14, and glycophorin C19,24. Soluble host proteins such as IgM25,26,27 and α2-macroglobulin28 also appear critical for at least some forms of rosetting, adding further to the complexity of the rosetting phenotype.
Blood group O (lack of blood group antigens A and B) has repeatedly been associated with protection from severe P. falciparum malaria29,30. This suggests that IE adhesion to A or B antigen, on erythrocytes (rosetting) or on endothelium (sequestration), is a risk factor for development of severe malaria. Identification of the parasite ligand(s) mediating binding to these receptors is therefore of interest. To achieve that, we selected IEs for their ability to adhere to blood group A, B and O blood group oligosaccharides in vitro by repeated panning on these antigens immobilized to plastic via bovine serum albumin, and examined associated changes in transcription of parasite genes encoding putative adhesion ligands.
Results
Plasmodium falciparum-infected erythrocytes can be selected in vitro for binding to ABO blood group antigens
The blood group antigens A and B are formed by addition of a terminal α-1,3-N-acetylgalactosamine or galactose residue, respectively, to the H antigen (blood group O). To test the ability of IEs to adhere to these antigens in the absence of interference from other potential adhesion receptors present on erythrocytes and endothelial cells, we used bovine serum albumin (BSA) neo-glycoproteins displaying A, B, or H antigens (BSA-A, BSA-B, and BSA-H) as receptors for IE adhesion. Whereas unselected erythrocytes infected by P. falciparum 3D7 bound only weakly to any of these antigens, selection for IE adhesion to either BSA-A or BSA-B resulted in IEs that bound significantly better to both receptors, whereas selection for IE adhesion to BSA-H had little effect (Fig. 1). Selection of four additional P. falciparum lines/clones yielded results similar to those obtained with 3D7 for two of them (FMG and FUP), whereas we were unable to improve the adhesion to blood group sugars of erythrocytes infected by P. falciparum FCR3 or HB3 (Supplementary Fig. 1).
Selection for IE adhesion to ABO antigens. Adhesion of erythrocytes infected with late-stage P. falciparum 3D7 to BSA, BSA-H, BSA-A, and BSA-B before (white) and after four rounds of selection on BSA-H (grey), BSA-A (red), and BSA-B (blue), respectively. Adhesion of uninfected erythrocytes to the receptors was always < 5% of the erythrocytes added. Error bars indicate standard deviations of mean results from the number of independent assays indicated in the figure. Statistically significant differences (P < 0.05) of post-hoc pairwise comparisons (Holm-Sidak method) following 1-way ANOVA (P < 0.001) are indicated by lines along the top of the panel.
The similar results obtained by IE panning on BSA-A and BSA-B prompted us to verify the uniqueness of the neo-glycoproteins used. Blood group A-specific antibody blocked adhesion of IEs to BSA-A, but had only limited effect on adhesion of the same IEs to BSA-B (Supplementary Fig. 2). In contrast, blood-group B-specific antibody efficiently inhibited adhesion of IEs to BSA-B, but had no effect on adhesion of the same IEs to BSA-A (Supplementary Fig. 2). These results confirm that the BSA-A and BSA-B neo-glycoprotein constructs were antigenically distinct. To assess the possibility that the shorter (three-atom) spacer used in the BSA-H construct than in the BSA-A and BSA-B constructs (six-atom spacer) negatively affected IE adhesion to BSA-H, we also tested IE adhesion to a BSA-B construct with a three-atom spacer (BSA-Bshort). Although selected P. falciparum 3D7 bound less well to BSA-Bshort, similarly selected FMG IEs adhered equally well to either construct (Supplementary Fig. 3). This suggests that the length of the spacer is of limited importance.
Formation of rosettes after selection for IE adhesion to ABO antigens. Frequency of rosettes formed by adhesion of uninfected blood group O, A, or AB erythrocytes to erythrocytes infected with late-stage P. falciparum 3D7 (A), FMG (B), or FUP (C) before selection (white) or after selection of IEs for adhesion to BSA-A (red) or BSA-B (blue). Data from one out of two independent experiments with similar results are shown.
Temporal stability of IE adhesion phenotype following selection. Adhesion of P. falciparum 3D7-IEs to BSA (circles) or BSA-A (diamonds) (A), or to BSA-H (triangles) or BSA-B (squares) (B) at various time-points following selection for IE adhesion to BSA-A (red symbols) or BSA-B (blue symbols). Individual data points (symbols), regression lines (heavy lines), and 95% confidence intervals for regression lines (thin lines) are shown.
Overall, the experiments indicate that some, but possibly not all P. falciparum parasites can express ligands on the surface of IEs that enable their adhesion to the blood group carbohydrate antigens A and B.
Plasmodium falciparum-IEs selected for adhesion to A and B blood group antigens form rosettes with corresponding uninfected erythrocytes
Several studies have implicated ABO antigens as receptors involved in the rosetting reaction around P. falciparum IEs. We therefore tested the impact on rosetting rates of selection for IE adhesion to ABO antigens. Without selection, rosettes did not form around erythrocytes infected by P. falciparum 3D7, FMG, or FUP, but selection of IEs for adhesion to either A or B yielded IEs that formed rosettes with A+ and AB+ erythrocytes, but not with O+ erythrocytes (Fig. 2 and Supplementary Table 1). These results support the importance of A and B blood group antigens in rosetting.
Adhesiveness of P. falciparum-infected erythrocytes to blood group A and B antigens is a stable phenotype
The increase in IE adhesion to blood group A and B antigens following repeated panning of IEs on these receptors suggests that the adhesion is mediated by ligands encoded by members of one of the several multi-gene families in malaria parasites. While only limited information is available regarding the temporal stability of IE adhesion mediated by the STEVOR (~ 30 variants/parasite) and RIFIN antigens (~ 150 variants/parasite), substantial variation is known to exist in the stability of adhesion mediated by members of the best-studied adhesion family PfEMP1 (~ 60 variants/parasite). To determine the stability of the blood group A- and B-adhering IE phenotypes, in vitro cultures of P. falciparum 3D7 that had been selected for IE adhesion to either receptor were maintained for extended periods without further selection. In both cases, the initial IE adhesion phenotype was essentially stable for more than three months (> 50 generations) after the last selection round (Fig. 3).
Plasmodium falciparum-infected erythrocytes selected for adhesion to A and B blood group antigens bind better to endothelial cells than unselected infected erythrocytes
Expression of ABO antigens is not restricted to erythrocytes, but is also widespread on vascular endothelium, where they are potential receptors for sequestering IEs. We therefore proceeded by comparing the ability of unselected IEs and IEs selected for adhesion to blood group A and B to adhere to cell lines and human primary endothelial cells. Erythrocytes infected by P. falciparum 3D7 (Fig. 4) or FMG (Supplementary Fig. 4A) and selected for adhesion to blood group A or B showed increased adhesion to primary human aortic and foreskin endothelial cells and to the choriocarcinoma cell line BeWo, compared to unselected IEs. In contrast, selected IEs did not bind better than unselected IEs to wild-type Chinese hamster ovary (CHO) cells (CHO-K1), to K1-derived glycosylation mutants A745 and D677, or to K1 cells transfected to express known P. falciparum adhesion receptors CD36 or CD54 (ICAM-1) (Supplementary Fig. 5).
Adhesion of IEs to cellular receptors. Adhesion of erythrocytes infected with late-stage P. falciparum 3D7 to monolayers of aorta endothelial cells (EC), foreskin EC, BeWo choriocarcinoma cells, or uncoated wells (No cells) before (white) and after four rounds of selection on BSA-A (red) or BSA-B (blue), respectively. Error bars indicate standard deviations of mean results from four (No cells, Aorta EC, Foreskin EC) or three (BeWo cells) independent assays. Statistically significant differences (P < 0. 05) of post-hoc pairwise comparisons (Holm-Sidak method) following 1-way ANOVA (P < 0.01) are indicated by lines along the top of the panel.
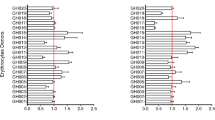
Transcription of var genes. Transcription of var genes in ring-stage P. falciparum 3D7 before (left) and after selection × 3 for IE adhesion to BSA-A (centre) or BSA-B (right). Genes are sorted according to structural groups 79 and to the transcript levels of individual genes relative to housekeeping gene in unselected parasites (this order is maintained in all panels). The size of the pies reflect the overall transcript levels relative to housekeeping genes. The var gene pf130003 is identified by an asterisk (*). For additional details regarding individual genes, see Supplementary Data.
Plasmodium falciparum HB3-IEs, which we were unable to select for increased IE adhesion to ABO antigens, adhered to the tested human endothelial cells to approximately the same degree as P. falciparum 3D7 and FMG after selection (Supplementary Fig. 4B).
Selection of P. falciparum-infected erythrocytes for adhesion to A and B blood group antigens does not lead to major qualitative changes in var gene transcription
We next considered whether IE selection for adhesion to A and B antigens caused qualitative changes and/or quantitative increases in the transcription of genes encoding adhesive parasite ligands that could explain the increased IE adhesion to these receptors. As PfEMP1 appears to be the major IE adhesion ligands, we first compared levels of PfEMP1-encoding var gene transcripts in P. falciparum 3D7 and FMG before and after selection. Selection of IEs for increased adhesion to BSA-A and BSA-B tended to cause a reduction in the level of var gene transcripts relative to transcripts of housekeeping genes for both the tested parasites (Fig. 5, Supplementary Fig. 6). Although unselected and selected parasites alike were predominantly ring stages, as evidenced by microscopy and 10–30 × increases in transcript number of the ring stage-specific gene sbp-131, fold increases tended to be lower for selected than for unselected parasites. Therefore, we cannot formally rule out that the apparent down-regulation was at least partially due to differences in the proportion of ring-stage parasites in the unselected versus the selected parasites. This notwithstanding, all data presented were obtained from a time point (15–16 h post-invasion), where transcription of both var and rif genes is active32.
Transcription of rif genes. Transcription of rif genes in ring-stage P. falciparum 3D7 before (left) and after selection × 3 for IE adhesion to BSA-A (centre) or BSA-B (right). Genes are sorted according to types77 (A: red; B: blue; uncharacterized: yellow) and to the transcript levels of individual genes relative to housekeeping gene in unselected parasites. The size of the pies reflect the overall transcript levels relative to housekeeping genes. For details regarding individual genes, see Supplementary Data.
We observed no qualitative differences in the transcription of individual var genes (Supplementary Data), or in the relative proportions of transcribed genes in the structural var gene groups A, B, C, and E (Fig. 5 and Supplementary Fig. 6) that could explain the change in IE adhesion before and after selection. We were thus unable to substantiate the hypothesis that the observed increased IE adhesion to A and B blood group antigens in response to in vitro selection is due to changes in transcription of var genes encoding particular PfEMP1 adhesion ligands.
Selection of P. falciparum-infected erythrocytes for adhesion to A and B blood group antigens does not lead to major qualitative changes in rif gene transcription
Particular RIFIN variants have also been implicated as parasite ligands involved in IE rosetting, and therefore by implication in endothelial IE adhesion17,18,33. We therefore proceeded to compare the levels of rif gene transcripts in P. falciparum 3D7 before and after selection. As for the var genes, selection tended to result in a reduction in rif gene transcript levels, in the absence of major qualitative differences at the level of individual genes or the proportion of genes in the structural rif gene groups A and B (Fig. 6, Supplementary Fig. 7). Increased IE adhesion to A and B blood group antigens in response to in vitro selection thus does not appear due to changes in rif transcription.
Immune IgG recognition of IEs. Immune IgG recognition of erythrocytes infected with late-stage P. falciparum 3D7 (A), FMG (B), or HB3 (C) before (white) and after four rounds of selection on BSA-A (red) or BSA-B (blue), respectively. Medians (centre lines), central 50% (boxes), central 90% (error bars), and outliers (circles) of levels of IgG in individual plasma samples from 93 Ghanaian children with P. falciparum malaria are shown. Recognition of the IEs by IgG in a pool of plasma from non-exposed donors was always < 5.5 (3D7) or < 1.5 (FMG and HB3).
Selection of P. falciparum-infected erythrocytes for adhesion to A and B blood group antigens does not affect immune IgG recognition of the parasite ligands expressed on the IE surface
Rosetting has repeatedly been associated with severe P. falciparum malaria. Furthermore, protective immunity to the disease has been associated with acquisition of IgG specific for parasite antigens on the surface of IEs, and acquired protection against severe disease generally precedes protection against uncomplicated malaria and asymptomatic infection. On this basis, we hypothesized that selection for IE adhesion to ABO antigens would lead to increased recognition of the IEs by IgG from semi-immune individuals living in areas with stable transmission of P. falciparum parasites. To test this, we labelled unselected IEs and IEs selected for adhesion to blood group A and B antigens with plasma IgG from 93 Ghanaian children with P. falciparum malaria. For all the tested parasites (3D7, FMG, and HB3), the median IE recognition by immune plasma IgG was similar for unselected, BSA-A- and BSA-B-selected IEs (Fig. 7). Furthermore, immune IgG recognition of unselected and selected IEs were very significantly correlated for all three parasite lines (0.65 < r < 0.93; 1 × 10–12 < P < 1 × 10–42 for all comparisons). Recognition of different parasite lines selected the same way generally correlated much less well (0.08 < r < 0.64; 0.5 < P < 1 × 10–12 for all comparisons of parasites selected the same way). We thus found no evidence that selection for increased IE adhesion to blood group A or B antigens led to increased IE recognition by immune IgG, or to a different recognition pattern.
Discussion
The majority of severe and fatal episodes of P. falciparum malaria occurs among young children in equatorial Africa. The resulting selection pressure on the human population has led to mutations and adaptations that confer some degree of clinical protection. Classical examples include sickle-cell anaemia and thalassaemia34,35. The ABO blood group antigens have also been implicated as determinants of malaria susceptibility, as blood group O appears to offer some degree of protection36. The association has mainly been attributed to the preferential involvement of the non-O blood group antigens in rosetting20. Rosetting can lead to vascular obstruction and endothelial inflammation, but likely also serves as a marker of adhesion of IEs to endothelial host receptors (sequestration), as blood group antigens are also widely expressed on vascular endothelium37.
Analysis of the role of ABO blood group antigens in malaria pathogenesis is complicated by the many other endothelial receptors known or suspected to be involved in sequestration, and by the high number and diversity of the parasite molecules that serve as sequestration ligands on IEs38. In the present study, we therefore sought to study the contribution of ABO antigens to IE adhesion and rosetting in the absence of other host receptors present on erythrocytes and endothelial cells. We also sought to gain insights regarding the parasite ligands mediating rosetting and sequestration.
To achieve this, we used five P. falciparum lines/clones (3D7, FCR3, FMG, FUP, and HB3) and commercially available BSA neo-glycoproteins, engineered to selectively display the H carbohydrate antigen (blood group O; BSA-H), blood group A antigen (BSA-A), or blood group B antigen (BSA-B). All the IEs used in our study initially showed minimal adhesion to each of the tested neo-glycoproteins (Fig. 1, and Supplementary Fig. 1). All except FCR3 and HB3 could be selected by repeated panning of IEs on BSA-A or BSA-B for markedly increased IE adhesion to these receptors. In contrast, attempts to select for increased adhesion to BSA-H (only 3D7) were unsuccessful. HB3 is genetically distinct from FCR3, whereas FCR3 is derived from FMG39, which responded rapidly to selection on BSA-A and BSA-B by changing its IE adhesion phenotype. FCR3 thus appears to have lost part(s) of its genome that is required for IE adhesion to ABO antigens. Alternatively, switching to that phenotype is for some reason a rare event in FCR3. Blood group O erythrocytes can form rosettes around IEs, but they tend to be smaller and weaker than rosettes involving A, B, or AB erythrocytes20,40. Several erythrocyte antigens have been implicated as receptors in rosetting41, and the most parsimonious explanation for our inability to select IEs for adhesion to the H antigen that defines blood group O is that the receptor mediating rosetting of blood group O erythrocytes is not the H antigen.
In our hands, selection on either BSA-A or BSA-B resulted in increased IE adhesion to both receptors (Fig. 1, and Supplementary Fig. 1) and in increased rosetting rates with both blood group A and blood group AB erythrocytes (Fig. 2). To our knowledge, selection for IE adhesion to ABO antigens in the absence of other potentially interfering receptors has not been reported before, but our results agree with reports implicating both blood group A and B antigens in rosetting20. The strong co-selection for IE adhesion to both A and B oligosaccharides by panning on either receptor indicates that the same parasite ligand(s) can bind both antigens, although this may not always be the case42. In addition to increased IE adhesion to BSA-A and BSA-B, selection on these receptors also lead to increased IE adhesion to several human cell lines (Fig. 4 and Supplementary Fig. 4). In contrast, the selection had little impact on IE adhesion to a range of CHO cells (Supplementary Fig. 5), including various glycosylation mutants and cells transfected to express human CD36 or ICAM-1, which are known P. falciparum IE adhesion receptors22,43. These findings support the hypothesis that the association between rosetting and severe malaria is not only mediated by rosetting per se, but also involves IE adhesion to endothelium12.
Proteins encoded by several parasite multi-gene families and expressed on the IE surface have been implicated in IE rosetting and endothelial adhesion38. We therefore sought to determine the role of these in the increased IE adhesiveness to BSA-A and BSA-B following selection. We first considered the PfEMP1 antigens, which are encoded by the approximately 60 var genes per genome. PfEMP1 is a key element in the characteristic clonal antigenic variation of P. falciparum that endows this species with the capacity for rapid changes in the antigenic and adhesive characteristics of IEs44,45,46. Furthermore, IE adhesion to a number of endothelial host receptors, including CD36, ICAM-1 (CD54), chondroitin sulphate A, and endothelial protein C receptor, has been attributed to particular PfEMP1 variants47,48,49,50. Severe disease is largely restricted to P. falciparum (where it has been associated with rosetting8,9,10, and although malaria-infected erythrocytes are generally able to form rosettes2,4,5,6,7, P. falciparum is the only malaria parasite infecting humans that possesses this type of antigens. Finally, PfEMP1 is believed to be the main target of naturally acquired protective antibodies targeting the IE surface51,52. Despite all these indicators, we did not find evidence of a marked change in var gene transcription profiles (Fig. 5 and Supplementary Fig. 6) that could explain the marked changes in IE adhesion to BSA-A and BSA-B (Fig. 1 and Supplementary Fig. 1) and in IE rosetting rates (Fig. 2) following selection for IE adhesion to blood group A and B. The only noteworthy difference observed was an increase in the proportion of pf13_0003 transcripts following selection (Fig. 5). However, the change appears insufficient to explain the marked phenotypic response to selection, despite the fact that the PfEMP1 encoded by pf13_0003 (and other genes with orthologous DBL1α domains, such as varO and it4var9) has previously been implicated in rosetting14,53,54. Indeed, transcription of it4var9 appeared to be very low and did not change in response to selection (Supplementary Fig. 6 and Supplementary Data). The same was true for it4var60, another var gene implicated in rosetting through blood group A and other receptors16. Overall, IE selection for adhesion to blood groups A and B on var gene transcription tended to cause down-regulation relative to transcription of housekeeping genes (Fig. 5, Supplementary Fig. 6). The second major multi-gene family encoding antigens implicated in IE rosetting and adhesion to ABO antigens is rif18,55. However, the rif gene transcriptional response to IE selection for adhesion to ABO antigens was also minimal, apart from a similar tendency towards overall down-regulation (Fig. 6 and Supplementary Fig. 7). For both gene families, it cannot be formally excluded that this was related to a less stringent dominance of ring stages in the selected populations analysed.
The repertoire of P. falciparum-IE surface-reactive plasma IgG varies markedly with age, exposure, and level of clinical immunity to malaria56,57,58,59,60. Furthermore, in vitro selection of IEs for adhesion to specific host receptors can lead to dramatic changes in the IgG recognition of IEs—changes that correlate with the clinical immune status of the IgG donor61,62. These findings indicate that IE adhesion is mediated by parasite-encoded variant antigens on the IE surface, and has led to the identification of PfEMP1 and RIFIN proteins that are selectively expressed by IEs with defined adhesion phenotypes18,48,49,50,63. Despite this, we found here that IgG-specific recognition of IEs before and after selection for adhesion to ABO antigens were remarkably similar (Fig. 7).
There are several possible explanations for our findings. One is that IE adhesion to blood group sugars is not mediated by members of the variant surface antigen families PfEMP1 and RIFINs. Instead, it might involve variant antigens not studied here, e.g., STEVOR19,64,65, or unidentified conserved antigens. Our study has little to offer with respect to the former of these alternatives. With respect to the latter, our selection for IE adhesion to ABO antigens might conceivably have favoured parasites with reduced expression of PfEMP1 and/or RIFIN proteins, which would otherwise interfere with the functionality of the unknown parasite ligand. The absence of a serological response to the selection might reflect an increased IgG response to that ligand that offset a reduced IgG recognition of PfEMP1 and RIFIN antigens. However, the marked reduction in IgG reactivity with the IE surface if PfEMP1 expression is selectively disrupted52 can be seen as an argument against this hypothesis. Furthermore, the fact that erythrocytes infected by parasites recovered from severely ill patients tend both to be particularly well recognized by plasma IgG59,60 and to form rosettes8,10 is difficult to explain under this hypothesis.
Another possibility that potentially overcomes the above difficulties is that IE adhesion to ABO antigens depends on post-translational modifications of variant antigens. These modifications might enable increased IE adhesion to ABO antigens despite reduced VSA expression. Although this idea is currently unsupported by direct evidence, it is consistent with our findings. The remarkable—and somewhat surprising—temporal stability of the selection-induced change in IE adhesion to ABO antigens (Fig. 3) can also be accommodated in this scenario, if these putative modifications are assumed not to be restricted to particular members or types within the affected variant antigen families. Finally, the hypothesis is compatible with the bulk of the evidence available in the literature, and thus deserves further study.
In conclusion, we have shown that the study of IE adhesion to ABO blood antigens in the absence of other, potentially confounding host receptors can improve our understanding of the processes and molecules involved in IE sequestration and rosetting, two central elements in the pathogenesis of P. falciparum malaria.
Methods
Plasmodium falciparum malaria parasites
Five P. falciparum lines/clones were used in the experiments reported here. 3D7 is a clonal derivative of an isolate (NF54) originally obtained from a malaria patient in the Netherlands66. P. falciparum FMG was originally obtained from a Gambian patient, and is the parent of the widely used FCR3 line39. HB3 is a clonal derivative of an isolate from Honduras67, while FUP is a line originally obtained by inoculation of Aotus monkeys with blood from a patient returning from Uganda, and subsequently adapted to in vitro culture68. All the parasites were maintained in type O erythrocytes in RPMI-1640 medium supplemented with Albumax II, as described in detail elsewhere69. Late-stage (trophozoite/schizont) IEs were purified by magnet-activated cell sorting as described previously70, except that RPMI-1640 was used throughout. To rule out cross-contamination among the lines, or unintentional outgrowth of minor genotypes in non-clonal lines, we regularly verified the genotype of our cultures by monitoring line-specific polymorphisms in the MSP1 and MSP2 loci71.
Cell lines
Primary human endothelial cells obtained from aorta and dermis of foreskin, and the choriocarcinoma cell line BeWo, were cultured in vitro according to recommended procedures as described elsewhere72,73. Chinese hamster ovary (CHO) cell lines A745, D677, K1 and K1-derived transgenic lines expressing human CD36 and CD54 (ICAM-1), respectively, were cultured in vitro as previously described73.
ABO blood group oligosaccharides
Blood group A tri-saccharide with 6-atom spacer (NGP6305), blood group B tri-saccharide with 6-atom (NGP6323) or 3-atom (NGP0323) spacer, and blood group O (H) di-saccharide with 3-atom spacer (NGP0205), all coupled to bovine serum albumin (BSA), were obtained from Dextra, UK (https://dextrauk.com/). The ability of the constructs to bind and present the relevant oligo saccharide epitopes were confirmed with human ABO-specific, biotinylated plant lectins, and with A- and B-specific mouse monoclonal antibodies (Sigma SAB4700677 and SAB4700676, respectively) (Supplementary Fig. 8).
In vitro selection of P. falciparum parasites for infected erythrocyte adhesion to ABO blood group antigens
ABO blood group saccharide-coupled BSA (10 µg/mL) was used to coat 24-well standard culture plates (400 µL/well, overnight, 4 °C). The wells were subsequently blocked with BSA (20 mg/mL, 2 h, room temp.), and washed × 3 in parasite culture medium. Purified late-stage IEs (~ 1 × 108/mL) were added to the wells (400 µL/well) and incubated on a shaking table (60 min, room temp., 100 RPM). Unbound IEs were removed by gentle washing (×4 to ×6) with parasite culture medium (1 mL/wash) without initial removal of 400 µL. The wells were inspected using an inverted microscope after every other wash, to optimize enrichment of adherent IEs). After the final wash, parasite culture medium (500 µL/well) and uninfected erythrocytes (40 µL/well) were added to the plates, which were then incubated overnight under standard parasite culture conditions to allow reinvasion, followed by transfer to standard culture flasks and further propagation.
Infected erythrocyte adhesion assays
The ability of IEs to adhere to ABO blood oligosaccharides and to cells was measured essentially as described previously72. Assays were done in 96-well Polysorb plates, coated (50 µL/well) and blocked (150 µL/well) as described for the selection procedure (BSA constructs), or in 96-well flat-bottomed tissue culture plates (cells), in which monolayers of the relevant cell type had been seeded approximately 48 h before. Wells with the appropriate cell culture medium but without cells were always included as negative controls. Purified late-stage IEs (1–2 × 107/mL, 100 µL/well) were added to duplicate (adhesion to ABO constructs) or triplicate wells (adhesion to cells), and incubated (30 min on a shaking table as above). After the incubation, the plates were placed upside-down in a container with PBS (2% foetal calf serum; 60 min; room temp.) to allow unbound IEs to sink to the bottom of the container under gravity only. The plates were subsequently removed from the container, dried with paper tissue, followed by lysis of bound IEs by addition of PBS (100 µL/well), supplemented with Triton-X-100 (1% v/v). A separate preparation of lysed IEs (same number and batch as used in the adhesion assay) was then added to two previously empty wells (total_IEs). Adherent IEs (adh_IEs) were quantified by adding tetramethylbenzidine (100 µL/well) ELISA substrate and measuring the optical density (OD) of wells at 450 nm in a standard ELISA reader. The enzymatic reaction was stopped with sulphuric acid (1 M) while ODtotal_IEs < 2 to ensure a linear relationship between the number of IEs OD. The fraction of IEs adhering was calculated as (ODadh_IEs − ODbackground)/(ODtotal_IEs − ODbackground), where ODbackground was the mean OD obtained in wells, where IEs had not been added. In assays measuring IE binding to cells, the background wells were seeded with the appropriate cells.
The ability of anti-A and anti-B antibodies to inhibit IE adhesion to BSA-A and BSA-B was tested using saturating concentrations of the above-mentioned monoclonal antibodies.
Rosetting assays
Purified late stage-IEs were mixed with uninfected erythrocytes with different ABO types. Incubation, staining with ethidium bromide and reading of the assays were as described previously26.
Assessment of human IgG reactivity with variant surface antigens on infected erythrocytes
Purified late stage-IEs (1 × 105/plasma sample) were first incubated with human immune plasma (5 µL; 30 min; room temp.), obtained from 93 ABO-typed (with commercially available agglutination kits (Fortress Diagnostics, UK)) children with acute P. falciparum malaria, living in an area of high and seasonal transmission of P. falciparum parasites74. Further processing and quantification of IgG binding to IEs by flow cytometry were essentially as described previously75, using FlowLogic software (https://www.inivai.com/).
var and rif gene transcription profiling
Parasite cDNA was generated from DNA-free total RNA prepared from ring-stage IEs (approximately 15–16 h post-invasion) and using SuperScript II reverse transcriptase and random primers, according to the manufacturer’s instructions (https://thermofisher.com). Levels of var and rif gene transcripts were measured using cDNA, QuantiTec SYBR Green PCR Master Mix (Qiagen), and gene-specific primer pairs for 58 (3D7)76 and 56 (FMG)71 var genes, 154 rif genes (3D7)77,78, and for the endogenous control genes seryl-tRNA synthetase (p90) and fructose-bisphosphate aldolase (p61)76. Transcript copy number of each gene in tested cDNA was calculated using quantitative measurements of tenfold dilutions of genomic DNA as previously described78.
Statistical analysis
The statistical significance of intergroup differences were first tested by 1-way analysis of variance. If that yielded a statistically significant (P < 0.05), statistically significant (P < 0.05) pairwise differences were identified by the Holm-Sidak method.
Ethics statement
The work described here was conducted in accordance with all relevant guidelines and regulations, and all donors provided their informed consent to participate prior collection of blood samples. The experimental protocols (including collection of the human blood samples used), were approved by the Ethics Committee of the Noguchi Memorial Institute for Medical Research, University of Ghana, by Ghana Health Service (GHS-ERC 08/05/14), and by the Regional Research Ethics Committees for the Capital Region of Denmark (Protocol H-4-2013-083).
References
World Health Organization, N. World malaria report 2018 (2018).
David, P. H., Handunnetti, S. M., Leech, J. H., Gamage, P. & Mendis, K. N. Rosetting: a new cytoadherence property of malaria-infected erythrocytes. Am. J. Trop. Med. Hyg. 38, 289–297 (1988).
Handunnetti, S. M., David, P. H., Perera, K. L. R. L. & Mendis, K. N. Uninfected erythrocytes form “rosettes” around Plasmodium falciparum infected erythrocytes. Am. J. Trop. Med. Hyg. 40, 115–118 (1989).
Udomsangpetch, R. et al. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J. Exp. Med. 169, 1835–1840 (1989).
Udomsangpetch, R., Thanikkul, K., Pukrittayakamee, S. & White, N. J. Rosette formation by Plasmodium vivax. Trans. R. Soc. Trop. Med. Hyg. 89, 635–637 (1995).
Angus, B. J., Thanikkul, K., Silamut, K., White, N. J. & Udomsangpetch, R. Rosette formation in Plasmodium ovale infection. Am. J. Trop. Med. Hyg. 55, 560–561 (1996).
Lowe, B. S., Mosobo, M. & Bull, P. C. All four species of human malaria parasites form rosettes. Trans. R. Soc. Trop. Med. Hyg. 92, 526–526 (1998).
Carlson, J. et al. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336, 1457–1460 (1990).
Doumbo, O. K. et al. High levels of Plasmodium falciparum rosetting in all clinical forms of severe malaria in African children. Am. J. Trop. Med. Hyg. 81, 987–993 (2009).
Rowe, A., Obeiro, J., Newbold, C. I. & Marsh, K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect. Immun. 63, 2323–2326 (1995).
Marin-Menendez, A. et al. Rosetting in Plasmodium vivax: a cytoadhesion phenotype associated with anaemia. PLoS Negl. Trop. Dis. 7, e2155. https://doi.org/10.1371/journal.pntd.0002155 (2013).
Wang, C. W. & Hviid, L. Rifins, rosetting, and red blood cells. Trends Parasitol. 31, 285–286. https://doi.org/10.1016/j.pt.2015.04.009 (2015).
Mercereau-Puijalon, O., Guillotte, M. & Vigan-Womas, I. Rosetting in Plasmodium falciparum: a cytoadherence phenotype with multiple actors. Transfus. Clin. Biol. 15, 62–71 (2008).
Rowe, J. A., Moulds, J. M., Newbold, C. I. & Miller, L. H. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388, 292–295 (1997).
Le Scanf, C., Fandeur, T., Bonnefoy, S., Guillotte, M. & Mercereau-Puijalon, O. Novel target antigens of the variant-specific immune response to Plasmodium falciparum identified by differential screening of an expression library. Infect. Immun. 67, 64–73 (1999).
Albrecht, L. et al. var gene transcription and PfEMP1 expression in the rosetting and cytoadhesive Plasmodium falciparum clone FCR3S1.2. Malar. J. 10, 17. https://doi.org/10.1186/1475-2875-10-17 (2011).
Helmby, H., Cavelier, L., Pettersson, U. & Wahlgren, M. Rosetting Plasmodium falciparum-infected erythrocytes express unique strain-specific antigens on their surface. Infect. Immun. 61, 284–288 (1993).
Goel, S. et al. RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nat. Med. 21, 314–317. https://doi.org/10.1038/nm.3812 (2015).
Niang, M. et al. STEVOR is a Plasmodium falciparum erythrocyte binding protein that mediates merozoite invasion and rosetting. Cell Host Microbe 16, 81–93. https://doi.org/10.1016/j.chom.2014.06.004 (2014).
Carlson, J. & Wahlgren, M. Plasmodium falciparum erythrocyte rosetting is mediated by promiscuous lectin-like interactions. J. Exp. Med. 176, 1311–1317 (1992).
Handunnetti, S. M. et al. Involvement of CD36 on erythrocytes as a rosetting receptor for Plasmodium falciparum-infected erythrocytes. Blood 80, 2097–2104 (1992).
Ockenhouse, C. F., Tandon, N. N., Magowan, C., Jamieson, G. A. & Chulay, J. D. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science 243, 1469–1471 (1989).
Rowe, A., Berendt, A. R., Marsh, K. & Newbold, C. I. Plasmodium falciparum: a family of sulphated glycoconjugates disrupts erythrocyte rosettes. Exp. Parasitol. 79, 506–516 (1994).
Lee, W. C. et al. Glycophorin C (CD236R) mediates vivax malaria parasite rosetting to normocytes. Blood 123, e100–e109. https://doi.org/10.1182/blood-2013-12-541698 (2014).
Rowe, J. A., Shafi, J., Kai, O. K., Marsh, K. & Raza, A. Nonimmune IgM, but not IgG binds to the surface of Plasmodium falciparum-infected erythrocytes and correlates with rosetting and severe malaria. Am. J. Trop. Med. Hyg. 66, 692–699 (2002).
Stevenson, L. et al. Investigating the function of Fc-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting. Cell. Microbiol. 17, 819–831 (2015).
Akhouri, R. R., Goel, S., Furusho, H., Skoglund, U. & Wahlgren, M. Architecture of human IgM in complex with P. falciparum erythrocyte membrane protein 1. Cell Rep. 14, 723–736. https://doi.org/10.1016/j.celrep.2015.12.067 (2016).
Stevenson, L. et al. a2-macroglobulin can crosslink multiple Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) molecules and may facilitate adhesion of parasitized erythrocytes. PLoS Pathog. 11, e1005022. https://doi.org/10.1371/journal.ppat.1005022 (2015).
Cserti, C. M. & Dzik, W. H. The ABO blood group system and Plasmodium falciparum malaria. Blood 110, 2250–2258 (2007).
Degarege, A. et al. Effect of ABO blood group on asymptomatic, uncomplicated and placental Plasmodium falciparum infection: systematic review and meta-analysis. BMC Infect. Dis. 19, 86. https://doi.org/10.1186/s12879-019-3730-z (2019).
Joice, R. et al. Inferring developmental stage composition from gene expression in human malaria. PLoS Comput. Biol. 9, e1003392. https://doi.org/10.1371/journal.pcbi.1003392 (2013).
Yam, X. Y., Niang, M., Madnani, K. G. & Preiser, P. R. Three is a crowd—new insights into rosetting in Plasmodium falciparum. Trends Parasitol. 33, 309–320. https://doi.org/10.1016/j.pt.2016.12.012 (2017).
Fernandez, V., Hommel, M., Chen, Q. J., Hagblom, P. & Wahlgren, M. Small, clonally variant antigens expressed on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J. Exp. Med. 190, 1393–1403 (1999).
Allison, A. C. Protection afforded by sickle cell trait against subtertian malarial infection. Br. Med. J. 1, 290–293 (1954).
Haldane, J. B. The rate of mutation of human genes. Hereditas 35, 267–273 (1949).
Degarege, A., Gebrezgi, M. T., Ibanez, G., Wahlgren, M. & Madhivanan, P. Effect of the ABO blood group on susceptibility to severe malaria: a systematic review and meta-analysis. Blood Rev. https://doi.org/10.1016/j.blre.2018.07.002 (2018).
Szulman, A. E. The histological distribution of blood group substances A and B in man. J. Exp. Med. 111, 785–800 (1960).
Wahlgren, M., Goel, S. & Akhouri, R. R. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat. Rev. Microbiol. 15, 479–491. https://doi.org/10.1038/nrmicro.2017.47 (2017).
Jensen, J. B. & Trager, W. Plasmodium falciparum in culture: establishment of additional strains. Am. J. Trop. Med. Hyg. 27, 743–746 (1978).
Barragan, A., Kremsner, P. G., Wahlgren, M. & Carlson, J. Blood group A antigen is a co-receptor in Plasmodium falciparum rosetting. Infect. Immun. 68, 2971–2975 (2000).
McQuaid, F. & Rowe, J. A. Rosetting revisited: a critical look at the evidence for host erythrocyte receptors in Plasmodium falciparum rosetting. Parasitology https://doi.org/10.1017/S0031182019001288 (2019).
Vagianou, C. D. et al. ABO blood group antigen decorated giant unilamellar vesicles exhibit distinct interactions with Plasmodium falciparum infected red blood cells. ACS Chem. Biol. 13, 2421–2426. https://doi.org/10.1021/acschembio.8b00635 (2018).
Berendt, A. R., Simmons, D. L., Tansey, J., Newbold, C. I. & Marsh, K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature 341, 57–59 (1989).
Baruch, D. I. et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82, 77–87 (1995).
Smith, J. D. et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82, 101–110 (1995).
Su, X. et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82, 89–100 (1995).
Baruch, D. I., Gormley, J. A., Ma, C., Howard, R. J. & Pasloske, B. L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. U.S.A. 93, 3497–3502 (1996).
Salanti, A. et al. Selective upregulation of a single distinctly structured var gene in CSA-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49, 179–191 (2003).
Turner, L. et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498, 502–505. https://doi.org/10.1038/nature12216 (2013).
Lennartz, F. et al. Structure-guided identification of a family of dual receptor-binding PfEMP1 that is associated with cerebral malaria. Cell Host Microbe 21, 403–414. https://doi.org/10.1016/j.chom.2017.02.009 (2017).
Hviid, L. The role of Plasmodium falciparum variant surface antigens in protective immunity and vaccine development. Hum. Vaccin 6, 84–89 (2010).
Chan, J. A. et al. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J. Clin. Invest. 122, 3227–3238. https://doi.org/10.1172/JCI62182 (2012).
Fandeur, T., Le Scanf, C., Bonnemains, B., Slomianny, C. & Mercereau-Puijalon, O. Immune pressure selects for Plasmodium falciparum parasites presenting distinct red blood cell surface antigens and inducing strain-specific protection in Saimiri sciureus monkeys. J. Exp. Med. 181, 283–295 (1995).
Vigan-Womas, I. et al. Allelic diversity of the Plasmodium falciparum erythrocyte membrane protein 1 entails variant-specific red cell surface epitopes. PLoS ONE 6, e16544. https://doi.org/10.1371/journal.pone.0016544 (2011).
Kyes, S. A., Rowe, J. A., Kriek, N. & Newbold, C. I. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 96, 9333–9338 (1999).
Marsh, K. & Howard, R. J. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231, 150–153 (1986).
Bull, P. C. et al. Parasite antigens on the infected red cell are targets for naturally acquired immunity to malaria. Nat. Med. 4, 358–360 (1998).
Fried, M., Nosten, F., Brockman, A., Brabin, B. T. & Duffy, P. E. Maternal antibodies block malaria. Nature 395, 851–852 (1998).
Bull, P. C. et al. Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J. Infect. Dis. 182, 252–259 (2000).
Nielsen, M. A. et al. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and non-severe malaria and is modified by acquired immunity. J. Immunol. 168, 3444–3450 (2002).
Staalsoe, T. et al. Variant surface antigen-specific IgG and protection against the clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363, 283–289 (2004).
Ricke, C. H. et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulphate A. J. Immunol. 165, 3309–3316 (2000).
Saito, F. et al. Immune evasion of Plasmodium falciparum by RIFIN via inhibitory receptors. Nature 552, 101–105. https://doi.org/10.1038/nature24994 (2017).
Cheng, Q. et al. stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol. Biochem. Parasitol. 97, 161–176 (1998).
Niang, M., Yan, Y. X. & Preiser, P. R. The Plasmodium falciparum STEVOR multigene family mediates antigenic variation of the infected erythrocyte. PLoS Pathog. 5, e1000307 (2009).
Delemarre, B. J. & Van der Kaay, H. J. Malaria tropica op natuurlijke wijze verkregen in Nederland. Ned Tijdschr Geneeskd 123, 1981–1982 (1979).
Bhasin, V. K. & Trager, W. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 33, 534–537 (1984).
Geiman, Q. M. & Meagher, M. J. Susceptibility of a New World monkey to Plasmodium falciparum from man. Nature 215, 437–439 (1967).
Cranmer, S. L., Magowan, C., Liang, J., Coppel, R. L. & Cooke, B. M. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 91, 363–365 (1997).
Staalsoe, T., Giha, H. A., Dodoo, D., Theander, T. G. & Hviid, L. Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry 35, 329–336 (1999).
Wang, C. W. et al. Evidence for in vitro and in vivo expression of the conserved VAR3 (type 3) Plasmodium falciparum erythrocyte membrane protein 1. Malar. J. 11, 129. https://doi.org/10.1186/1475-2875-11-129 (2012).
Hempel, C., Boisen, I. M., Efunshile, A., Kurtzhals, J. A. & Staalso, T. An automated method for determining the cytoadhesion of Plasmodium falciparum-infected erythrocytes to immobilized cells. Malar. J. 14, 112. https://doi.org/10.1186/s12936-015-0632-4 (2015).
Haase, R. N. et al. Plasmodium falciparum parasites expressing pregnancy-specific variant surface antigens adhere strongly to the choriocarcinoma cell line BeWo. Infect. Immun. 74, 3035–3038 (2006).
Partey, F. D. et al. Kinetics of antibody responses to PfRH5-complex antigens in Ghanaian children with Plasmodium falciparum malaria. PLoS ONE 13, e0198371. https://doi.org/10.1371/journal.pone.0198371 (2018).
Staalsoe, T. et al. In vitro selection of Plasmodium falciparum 3D7 for expression of variant surface antigens associated with severe malaria in African children. Parasite Immunol. 25, 421–427 (2003).
Dahlbäck, M. et al. Changes in var gene mRNA levels during erythrocytic development in two phenotypically distinct Plasmodium falciparum parasites. Malar. J. 6, 78 (2007).
Joannin, N., Abhiman, S., Sonnhammer, E. L. & Wahlgren, M. Sub-grouping and sub-functionalization of the RIFIN multi-copy protein family. BMC Genomics 9, 19 (2008).
Wang, C. W., Magistrado, P. A., Nielsen, M. A., Theander, T. G. & Lavstsen, T. Preferential transcription of conserved rif genes in two phenotypically distinct Plasmodium falciparum parasite lines. Int. J. Parasitol. 39, 655–664 (2009).
Rask, T. S., Hansen, D. A., Theander, T. G., Pedersen, A. G. & Lavstsen, T. Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes-divide and conquer. PLoS Comput. Biol. 6, e1000933 (2010).
Acknowledgements
Maiken Visti is thanked for excellent technical assistance. This work was funded by The Consultative Committee for Development Research [Grant Numbers DFC 12-081RH and 17-02-KU], by the Danish Council for Independent Research [DFF-4183-00539], and by Rigshospitalet [E-22071-01]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
W.P., C.H., J.A.L.K., and T.S. conceived and designed the study. W.P., C.W.W., S.S., R.W.O., N.D., and T.S. performed the experiments. W.P., L.H., and M.F.O. organised and conducted the study-related fieldwork. W.P., J.K., L.H., and T.S. analysed the data. W.P., T.S. and L.H. wrote the first draft of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Puije, W., Wang, C.W., Sudharson, S. et al. In vitro selection for adhesion of Plasmodium falciparum-infected erythrocytes to ABO antigens does not affect PfEMP1 and RIFIN expression. Sci Rep 10, 12871 (2020). https://doi.org/10.1038/s41598-020-69666-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69666-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.