Abstract
Our data about pathogenesis of hepatic steatosis after liver transplantation is scarce. This study aimed to investigate the association between serum adipokines and insulin resistance with hepatic steatosis in liver transplant recipients. We investigated the association between insulin resistance, serum adiponectin, insulin, and leptin with hepatic steatosis in a cohort of liver transplant recipients. Homeostatic model assessment of insulin resistance 2 (HOMA 2-IR) was used for estimation of insulin resistance. Hepatic steatosis was determined using ultrasound and controlled attenuation parameter (CAP). A total of 178 patients were included. 79 patients (44.4%) had hepatic steatosis. Serum adiponectin (OR: 0.912; 95% CI 0.869–0.957; P < 0.001), serum leptin (OR: 1.060; 95% CI 1.017–1.102; P = 0.005), HOMA2-IR (OR: 1.671; 95% CI 1.049–2.662; P = 0.031), and post-transplant diabetes mellitus (PTDM) (OR: 5.988; 95% CI 1.680–21.276; P = 0.006) were independently associated with hepatic steatosis after liver transplantation. CAP values were negatively correlated with serum adiponectin (P = 0.011) and positively correlated with serum insulin (P = 0.001), leptin (P < 0.001) and HOMA2-IR (P < 0.001). Insulin resistance and alterations in adipokines might have central role in pathogenesis of hepatic steatosis after liver transplantation and can be targeted for diagnostic and therapeutic purposes.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is rapidly surpassing other indications of liver transplantation during recent years and is going to become the leading cause of liver transplantation worldwide1. This is mainly due to the increasing prevalence of NAFLD secondary to the epidemics of metabolic syndrome and obesity2. NAFLD is not only an increasing cause of liver transplantation but also might occur after transplantation as recurrence of the disease or de novo hepatic steatosis3. The prevalence of hepatic steatosis after liver transplantation has been ranged from 30–60% in different studies4,5.
While pathogenesis of NAFLD and nonalcoholic steatohepatitis (NASH) has been thoroughly investigated in recent years6,7, our data regarding pathogenesis of post-transplant hepatic steatosis is limited. Several risk factors for hepatic steatosis after liver transplantation have been hypothesized. Post-transplant metabolic syndrome and obesity is prevalent after liver transplantation promoting the process of hepatic steatosis8. Weight gain is occurred in the majority of patients after liver transplantation irrespective of the underlying cause of cirrhosis and is probably one of the main contributing factors for hepatic steatosis after liver transplantation9,10. Hyperlipidemia, post-transplant diabetes mellitus (PTDM) and hypertension are traditional risk factors for fatty liver disease that might occur after liver transplantation11,12. Insulin resistance has a central role in the pathogenesis of NAFLD. Insulin resistance is involved in progression of NAFLD by increasing release of free fatty acids from the adipose tissue and de novo lipogenesis within hepatocytes secondary to hyperinsulinemia13,14. Insulin resistance can also cause flux of free fatty acids from adipocytes in to the liver14. Serum adipokines including adiponectin and leptin have been reported to be involved in the pathogenesis of NAFLD. Serum leptin level is increased and serum adiponectin is decreased in patients with NAFLD in parallel to the severity of disease15.
To date, only few data are available about association of post-transplant hepatic steatosis with serum adipokines and insulin resistance as the main mechanisms of hepatic steatosis. In this study we aimed to investigate the impact of insulin resistance and serum adipokines on post-transplant hepatic steatosis.
Results
Association of hepatic steatosis with serum adipokines and insulin resistance after liver transplantation
178 liver transplant recipients were included in the study. There were 99 men (55.6%) and 79 women (44.4%). Baseline characteristics of patients are outlined in Table 1. The meantime for evaluation of fatty change after liver transplantation and blood sampling was 38.83 ± 34.43 months. Using ultrasound, 79 patients (44.4%) were diagnosed to have hepatic steatosis after liver transplantation. Twenty eight patients (35%) had grade 1 steatosis, 29 patients (36.7%) had grade 2 steatosis and 22 patients were diagnosed to have grade 3 steatosis in ultrasound. CAP values were statistically different between patients without steatosis (189.95 ± 51.88 dB/m), with grade 1 stetaosis (229.52 ± 65.40 dB/m), and with grade 2 stetaosis (275.20 ± 39.87 dB/m) (P < 0.001) in ultrasound (Fig. 1). Univariate analysis of risk factors for hepatic steatosis after liver transplantation are outlined in Table 2. Mean serum concentration of adiponectin after liver transplantation was 13.30 ± 10.59 µg/ml in patients with steatosis compared to 24.91 ± 17.42 µg/ml in patients without steatosis (P < 0.001). Serum concentration of leptin (20.64 ± 22.02 ng/ml versus 9.97 ± 11.97 ng/ml) and insulin (15.81 ± 17.73 µU/ml versus 8.80 ± 7.07 µU/ml) were significantly higher in patients with hepatic steatosis compared to those without hepatic steatosis (P < 0.001). In regression analysis, serum adiponectin (OR: 0.912; 95% CI 0.869-0.957; P < 0.001), serum leptin (OR: 1.060; 95% CI 1.017–1.102; P = 0.005), HOMA2-IR as a marker of insulin resistance (OR: 1.671; 95% CI 1.049–2.662; P = 0.031), and PTDM (OR: 5.988; 95% CI 1.680–21.276; P = 0.006), were independently associated with hepatic steatosis after liver transplantation (Table 2).
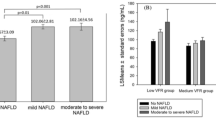
Serum adiponectin level was statistically different over different grades of steatosis in ultrasound (F (2, 166) = 7.052, P = 0.001). Serum adiponectin level was significantly lower in patients with grade 2 steatosis (8.89 ± 6.52 µg/ml) and grade 1 steatosis (15.97 ± 12.72 µg/ml) compared to the patients without steatosis (23.48 ± 17.37 µg/ml). Serum leptin level was statistically different over different grades of steatosis in ultrasound (F (2, 166) = 8.698, P < 0.001). Serum leptin level was significantly higher in patients with grade 2 steatosis (28.74 ± 28.68 ng/ml) and grade 1 steatosis (18.37 ± 20.54 ng/ml) compared to the patients without steatosis (10.29 ± 11.53 ng/ml) (Fig. 2). Serum insulin level was not statistically different over different grades of steatosis (F (2, 166) = 1.390, P = 0.252) (Fig. 2).
Liver fat contents measured by CAP are associated with serum adipokines and insulin resistance
CAP was measured in 172 patients. Mean CAP values in our patients was 208.19 ± 61.64 dB/m. The correlation of CAP with serum adiponectin, insulin, leptin, HOMA2-IR and other metabolic indices are outlined in Table 3. Using Pearson correlation test, CAP was negatively correlated with serum adiponectin (P = 0.011) and positively correlated with serum insulin (P = 0.001), leptin (P < 0.001) and HOMA2-IR (P < 0.001) (Fig. 3). Serum tacrolimus level was not correlated with CAP values (r = − 0.102, P = 0.253). CAP was also positively associated with BMI (P < 0.001), serum triglyceride (P < 0.001) and FBS (P < 0.001) (Table 3). In linear regression analysis, serum adiponectin, leptin, HOMA-IR and serum triglyceride were independently associated with CAP (Table 3).
The association between serum adipokines and hepatic steatosis diagnosed by TE is outlined in Fig. 4. Patients with hepatic steatosis diagnosed by TE had significantly higher serum levels of leptin (P < 0.001), insulin (P = 0.002), HOMA2-IR (P < 0.001) and lower levels of serum adiponectin (P = 0.015) compared to the patients without hepatic steatosis in TE.
Hepatic steatosis in lean patients after liver transplantation and the impact of low serum adiponectin
Ninety two patients (53.5%) were lean (BMI < 25 kg/m2). Among lean subjects, 20 patients (22.2%) had hepatic steatosis (11.2% of all the study population). In univariate analysis, post-transplant hyperlipidemia (odds ratio = 3.47: 95% CI 1.092–11.030; P-Value = 0.029), PTDM (odds ratio = 4.23: 95% CI 1.480–12.128; P-Value = 0.005), and low serum adiponectin level (P = 0.045) were associated with hepatic steatosis in lean liver transplant recipients (Table 4). In regression analysis, PTDM (odds ratio = 1.05: 95% CI 0.078–1.333; P-Value = 0.014), and low serum adiponectin level (odds ratio = 0.94: 95% CI 0.902–0.996; P-Value = 0.032) were independently associated with hepatic steatosis among lean liver transplant recipients (Table 4).
Discussion
Our results demonstrate that hepatic steatosis diagnosed by ultrasound and TE in liver transplant recipients is associated with decreased serum adiponectin and increased serum leptin and insulin levels. Liver transplant recipients with hepatic steatosis had higher insulin resistance as assessed by HOMA2-IR. PTDM was also an independent predictor of hepatic steatosis after liver transplantation. We used CAP for non-invasive estimation of liver fat content. CAP values were different over different grades of hepatic steatosis in ultrasound and was correlated negatively with serum adiponectin and positively with serum leptin and HOMA2-IR. After adjustment for age, gender, BMI and other metabolic indices, serum adipokines and insulin resistance were independently associated with hepatic steatosis after liver transplantation highlighting their role in pathogenesis of hepatic steatosis after liver transplantation. In a subgroup of lean patients, decreased serum adiponectin level was an independent predictor of hepatic steatois after liver transplantation.
Recurrent or de novo hepatic steatosis after liver transplantation has been reported to be associated with PTDM, higher BMI and other components of metabolic syndrome16,17,18. However, limited data is available about pathogenesis of hepatic steatosis after liver transplantation. Increased fasting hepatic gluconeogenesis and impairment of suppression of hepatic glucose production are two components of hepatic insulin resistance19. Diacylglycerol (DAG) and ceramide have been suggested to be the two major lipids involved in hepatic insulin resistance via activation of protein kinase and downstream impairment of hepatic insulin signaling20. Total hepatic DAG and ceramide have been correlated with HOMA-IR as a marker of insulin resistance21,22. It is well known that insulin resistance is strongly associated with hepatic steatosis23. In a population based cohort, HOMA- IR was closely associated with liver fat contents assessed by magnetic resonance spectropscopy24. However, the role of insulin resistance in the development of hepatic steatosis after liver transplantation is controversial.
In contrast to our findings, Andrade and colleagues reported that HOMA-IR, as a marker of insulin resistance, was not a predictor of NAFLD after liver transplantation25. In this study, we used HOMA2-IR (the updated HOMA model) that is a better indicator of hepatic insulin resistance as it accounts for variations in both peripheral and hepatic glucose output26. Insulin resistance is prevalent among patients undergoing liver transplantation for hepatitis C virus (HCV) cirrhosis27 and other causes of liver cirrhosis28. A cross-sectional study showed that insulin resistance is highly prevalent and associated with new onset diabetes after liver transplantation29. None of these studies reported the impact of insulin resistance on the development of hepatic steatosis after liver transplantation.
Leptin and adiponectin are two major adipokines that are produced and secreted mainly from adipocytes and are involved in the pathogenesis of hepatic steatosis15. Leptin acts in liver cells via activation of the Janus kinase (JAK) 2/signal transducer and activator of transcription (STAT)-3 pathway resulting in increased expression of suppressor of cytokine signaling (SOCS)-3 which is a feedback inhibitor of leptin signaling30. It has been shown that inhibition of SOCS-3 improves leptin resistance and hepatic steatosis31. A meta-analysis of 33 studies has shown that serum leptin level was higher in patients with simple steatosis and NASH compared to the healthy controls32. Adiponectin is decreased when adipose tissue mass increases and has anti-steatotic properties on hepatocytes. Adiponectin promotes oxidation of free fatty acids, inhibits gluconeogenesis and prevents apoptosis of hepatocytes. It has also anti-fibrotic and anti-inflammatory effects by acting on Kupffer cells and hepatic satellite cells33,34,35.
Alterations of serum adipokines after liver transplantation have been reported previously in few studies, none of them focused on hepatic steatosis. Watt et al. reported that increased serum leptin and decreased serum adiponectin levels were seen in liver recipients with cardiovascular events36. A recent study reported that hypoadiponectinemia was an independent predictor of future cardiovascular events in liver transplant recipients37. However, a cross-sectional study among a small group of patients failed to demonstrate any correlation between serum adipokines and metabolic syndrome after liver transplantation38. In a previous study no association was demonstrated between hepatic steatosis and serum leptin after liver transplantation39. In the mentioned study, authors only included patients with HCV infection and did not evaluate the impact of serum adiponectin. It has been recently suggested that patients with NAFLD and adiponectin gene polymorphisms were susceptible to hepatic steatosis after liver transplantation40.
In this study, we showed, for the first time, that alterations in serum adiponectin and leptin as well as insulin resistance were correlated with hepatic steatosis and hepatic fat contents in liver transplant recipients independent of traditional risk factors of hepatic steatosis. We used ultrasound and CAP for non-invasive estimation of hepatic steatosis. CAP is a non-invasive, cheap and easy technique (compared to other methods) for quantitative measurement of liver fat content during transient elastography41,42. This parameter has been increasingly measured in patients with NAFLD43 and suggested to have good efficacy for assessment of hepatic steatosis after liver transplantation44.
Finally, cumulative evidence suggests that hepatic steatosis is prevalent after liver transplantation, however, its long term impact on allograft and patient outcomes is less evident. Our study expands current knowledge about pathophysiology of hepatic steatosis after liver transplantation. Liver transplant recipients with insulin resistance and alterations in adiponectin and leptin are susceptible to hepatic steatosis and should be actively diagnosed and treated. Targeting insulin resistance and adipokines can be considered as therapeutic options for prevention and management of hepatic steatosis after liver transplantation.
Methods and materials
Patients
In a cross-sectional study, all adult (> 18 years) liver transplant recipients who referred for their routine post-transplant follow-up between July 2017 and November 2017 were included in the study. All patients had undergone liver transplantation from deceased donors at Shiraz Organ Transplant Center, Shiraz, Iran. We included patients who have passed at least 6 months after transplant surgery. Tacrolimus based immunosuppressive regimen was used for the maintenance of immunosuppression after liver transplantation for all patients. Patients with acute rejection episodes and those with serum aminotransferase levels higher than 5 times upper limit of normal range were excluded from the study. Clinical characteristics of patients were recorded at the time of study inclusion. Body mass index (BMI) was calculated using this formula: weight (kg) divided by height (m2) squared. Patients with BMI < 25 kg/m2 were defined to be lean. Hepatic steatosis in transplant recipients was assessed by ultrasound and classified as grades 0, 1, 2, and 3 based on the severity of liver echogenicity as a marker of severity of hepatic steatosis.
Measurement of serum adiponectin, leptin and insulin
Peripheral venous blood samples were collected from patients in the morning after an overnight fasting. Blood samples were centrifuged immediately at 4 °C and separated serum samples were stored at − 80 °C. Serum adiponectin levels were measured by enzyme linked immunoassay (ELISA) method using human adiponectin ELISA kit (Mediagnost, Reutlingen, Germany). Serum insulin levels were measured by ELISA method using human insulin kit (Monobind Inc., Lake Forest, CA (92630), USA). Serum leptin levels were measured using human leptin ELISA kit (Mediagnost, Reutlingen, Germany). Homeostatic model assessment of insulin resistance (HOMA-IR) is a method for assessment of insulin resistance and β-cell function based on the fasting plasma insulin and glucose concentrations. Insulin resistance was estimated using HOMA 2-IR software, as described before45. Blood sampling was performed on the day of clinical visit and assessment of hepatic steatosis by ultrasound and CAP measurement.
Measurement of controlled attenuation parameter (CAP)
Non-invasive estimation of hepatic fat content was measured for all patients using vibration‐controlled transient elastography (VCTE) and expressed as CAP. Measurement of CAP was done on the day of clinical visit and blood sampling and after an overnight fasting. All CAP measurements was done by one person. CAP was obtained along the inter-axillary line and intercostal spaces on the liver while the patient was in supine position. CAP measurement was considered to be reliable if 10 valid successful acquisitions were made correctly. TE was performed using M probe in all patients and XL probe was used in obese patients in whom use of M probe was failed. CAP measurement was expressed in decibel per meter (dB/m)46. The cutoff scores for CAP estimation of hepatic steatosis were as follows: ≥ 238 dB/m for S1 (corresponding to 11–32% liver fat), ≥ 259 dB/m for S2 (33–65% liver fat), and ≥ 292 dB/m for S3 (≥ 66% liver fat), as previously determined.
Statistical analysis
Continuous and categorical variables were compared using Student’s t-test and Chi-square test, respectively. Data were presented using means ± standard deviation for numeric variables, and percents and counts for categorical variables. A one way analysis of variance (ANOVA) and post-hoc Tukey test were used to compare serum levels of adiponectin, leptin and insulin with different grades of hepatic steatosis. Logistic regression analysis was used to identify the independent variables and association of serum adipokines and insulin resistance with hepatic steatosis after liver transplantation. Pearson’s and Spearman’s correlation coefficients were used to assess correlation of serum adipokines and insulin resistance with CAP values after liver transplantation. Linear regression analysis was used to identify continuous variables independently associated with CAP values. Statistical analysis was performed with SPSS 20.0 (SPSS Inc.; Chicago, IL, USA). A P- value of < 0.05 was considered statistically significant.
Ethics and consent
The study protocol was approved by the institutional review board of Organ Transplant Center, Shiraz University of Medical Sciences on 5/11/2016. The study protocol was performed in accordance with the Helsinki Declaration as revised in Seoul 2008. Written informed consents were obtained from each patient after explaining the study harms and benefits.
Data availability
Due to patient privacy the datasets in the current study are not publicly available but can be available by corresponding author on reasonable request.
Abbreviations
- HOMA-IR:
-
Homeostatic model assessment of insulin resistance
- CAP:
-
Controlled attenuation parameter
- PTDM:
-
Post-transplant diabetes mellitus
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
References
Holmer, M. et al. Nonalcoholic fatty liver disease is an increasing indication for liver transplantation in the Nordic countries. Liver Int. 38(11), 2082–2090 (2018).
Kim, D., Touros, A. & Kim, W. R. Nonalcoholic fatty liver disease and metabolic syndrome. Clin. Liver Dis. 22(1), 133–140 (2018).
Kappus, M. & Abdelmalek, M. D. Novo and recurrence of nonalcoholic steatohepatitis after liver transplantation. Clin. Liver Dis. 21(2), 321–335 (2017).
Filipec-Kanizaj, T. et al. Nonalcoholic fatty liver disease and liver transplantation—Where do we stand?. Mikolasevic I World J. Gastroenterol. 24(14), 1491–1506 (2018).
Kim, H. et al. Histologically proven non-alcoholic fatty liver disease and clinically related factors in recipients after liver transplantation. Clin. Transplant. 28(5), 521–529 (2014).
Buzzetti, E., Pinzani, M. & Tsochatzis, E. A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65(8), 1038–1048 (2016).
Schuppan, D. & Schattenberg, J. M. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J. Gastroenterol. Hepatol. 28(Suppl 1), 68–76 (2013).
Chascsa, D. M. & Vargas, H. E. The gastroenterologist’s guide to management of the post-liver transplant patient. Am. J. Gastroenterol. 113(6), 819–828 (2018).
Richards, J., Gunson, B., Johnson, J. & Neuberger, J. Weight gain and obesity after liver transplantation. Transpl. Int. 18(4), 461–466 (2005).
Barone, M. et al. Obesity as predictor of postoperative outcomes in liver transplant candidates: Review of the literature and future perspectives. Dig. Liver Dis. 49(9), 957–966 (2017).
Dehghani, S. M. et al. Hyperlipidemia in Iranian liver transplant recipients: prevalence and risk factors. J. Gastroenterol. 42(9), 769–774 (2007).
Jenssen, T. & Hartmann, A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat. Rev. Endocrinol. 15(3), 172–188 (2019).
Jelenik, T. et al. Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes 66, 2241–2253 (2017).
Lambert, J. E., Ramos-Roman, M. A., Browning, J. D. & Parks, E. J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146, 726–735 (2014).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Adipokines in nonalcoholic fatty liver disease. Metabolism 65(8), 1062–1079 (2016).
Vallin, M. et al. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation: natural history based on liver biopsy analysis. Liver Transpl. 20(9), 1064–1071 (2014).
Galvin, Z. et al. Predictors of de novo nonalcoholic fatty liver disease after liver transplantation and associated fibrosis. Liver Transpl. 25(1), 56–67 (2019).
Seo, S. et al. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl 13(6), 844–847 (2007).
Birkenfeld, A. L. & Shulman, G. I. Non alcoholic fatty liver disease, hepatic insulin resistance and type 2 diabetes. Hepatology 59(2), 713–723 (2014).
Petersen, M. C. & Shulman, G. I. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol. Sci. 38(7), 649–665 (2017).
Ter Horst, K. W. et al. Hepatic diacylglycerol-associated protein kinase Cε translocation links hepatic steatosis to hepatic insulin resistance in humans. Cell. Rep. 19(10), 1997–2004 (2017).
Luukkonen, P. K. et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 64, 1167–1175 (2016).
Alves, T. C. et al. Regulation of hepatic fat and glucose oxidation in rats with lipid-induced hepatic insulin resistance. Hepatology 53, 1175–1181 (2011).
Isokuortti, E. et al. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: a population-based and inter-laboratory study. Diabetologia 60(10), 1873–1882 (2017).
Andrade, A. R. et al. New onset diabetes and non-alcoholic fatty liver disease after liver transplantation. Ann. Hepatol. 16(6), 932–940 (2017).
Rudenski, A. S., Matthews, D. R., Levy, J. C. & Turner, R. C. Understanding “insulin resistance”: both glucose resistance and insulin resistance are required to model human diabetes. Metabolism 40, 908–917 (1991).
Delgado-Borrego, A. et al. Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation 77(5), 703–710 (2004).
Da Silva Alves, V., Hack Mendes, R. & Pinto Kruel, C. D. Nutritional status, lipid profile and HOMA-IR in post-liver transplant patients. Nutr Hosp 29(5), 1154–1162 (2014).
Martínez-Díaz-Guerra, G. et al. Serum levels of osteocalcin and insulin resistance in patients with impaired glucose tolerance or new-onset diabetes mellitus after liver transplantation. Horm. Metab. Res. 48(5), 325–330 (2016).
Robertson, S. A., Leinninger, G. M. & Myers, M. G. Jr. Molecular and neural mediators of leptin action. Physiol. Behav. 94(5), 637–642 (2008).
Zhang, R. et al. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology 149(11), 5654–5661 (2008).
Polyzos, S. A. et al. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia 59(1), 30–43 (2016).
van der Poorten, D. et al. Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause?. Hepatology 57(6), 2180–2188 (2013).
Saxena, N. K. & Anania, F. A. Adipocytokines and hepatic fibrosis. Trends Endocrinol. Metab. 26(3), 153–161 (2015).
Handy, J. A. et al. Adiponectin activation of AMPK disrupts leptin-mediated hepatic fibrosis via suppressors of cytokine signaling (SOCS-3). J. Cell Biochem. 110(5), 1195–1207 (2010).
Watt, K. D. et al. Serum adipokine and inflammatory markers before and after liver transplantation in recipients with major cardiovascular events. Liver Transpl. 20(7), 791–797 (2014).
Siddiqui, M. B. et al. The relationship between hypoadiponectinemia and cardiovascular events in liver transplant recipients. Transplantation https://doi.org/10.1097/TP.0000000000002714 (2019).
Anastácio, L. R. et al. Adipokines, inflammatory mediators, and insulin-resistance parameters may not be good markers of metabolic syndrome after liver transplant. Nutrition 32(9), 921–927 (2016).
Veldt, B. J. et al. Insulin resistance, serum adipokines and risk of fibrosis progression in patients transplanted for hepatitis C. Am. J. Transplant 9(6), 1406–1413 (2009).
John, B. V. et al. Recipient but not donor adiponectin polymorphisms are associated with early posttransplant hepatic steatosis in patients transplanted for non-nonalcoholic fatty liver disease indications. Exp. Clin. Transplant 16(4), 439–445 (2018).
Shi, K. Q. et al. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: A meta-analysis of diagnostic accuracy. J. Gastroenterol. Hepatol. 29, 1149–1158 (2014).
Fujimori, N. et al. Controlled attenuation parameter is correlated with actual hepatic fat content in patients with non-alcoholic fatty liver disease with none-to-mild obesity and liver fibrosis. Hepatol. Res. 46(10), 1019–1027 (2016).
de Lédinghen, V., Vergniol, J., Foucher, J., Merrouche, W. & le Bail, B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver. Int. 32(6), 911–918 (2012).
Karlas, T. et al. Noninvasive characterization of graft steatosis after liver transplantation. Scand. J. Gastroenterol. 50(2), 224–232 (2015).
Levy, J. C., Matthews, D. R. & Hermans, M. P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21(12), 2191–2192 (1998).
Sasso, M. et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 36, 1825–1835 (2010).
Acknowledgements
We would like to thank Shiraz Transplant Center personnel, Mrs. Dehghan, Mrs. Mehrdad, Mr. Gholami and Mrs. Ebrahimi for their support of the project with informing and referring patients. We would also like to thank Mr. Amirzadeh for doing laboratory tests. The study was supported by a research grant from vice chancellery of research, Abu-Ali Sina Center for Medicine and Organ Transplant, Shiraz, Iran.
Author information
Authors and Affiliations
Contributions
A.E.: Study concept and design; acquisition of data; experiments and procedures; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. S.N., A.S., K.K., M.R.F., S.A.M.-H.: Acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. ALL authors approved the final version of the article, including the authorship list.
Corresponding author
Ethics declarations
Competing interests
The author declares that no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eshraghian, A., Nikeghbalian, S., Shamsaeefar, A. et al. Hepatic steatosis and liver fat contents in liver transplant recipients are associated with serum adipokines and insulin resistance. Sci Rep 10, 12701 (2020). https://doi.org/10.1038/s41598-020-69571-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69571-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.