Abstract
Recent studies establish a crucial role of the circadian clock in regulating plant defense against pathogens. Whether pathogens modulate host circadian clock as a potential strategy to suppress host innate immunity is not well understood. Coronatine is a toxin produced by the bacterial pathogen Pseudomonas syringae that is known to counteract Arabidopsis defense through mimicking defense signaling molecules, jasmonates (JAs). We report here that COR preferentially suppresses expression of clock-related genes in high throughput gene expression studies, compared with the plant-derived JA molecule methyl jasmonate (MJ). COR treatment dampens the amplitude and lengthens the period of all four reporters tested while MJ and another JA agonist JA-isoleucine (JA-Ile) only affect some reporters. COR, MJ, and JA-Ile act through the canonical JA receptor COI1 in clock regulation. These data support a stronger role of the pathogen-derived molecule COR than plant-derived JA molecules in regulating Arabidopsis clock. Further study shall reveal mechanisms underlying COR regulation of host circadian clock.
Similar content being viewed by others
Introduction
Plants have evolved sophisticated mechanisms to respond to daily attacks of pathogens and pests of different lifestyles. Increasing evidence has established that the circadian clock is an integral part of the plant innate immune system. In addition to being crucial for plant growth and development, the circadian clock regulates multiple layers of defense responses, including stomatal opening and closure, pathogen recognition, and defense signal activation1,2. Whether the circadian clock is modulated by pathogens and pests as a potential strategy to circumvent host defense has not been well understood.
Jasmonates (JAs) are lipid-derived molecules that are important for defense signaling. Recent studies demonstrate a circadian regulation of JA signaling. The JA level and expression of some key JA biosynthetic and signaling genes oscillate in a day3,4. Two Arabidopsis clock genes, LUX5 and TIC4 were shown to regulate clock output to affect JA signaling. In turn, JA signal activation dampens the amplitude and lengthens the period of some clock reporters5, suggesting a reciprocal regulation of the circadian clock by JA signaling. Such a reciprocation relationship is also found between the circadian clock and other biological processes, including nutrient uptake and signaling mediated by salicylic acid, reactive oxygen species, and phytohormones6,7,8,9, and it suggests an adaptive nature of plants to coordinate limited resources for growth, development, and environmental stimuli.
While being important for plant defense, JA signaling succumbs to pathogen interference. The bacterium pathogen, Pseudomonas syringae, is known to employ several mechanisms to manipulate host JA signaling, for instance, using the phytotoxin compound coronatine (COR) to mimic JA or using effector proteins to interfere with JA signaling10,11,12,13. COR structurally mimics JA-isoleucine (JA-Ile), a bioactive form of JA. Both COR and JA-Ile bind to the JA receptor, CORONATINE INSENSITIVE 1 (COI1), to activate JA signaling. COR is also known to regulate other defense pathways independently of its promotion of JA signaling14. Thus, COR and JA-Ile have overlapping and also distinct function in regulating biological processes. We recently showed that activation of JA signaling, using JA-Ile or another JA analog methyl jasmonate (MJ), reciprocally regulates clock activity5. Whether pathogen-derived COR could modulate clock activity has not been tested prior to this study. We report here that compared with plant-derived JA molecules, COR shows a stronger regulation of the circadian clock in Arabidopsis.
Results
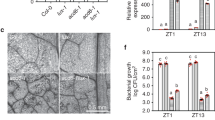
COR is a phytotoxin produced by pathovars of P. syringae and is important for the pathogenesis of the bacteria. The lack of COR makes P. syringae less virulent under a diurnal light and dark (LD) cycle15,16. In continuous light (LL), a free-running condition often used to test clock activity, we found that compared with P. syringae strain DC3000, the isogenic P. syringae strain DC3118 that does not produce COR, grew much less and induced less chlorosis and lesion in the infected Arabidopsis leaves (Figure S1).
One way that COR promotes bacterial virulence is through interfering with JA signaling. A number of studies demonstrated crosstalk between JA signaling and the circadian clock3,4,5,17. We are interested in elucidating in this report whether P. syringae-derived COR can regulate plant circadian clock. Toward this goal, we first compared expression of a set of circadian genes (Table S1), using two sets of time-series RNA-seq data from samples treated with 100 µM MJ or 5 µM COR18,19. Heatmap analysis showed that COR induced more changes in expression of the circadian genes than MJ (Fig. 1A,B). We estimated the relative number of affected gene expression by normalizing the total number of affected expression events with the number of time points. While the number of induced circadian genes was similar with the two treatments, COR showed a stronger suppression of circadian genes than MJ, including some core clock genes (Fig. 1C; Figure S2). We performed a similar analysis with a set of defense genes (Table S2). Interestingly, MJ and COR showed less difference in affecting defense gene expression (Fig. 1C; Figure S3).
COR exerts stronger suppression on expression of circadian genes than MJ. (A) Heatmap analysis of expression of circadian genes in MJ-treated samples. (B) Heatmap analysis of expression of circadian genes in COR-treated samples. For (A) and (B), Log2 transformed fold change of gene expression (100 µM MJ or 5 µM COR treatment vs. mock treatment) was used to generate the heatmaps with the heatmap.2 function in R package gplots. For MJ treatment, the mock solution contained 0.015% (v/v) Silwet L77 and 0.1% ethanol19. For COR treatment, water was used as a mock treatment18. (C) Relative number of genes affected by MJ or COR. Expression of each gene at a time point was considered as one gene expression event. The total number of defense or clock gene expression events in each category was normalized by the total number of time points.
While these analyses suggest that COR exerts a stronger suppression of circadian genes than MJ, we recognized that the two RNA-seq experiments were conducted in two different laboratories that used plants grown in different conditions18,19. In addition, both experiments were conducted under diurnal cycles. To reconcile these differences and examine the role of COR in clock regulation, we grew seedlings for 7 days under a 12 h light and 12 h dark (LD) cycle for entrainment. We then transferred the seedlings to continuous light (LL) for 1 day and treated them with MJ (100 μM) or COR (10 μM) for gene expression analysis. qRT-PCR results showed that both MJ and COR suppressed expression of selected core clock genes (Figure S4 and5), supporting that both MJ and COR regulate clock activity.
To further test the clock regulatory role of COR, we performed the luciferase (LUC) assay with plants expressing the LUC gene driven by promoters of different clock genes, including CCA1, TOC1, PRR7, and GRP7. Similar to MJ, we found that COR suppressed seedling growth in our clock assay condition (Figure S5 and5). After normalizing the LUC amplitude to relative leaf area of seedlings, we observed that COR dampened the amplitude and lengthened the period of all four reporters largely in a dosage dependent manner, regardless COR was applied at 25 or 37 h after light onset (subjective dawn or subjective dusk, respectively) (Fig. 2). COR further induced a lagging phase with the TOC1:LUC and GRP7:LUC reporters (Fig. 2D2 and 2D4), suggesting a higher sensitivity of these two reporters to COR than other reporters tested. To test if this clock regulatory role of COR requires intact JA signaling, we used the JA receptor mutant (coi1-17) expressing CCA1:LUC5,20. COR did not affect seedling growth and rhythmicity of the CCA1:LUC reporter in coi1-17 (Fig. 2A5, 2B5, 2C5, 2D5; Figure S5A). Thus, these results support that the role of COR in regulating clock activity requires a functional JA receptor.
COR treatment affects clock activity. LD-entrained 5 days old seedlings were transferred to LL for 1 day and were treated with COR or water at 25 h (top of each panel) or 37 h (bottom of each panel). Luminescence was recorded at 1 h intervals for 5 days and analyzed for clock activity. (A1)–(D1) Expression of CCA1:LUC in Col-0. (A2)–(D2) Expression of TOC1:LUC in Col-0. (A3)–(D3) Expression of PRR7:LUC in Col-0. (A4)-(D4) Expression of GRP7:LUC in Col-0. (A5)-(D5) Expression of CCA1:LUC in coi-17. (A1)–(A5) Luminescence traces. RLU relative luminescence units. The color indicates COR concentration, black for 0, magenta for 1 µM, and gray for 10 µM. (B1)–(B5) Normalized amplitude. The amplitude of the reporter was normalized to the relative leaf area shown in Figure S5. (C1)–(C5) Period. (D1)–(D5) Phase shift. Data represent mean ± SEM (n = 12). Statistical analysis was performed by One-way ANOVA post-hoc Tukey HSD test. Different letters indicate significant difference among the samples (P < 0.05). These experiments were repeated three times with similar results.
Like COR, MJ also affects seedling growth (Figure S5B). We previously showed that MJ only affects the amplitude but not the period and phase of the CCA1:LUC reporter5. We report here that three additional reporters (TOC1:LUC, PRR7:LUC, and GRP7:LUC) showed an amplitude dampening in the presence of MJ (Fig. 3). The PRR7:LUC and GRP7:LUC reporters also displayed period lengthening, depending on MJ dosages. Furthermore, MJ induced phase lagging in TOC1:LUC and GRP7:LUC. Unlike COR, MJ did not affect the period of CCA1:LUC and TOC1:LUC. These results suggest a stronger effect of COR than MJ in regulating clock activity, at least for some clock genes. They also illustrate differential sensitivity of different clock reporters to COR, MJ, and JA-Ile.
MJ treatment affects clock activity. LD-entrained 5 days old seedlings were transferred to LL for 1 day and were treated with MJ or water at 25 h (top of each panel) or 37 h (bottom of each panel). Luminescence was recorded at 1-h intervals for 5 days and analyzed for clock activity. (A1)–(D1) Expression of TOC1:LUC in Col-0. (A2)-(D2) Expression of PRR7:LUC in Col-0. (A3)–(D3) Expression of GRP7:LUC in Col-0. (A1)–(A3) Luminescence traces. RLU relative luminescence units. The color indicates MJ concentration, black for 0, magenta for 10 µM, and gray for 100 µM. (B1)-(B3) Normalized amplitude. The amplitude of the reporter was normalized to the relative leaf area shown in Figure S5. (C1)–(C3) Period. (D1)–(D3) Phase shift. Data represent mean ± SEM (n = 12). Statistical analysis was performed by One-way ANOVA post-hoc Tukey HSD test. Different letters indicate significant difference among the samples (P < 0.05). These experiments were repeated three times with similar results.
We previously reported that another plant-derived jasmonate, JA-Ile, acts through COI1 to suppress the amplitude, lengthen the period, but not affect the phase of CCA1:LUC and GRP7:LUC reporters in Col-05. Seedling growth was not affected by JA-Ile (Figure S5C and5). We confirmed these results with the PRR7:LUC reporter (Figure S5C and S6). Interestingly, the TOC1:LUC reporter showed less sensitivity to JA-Ile than other reporters tested, only showing a dampened amplitude but no change in the period and the phase. These results support a stronger role of COR than JA-Ile in clock regulation and differential sensitivity of clock reporters to COR and plant JA derivatives.
Discussion
Growing evidence indicates that pathogens can reprogram the circadian clock of the host. For instance, the bacterium P. syringae, the oomycete Hyaloperonospora arabidopsidis, and the fungus Botrytis cinerea were shown to manipulate the circadian clock of Arabidopsis9,21,22,23. Even gut microbiota in the animal host are capable of reprograming their animal host clock24,25. The key question remains how pathogens affect host clock activity and defense responses. Pathogens are known to secrete a vast range of molecules to interfere with host immunity. Studies just begin to reveal that some signals emanating from pathogens modulate the circadian clock of the host. Pathogen associated molecular patterns (PAMPs), including bacteria lipopolysaccharide (LPS) and flg22, were shown to affect the circadian system of animals and Arabidopsis, respectively21,26. We report here that the P. syringae-produced toxin molecule COR exerts a stronger influence on Arabidopsis clock than some plant-derived JA molecules. Our conclusion is strongly supported by experimental evidence. First, large-scale gene expression analysis showed a stronger suppression of circadian genes by COR than by MJ (Fig. 1; Figures S2 and S3). Second, luciferase assays using marker gene promoters fusing to the luciferase reporter showed stronger effect of COR than JA-Ile and MJ in regulating clock activity (Figs. 2, 3; Figure S6). Third, COR also exerted a stronger effect than MJ and JA-Ile on seedling growth in LL (Figure S5). And finally, we found that COR is critical for pathogen virulence in LL (Figure S1). These various biological processes impacted by COR, JA-Ile, and/or MJ are all regulated by the circadian clock.
Such a stronger role of COR in clock regulated events than that of JA-Ile and MJ is consistent with previous studies that show more potent effect of COR than some JA molecules on other physiological processes14,27,28. It is possible that pathogens use COR through a specific mechanism(s) to hyperactivate the JA signaling. Indeed, COR was shown to bind with a higher affinity to the JA receptor COI1 than plant-derived JA molecules29,30. Downstream of COI1, the JA signaling is highly modular; both the JA signaling repressors (JAZ proteins) and activators (MYC proteins) belong to protein families, members of which interact with different proteins to influence multiple biological processes31. Therefore, it is possible that the COR-COI1 complex could selectively target some JAZ proteins for degradation, leading to a stronger or differential impact on MYC proteins and other signaling targets, such as the circadian clock. In addition to a differential perception of COR and JA molecules that could cause differences in regulating the circadian clock and other biological processes, the different efficacy between COR and other JA molecules could also be due to the solubility, uptake efficiency, stability, and catabolism of each compound in plants.
Our data further demonstrate that the four clock reporters used in this study showed different responses to COR, MJ, and JA-Ile treatments. Such a differential response of clock reporter genes to external treatments has been reported previously in response to nutrient status, ROS, phytohormones, temperature, and photoperiod6,7,8,32,33,34,35. The differential response of these reporters may reflect tissue specific gene expression that allows differential clock response to the chemicals in separate tissues36. Alternatively, there may be different clocks functioning simultaneously with different rhythms in the same tissue or even in the same cell37. Together they support the plasticity of the circadian clock that may create flexibility for plants to respond to various external stimuli38.
Manipulation of host circadian clock may represent a common strategy of microbes to suppress host immunity. How pathogen-produced specific molecules modulate host clock activity and defense responses still remains largely unknown. Our finding of the role of the pathogen-derived molecule COR in modulating Arabidopsis clock opens a new and exciting research direction to elucidate the molecular mechanisms underlying clock-defense interplay during host–pathogen interactions. Our data also illustrate the circadian clock being decentralized, which likely allows organisms to adapt to the changing environment in the presence of pathogens and other biotic and abiotic stresses.
Methods
Plant materials
All plants used in this report are in the Col-0 background. Plants were grown in growth chambers with 180 µmol m−2 s−1 photo flux density, 60% humidity, 22 °C, and a 12 h light/12 h dark (LD) cycle. The clock reporter lines, CCA1:LUC in Col-0 or in coi1-17 and GRP7:LUC in Col-0, were described previously5. Col-0 expressing TOC1:LUC or PRR7:LUC were kindly provided by C. R. McClung at Dartmouth College.
RNA-seq analysis
Two sets of high-resolution RNA-seq data from 100 µM MJ or 5 µM COR treated samples were used for gene expression analysis18,19. The log2 transformed fold changes of expression of circadian genes (Table S1) and defense genes (Table S2) were used to generate the heatmap using the heatmap.2 function in R package gplots. The circadian genes were annotated to be related to rhythmic processes according to Arabidopsis Information Resources (Table S1) and the defense genes were reported previously21 (Table S2).
qRT-PCR analysis
RNA extraction and qRT-PCR were performed as previously described39,40. Primers used in qRT-PCR are listed in Table S3.
Luciferase assay
Seedlings expressing the reporter gene LUCIFERASE (LUC) under the control of a clock-regulated promoter were grown on 1/2 MS media with 1% sucrose in LD and at 22 °C for 5 days. Seedlings were transferred to 96-well plates containing 200 µl of 1/2 MS medium with 0.5% sucrose, 0.4% agar, and 0.25 mM D-luciferin for 1 day in LD followed by 1 day in LL with a light intensity of 180 µmol m−2 s−1. Each well contained one seedling. Seedling treatments were conducted 25 or 37 h after light onset by adding to each well 15 µl of a chemical, using COR (1 µM or 10 µM), MJ (10 µM or 100 µM), JA-Ile (10 µM or 100 µM), or sterile water as mock treatment. The dosages used for MJ, JA-Ile, and COR were chosen based on the published literature (for examples15,16,18,19,27,29,41,42,43) and our preliminary experiments to test the concentrations for each chemical that induced changes of clock activity in a dosage dependent manner but did not cause overstress in plants. MJ and JA-Ile treatments with 10 µM and 100 µM demonstrated dosage-dependent phenotypes, including clock activity and seeding growth. But 100 µM coronatine drastically stunted plant growth and induced high anthocyanin production, suggesting plants under extreme stress. Thus, we used 1 and 10 µM for COR in this report.
Immediately after the treatments, the plants were measured for luminescence with an Omega Luminescence Reader (BMG LABTECH, Inc.) in LL with 90 µmol m−2 s−1 photon flux density. LUC activity was measured at 1-h intervals for 5 days and analyzed for amplitude, period, and phase with the R package MetaCycle44. All luciferase assay experiments were repeated three times with similar results.
References
Lu, H., McClung, C. R. & Zhang, C. Tick tock: circadian regulation of plant innate immunity. Annu. Rev. Phytopathol. 55, 287–311 (2017).
Greenham, K. & McClung, C. R. Integrating circadian dynamics with physiological processes in plants. Nat. Rev. Genet. 16, 598–610 (2015).
Goodspeed, D., Chehab, E. W., Min-Venditti, A., Braam, J. & Covington, M. F. Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc. Natl. Acad. Sci. U.S.A. 109, 4674–4677 (2012).
Shin, J., Heidrich, K., Sanchez-Villarreal, A., Parker, J. E. & Davis, S. J. TIME FOR COFFEE represses accumulation of the MYC2 transcription factor to provide time-of-day regulation of jasmonate signaling in Arabidopsis. Plant Cell 24, 2470–2482 (2012).
Zhang, C. et al. LUX ARRHYTHMO mediates crosstalk between the circadian clock and defense in Arabidopsis. Nat. Commun. 10, 2543 (2019).
Lai, A. G. et al. CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc. Natl. Acad. Sci. U.S.A. 109, 17129–17134 (2012).
Hanano, S., Domagalska, M. A., Nagy, F. & Davis, S. J. Multiple phytohormones influence distinct parameters of the plant circadian clock. Genes Cells 11, 1381–1392 (2006).
Hong, S., Kim, S. A., Guerinot, M. L. & McClung, C. R. Reciprocal interaction of the circadian clock with the iron homeostasis network in Arabidopsis. Plant Physiol. 161, 893–903 (2013).
Li, Z., Bonaldi, K., Uribe, F. & Pruneda-Paz, J. L. A localized Pseudomonas syringae infection triggers systemic clock responses in Arabidopsis. Curr. Biol. 28, 630–639 e634 (2018).
Gimenez-Ibanez, S. et al. The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol. 12, e1001792 (2014).
Jiang, S. et al. Bacterial effector activates jasmonate signaling by directly targeting JAZ transcriptional repressors. PLoS Pathog. 9, e1003715 (2013).
Katsir, L., Schilmiller, A. L., Staswick, P. E., He, S. Y. & Howe, G. A. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. U.S.A. 105, 7100–7105 (2008).
Bender, C. L., Alarcon-Chaidez, F. & Gross, D. C. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 63, 266–292 (1999).
Geng, X., Jin, L., Shimada, M., Kim, M. G. & Mackey, D. The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta 240, 1149–1165 (2014).
Panchal, S. et al. Coronatine facilitates pseudomonas syringae infection of arabidopsis leaves at night. Front. Plant Sci. 7, 880 (2016).
Melotto, M., Underwood, W., Koczan, J., Nomura, K. & He, S. Y. Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980 (2006).
Covington, M. F. & Harmer, S. L. The circadian clock regulates auxin signaling and responses in Arabidopsis. PLoS Biol. 5, e222 (2007).
Attaran, E. et al. Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Plant Physiol. 165, 1302–1314 (2014).
Hickman, R. et al. Architecture and dynamics of the jasmonic acid gene regulatory network. Plant Cell 29, 2086–2105 (2017).
Devoto, A. et al. COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J. 32, 457–466 (2002).
Zhang, C. et al. Crosstalk between the circadian clock and innate immunity in Arabidopsis. PLoS Pathog. 9, e1003370 (2013).
Wang, W. et al. Timing of plant immune responses by a central circadian regulator. Nature 470, 110–114 (2011).
Windram, O. et al. Arabidopsis defense against Botrytis cinerea: chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. Plant Cell 24, 3530–3557 (2012).
Marcinkevicius, E. V. & Shirasu-Hiza, M. M. Message in a biota: gut microbes signal to the circadian clock. Cell Host Microbe 17, 541–543 (2015).
Thaiss, C. A. et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 167, 1495-1510 e1412 (2016).
Curtis, A. M., Bellet, M. M., Sassone-Corsi, P. & O’Neill, L. A. Circadian clock proteins and immunity. Immunity 40, 178–186 (2014).
Uppalapati, S. R. et al. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 42, 201–217 (2005).
Koda, Y. et al. Similarities of the biological activities of coronatine and coronafacic acid to those of jasmonic acid. Phytochemistry 41, 93–96 (1996).
Fonseca, S. et al. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5, 344–350 (2009).
Sheard, L. B. et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468, 400–405 (2010).
Howe, G. A., Major, I. T. & Koo, A. J. Modularity in jasmonate signaling for multistress resilience. Annu. Rev. Plant Biol. 69, 387–415 (2018).
Haydon, M. J., Mielczarek, O., Robertson, F. C., Hubbard, K. E. & Webb, A. A. Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 502, 689–692 (2013).
Flis, A. et al. Photoperiod-dependent changes in the phase of core clock transcripts and global transcriptional outputs at dawn and dusk in Arabidopsis. Plant Cell Environ. 39, 1955–1981 (2016).
Michael, T. P., Salome, P. A. & McClung, C. R. Two Arabidopsis circadian oscillators can be distinguished by differential temperature sensitivity. Proc. Natl. Acad. Sci. U.S.A. 100, 6878–6883 (2003).
Shimizu, H. et al. Decentralized circadian clocks process thermal and photoperiodic cues in specific tissues. Nat. Plants 1, 15163 (2015).
Inoue, K., Araki, T. & Endo, M. Oscillator networks with tissue-specific circadian clocks in plants. Semin. Cell Dev. Biol. 83, 78–85 (2017).
Gould, P. D. et al. Coordination of robust single cell rhythms in the Arabidopsis circadian clock via spatial waves of gene expression. Elife 7, e31700 (2018).
Webb, A. A. R., Seki, M., Satake, A. & Caldana, C. Continuous dynamic adjustment of the plant circadian oscillator. Nat. Commun. 10, 550 (2019).
Ng, G. et al. Genetic dissection of salicylic acid-mediated defense signaling networks in Arabidopsis. Genetics 189, 851–859 (2011).
Hamdoun, S. et al. Differential roles of two homologous cyclin-dependent kinase inhibitor genes in regulating cell cycle and innate immunity in Arabidopsis. Plant Physiol. 170, 515–527 (2016).
Feys, B. J. F., Benedetti, C. E., Penfold, C. N. & Turner, J. G. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6, 751–756 (1994).
Weiler, E. W. et al. The Pseudomonas phytotoxin coronatine mimics octadecanoid signalling molecules of higher plants. FEBS Lett. 345, 9–13 (1994).
Koo, A. J., Cooke, T. F. & Howe, G. A. Cytochrome P450 CYP94B3 mediates catabolism and inactivation of the plant hormone jasmonoyl-l-isoleucine. Proc. Natl. Acad. Sci. U.S.A. 108, 9298–9303 (2011).
Wu, G., Anafi, R. C., Hughes, M. E., Kornacker, K. & Hogenesch, J. B. MetaCycle: an integrated R package to evaluate periodicity in large scale data. Bioinformatics 32, 3351–3353 (2016).
Acknowledgements
We thank the members in the Lu laboratory for their assistance in this work. We thank Drs. C. Robertson McClung, Gregg Howe, and Alex Webb for helpful discussions about this report. This work was partially supported by a grant from National Science Foundation (NSF 1456140) to HL.
Author information
Authors and Affiliations
Contributions
M.G. did clock assays, qRT-PCR, and bacterial growth. C.Z. did bioinformatics analysis of gene expression using RNAseq data. H.L. designed experiments and wrote the manuscript with help from M.G. and C.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, M., Zhang, C. & Lu, H. Coronatine is more potent than jasmonates in regulating Arabidopsis circadian clock. Sci Rep 10, 12862 (2020). https://doi.org/10.1038/s41598-020-69627-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69627-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.