Abstract
Aquatic mammals play an important role in community structure. The present study applied stable isotope analysis (SIA) to evidence trophic relationships and resource partitioning among aquatic mammals inhabiting different environments in the Amazon estuarine complex and adjacent coastal zone (AE) and Northeastern coast (NC) of Brazil. In addition, isotopic niche partitioning among Sotalia guianensis, Inia spp. and Trichechus inunguis within the AE was also evaluated, and ecological S. guianensis stocks were characterized. Among marine delphinids, the carbon isotopic composition in offshore species mirrored that of nearshore species, contradicting the pattern of decreasing δ13C values characteristic of many areas around the world including areas in Southeastern and Southern Brazil. Isotopic niches were highly distinct, with no overlap among the assessed species inhabiting the AE. Inia spp. and T. inunguis occupied significantly larger isotopic niche spaces, suggesting high habitat plasticity. S. guianensis inhabited two coastal regions indicating an ecological distinction. Nitrogen values were similar between S. guianensis from the NC and AE, indicating comparable trophic positions. However, NC specimens presented more variable δ13C values compared to those from AE. SIA results also allowed for insights concerning habitat use and the trophic ecology of dolphin species inhabiting different oceanographic regions off Northern/Northeast Brazil. These findings provide novel data on the stable isotope composition for cetaceans and sirenians from this region, and aid in furthering knowledge on the trophic ecology and habitat use of the investigated species.
Similar content being viewed by others
Introduction
Understanding the ecological roles of aquatic mammals in a given ecosystem is important for the conservation of both the assessed species and their environments. However, information on aquatic mammal trophic ecology and habitat use is generally scarce, mainly due to the intrinsic difficulty of studying these animals in their remote environments. Nevertheless, several studies reported the coexistence and resource partitioning among aquatic mammals in the last few years1,2,3,−4.
Recently, stable isotope analysis (SIA) has become the most common biochemical method used to study aquatic mammal trophic ecology (see5). This method is based on the principle that the isotopic composition in consumer tissues reflects the isotopic composition of their assimilated prey6,7. Furthermore, nitrogen isotopic values predictably increase from prey to predator7,8 and are, thus, applied as consumer trophic position indicators9,10,−11. Carbon isotopic values show a lower increase along the food chain, so, they are mainly applied to infer basal sources in their foraging habitats (e.g.12). Thus, using this dual isotope approach allows the assessment of the species diet and habitat use which are, in turn, dimensions of its ecological niche13,14. Indeed, ecological niches have been described as the “n-dimensional hypervolume”, where dimensions may include both scenopoetic (usually environmental variables) and bionomic (resources) axes15. In a similar way, the δ-space formed by the axis δ13C and δ15N, can be comparable to these scenopoetic and bionomic dimensions of the ecological niche, as isotopic values in a predator’s tissues represent both the prey it consumes (bionomic) and the habitat where it forages (scenopoetic)13,14,16. This “isotopic niche” and its dimensions have been recently applied as an approach to study the trophic ecology and resource partitioning of several marine predators, such as baleen whales17, toothed cetaceans2,3,18, and sharks19.
In order to apply SIA to the study of the trophic and spatial ecology of marine predators, the patterns of baseline isotopic values along geographical gradients must be considered. These basal values are commonly used to generate maps of isotopic values (i.e. isoscapes) which in turn provide a powerful approach to understanding the habitat use, foraging ecology and movements of predators20 as these baseline differences cascade up, with modifications associated with trophic transfer, to the top of the food webs (e.g.20,21. Differences in baseline δ13C and δ15N values have been reported in particulate organic matter (POM) and plankton along latitudinal and longitudinal gradients in different parts of the world22,23,−24. Phytoplankton nitrogen isotopic values are mainly influenced by their nutrient source (i.e. nitrate, ammonium or N2), isotopic fractionation during N assimilation and nutrient pool size25,26. In general, relatively higher δ15N values are found in regions where nitrate is the main source of N for primary producers, while lower δ15N values are observed in areas where primary production is mainly supported by N-fixation25,26. Basal carbon isotopic values (i.e. phytoplankton δ13C), on the other hand, reflect those of the dissolved inorganic C, [CO2]aq, temperature, cell size, geometry, and growth rate, and CO2 drawdown27,28. Nutrient availability also influences phytoplankton δ13C values by affecting the growth rate and taxonomic composition of the primary producers28. Regions with low nutrient loads have typically low δ13C values. Furthermore, the presence of 13C-enriched sources of organic carbon (i.e. coastal macrophytes) in coastal regions usually result in a gradient of decreasing δ13C basal values towards oceanic waters20,29. This typical inshore-offshore gradient of decreasing δ13C basal values, however, is not ubiquitous and in regions where large rivers deliver a considerable amount of organic matter to the coastal regions (i.e. Amazon plume), more complex patterns are observed30,31,−32. In the Amazon estuary and the marine region under its influence, low δ13C values and high δ15N values are reported near the estuary and within the most coastal core of the plume, mainly due to the terrestrial origin of the organic matter. Basal carbon isotope values then increase with salinity31,32,−33 while the increasing proportion of production based on N-fixation towards oceanic waters generates a decreasing trend in δ15N values32,34. Nevertheless, the influence of the organic input of the Amazon plume, can reach hundreds of kilometers from the mouth of the estuary, thus influencing the basal isotopic values of large estuarine and marine areas32,33.
The Northern/Northeastern Brazilian region, besides the Amazon River Estuary, encompasses a diverse arrangement of habitats, including the longest contiguous mangrove area in the world35, with low human population density, reflecting in highly conserved mangroves36. In turn, aquatic faunal composition is directly influenced by coastal habitat diversity, fluvial deposit dynamics and river discharge influence37,38.
In this region, at least 52 cetacean species39,40,41,−42 and two sirenians43,44 have been reported, including several species that may be eligible to be listed as threatened or endangered, but the lack of biological population data hinders the correct evaluation of their conservation status45,46,−47. The Northern region is the least surveyed coastal area in Brazil and only two studies present compiled information obtained from stranding and beach surveys39,48. Although stranded carcasses are usually a source of biological and ecological data, the high temperatures registered in these tropical regions and the logistical limitations for monitoring these coastal areas usually result in an advanced decomposition state of the carcasses, precluding the access to important biological data, including stomach content analysis, mainly in the case of oceanic species. A better understanding of the trophic and spatial ecology of these aquatic mammals is therefore needed to fill this knowledge gap.
Four cetaceans and two sirenians (Antillean manatee, Trichechus manatus manatus and Amazonian manatee, T. inunguis) coexist in sympatry in the Amazon estuarine complex, including the Pará River Estuary49,50,−51, commonly referred to as Marajó Bay. The cetaceans belong to the Delphinidae (Guiana dolphin Sotalia guianensis, and tucuxi Sotalia fluviatilis) and Iniidae (Amazon river dolphin, Inia geoffrensis, and Araguaian boto Inia araguaiaensis) families. Despite decades of surveys, questions regarding habitat use and a possible sympatry between S. guianensis and S. fluviatilis remain unknown in this region. Recently, the presence of the river dolphins I. geoffrensis and the newly described I. araguaiaensis52 in the Amazon estuarine complex was confirmed through stranding events and molecular analyses42.
SIA can lead to important insights into the trophic relationships and foraging habits of these animals. Therefore, in this study carbon and nitrogen isotopes were analyzed in the bone/tooth collagen of aquatic mammals from different habitat use: oceanic (Sperm whale Physeter macrocephalus), coastal (e.g., Bottlenose dolphin Tursiops truncatus, Rough-toothed dolphin Steno bredanensis, Guiana dolphin S. guianensis), continental shelf (e.g., Fraser's dolphin Lagenodelphis hosei, Risso's dolphin Grampus griseus, Melon-headed whale Peponocephala electra; Common dolphin Delphinus sp.) and freshwater/estuarine (Amazon river dolphin I. geoffrensis, Araguaian boto I. araguaiaensis, Amazonian manatee T. inunguis). The analysis of low-turnover tissues, such as bone and teeth, can inform on the feeding ecology of an individual over almost its entire lifetime53,54, and has, therefore, been widely applied to clarify aspects regarding marine mammal trophic ecology55,56,−57.
The main goals herein were to: (1) investigate the habitat use and trophic relationships among freshwater and marine aquatic mammal species, (2) evaluate isotopic niche partitioning among the most representative species (Sotalia guianensis, Inia spp. and Trichechus inunguis) within the Amazon estuarine complex and (3) characterize differences in isotopic niches between ecological S. guianensis stocks.
Material and methods
Study sites
The samples assessed herein were obtained from stranded carcasses recovered along three main sectors: (1) the Amazon lowland (AL); (2) the Amazon estuarine complex and adjacent coastal zones (AE) and, (3) the Northeastern coast of Brazil (NC) (Fig. 1).
Map of the study sites representing the sampling sectors along the Northern and Northeastern Brazil (1) indicating the 10, 50, 100 and 200 m isobaths. Detailed maps of the sampling regions: (2) Amazon Lowland (AL), (3) Northeastern coast (NC), on the coast of the Maranhão (MA) and Piauí (PI) states, and (4) Amazon Estuary and adjacent coastal zones (AE). Amapá (AP), Pará (PA), Maranhão (MA), Piauí (PI) and Ceará states (CE). Sector AE only includes the study sampling area and not the entire Northern region. Dots indicate the sampling locations.
The Amazon lowland (AL) sector comprises the floodplain habitat named várzea, consisting of clearwater rivers58. Specimens from this region were collected in rivers around the Santarém municipality, Ayaya River, a small Amazon River tributary; at Oriximiná, Trombetas River; at Vitória do Xingu, in the lower Xingu River, and near Belém, Guajará Bay.
The Amazon estuarine complex and adjacent coastal zones (AE) covered in this study belong to the Northern and Eastern coast of Marajó Island (i.e., Marajó Bay), as well as the Eastern coast of the state of Pará. Marajó Bay is formed mainly by Pará discharges and the Araguaia-Tocantins River Basin can be considered as the main freshwater source to this bay59. This area receives a superficial saline intrusion during low river discharge60 and undergoes a macrotidal regime, with maximum tides around 4 m on both sides of the bay61. The continental Amazon shelf is influenced by factors such as proximity to the Equator, strong tides (semidiurnal tides) and oceanic currents and winds (e.g., North Brazil Current, easterly trades), as well as the substantial discharge of the Amazon River (e.g., water, solutes and particulate materials). The region is considered part of the wet tropics, due to high precipitation rates and temperature62.
The Northeastern coast (NC) covers the coastline of the state of Piauí and the easternmost coastline of the state of Maranhão. The Parnaíba River discharge into the Atlantic Ocean forms a delta with five tidally influenced bays, Tutóia, Caju, Melancieiras, Canárias and Igaraçu, that form the Parnaíba Delta63. The region comprises a mesotidal coast, with tides ranging from 1.1 m to 3.3 m64 and vast mangrove forest areas.
Sample collection
Samples were obtained from floating or stranded carcasses found during sampling surveys or from incidental catches in fishing gear (i.e., S. guianensis). Carcasses were recovered regardless of decomposition stage and taken to the Museu Paraense Emílio Goeldi (MPEG, Belém, Pará, Brazil) where hard parts (i.e., bones and teeth) were cleaned from outer soft tissues and stored dry. Most samples were collected between 2005 and 2014. However, older specimens of Inia spp. and T. inunguis collected by naturalists (i.e., Émil August Goeldi and Gottfried Hagmann) and MPEG researchers from the state of Pará (AL) in the 1910s, 1970s and 1980s were also included.
A total of 270 bone and teeth samples from aquatic mammals, representing 14 taxa and four families were assessed, namely Physeteridae (Physeter macrocephalus); Delphinidae (Delphinus sp., Globicephala macrorhynchus, Grampus griseus, Lagenodelphis hosei, Peponocephala electra, Pseudorca crassidens, Sotalia guianensis, Stenella attenuata, Steno bredanensis, Tursiops truncatus), Iniidae (Inia geoffrensis, I. araguaiaensis) and Trichechidae (Trichechus inunguis).
Most analyzed bone samples were obtained from the internal portion of the skull using pliers. In the absence of a skull, other available bones (n = 67) were used, following the priority order: 1st caudal vertebrae, 2nd chevrons, 3rd teeth and other bones (e.g., scapula, mandible, flipper). Tooth fragments were removed after demineralization and lipid extraction was performed for the whole tooth. Previous studies reported a difference of 0.2‰ in carbon and 0.3‰ in nitrogen between the teeth and bones of the same individual65. Therefore, the isotopic values of the sampled tissues were considered comparable.
Stable isotope analysis
Bone fragments were demineralized by repeated baths in hydrochloric acid (HCl, 0.5N for approximately 72 to 96 h66 in order to isolate collagen. Each bone fragment (or tooth) was placed in a glass vial covered with acid and stored at 4°C overnight, with acid replacement every 24 h. Samples were subjected to successive baths with distilled water to achieve a neutral pH after a rubber-like flexibility was reached. After this process, lipid extraction was performed by manual rinsing of the samples three times in a 2:1 methanol:chloroform solution, discarding the old solution each time and replacing it with a new one. Samples were then washed in distilled water and dried for at least 24 h at 60°C56. Dried samples were then ground to a fine powder using a mortar and pestle. Bone/teeth collagen samples were finally weighed (0.5 to 0.6 mg) in tin capsules (Costech Analytical).
Nitrogen and carbon isotope ratios were measured by Elemental Analyzer Continuous Flow Isotope Ratio Mass Spectrometry in the Center for Stable Isotopes, University of New Mexico using a Costech ECS 4010 Elemental Analyzer coupled to a ThermoFisher Scientific Delta V Advantage mass spectrometer via a CONFLO IV interface. Isotope ratios are reported using the standard delta (δ) notation relative to V-AIR and to Vienna Pee Dee Belemnite (V-PDB), respectively. The three internal laboratory standards are: UNM-CSI Protein std#1, casein purchased from Sigma Aldrich with δ15N and δ13C values of 6.43‰ and − 26.52‰; UNM-CSI Protein std#2, soy protein purchased from Sigma Aldrich with δ15N and δ13C values of 0.98‰ and − 25.78‰; UNM-CSI protein Std#4, house made tuna protein with δ15N and δ13C values of 13.32‰ and − 16.17‰. Analyses were normalized to the laboratory standards which were calibrated against IAEA N1, IAEA N2 and USGS 43 for δ15N and NBS 21, NBS 22 and USGS 24 for δ13C.
Data analysis
For T. inunguis (n = 11) and Inia spp. (n = 10) we obtained samples from museum specimens of distinct years (1910′s to 2010), therefore, before pooling samples from different decades, possible temporal trends were tested. An ordinary least squares linear trend performed for both taxa didn't detect significant differences in historical trends for Inia spp. (δ13C: r2 = 0.25, p = 0.13; δ15N: r2 = 0.25, p = 0.13) and for T. inunguis (δ13C: r2 = 0.06, p = 0.45; δ15N: r2 = 0.24, p = 0.12). Before pooling different bones from the same species (i.e., Sotalia guianensis, the only species with enough sampling to perform the test: n = 220), possible differences in stable isotope signatures were assessed using a permutational multivariate analysis of variance test and no differences were detected (pseudo-F = 1.60, p = 0.11). So, we assume that for our data set distinct bone tissue of samples didn’t affect results. Due to the small sample size for some species and lack of essential data (i.e., age, standard length, sex), neither sex or maturity stage were considered for the analyses.
δ13C and δ15N data normality and homogeneity of variance were tested by the Shapiro–Wilk and Levene tests, respectively. The Student’s t-test was used to compare isotopic values between S. guianensis populations and between marine and freshwater species. A significance level of 0.05 was assumed for all tests.
To investigate isotopic niche variations, species were grouped into Delphinids (all delphinids except S. guianensis), Inia spp. (I. geoffrensis and I. araguaiaensis) and T. inunguis. S. guianensis specimens were grouped according to their sampling sector: Sgui_AE for individuals from AE and Sgui_NC, for specimens from NC. Isotopic niche areas were calculated through standard ellipse areas corrected for small sample sizes (SEAc) using Stable Isotope Bayesian Ellipses (SIBER routine in SIAR package in R67). Overlaps among ellipses were also calculated, in order to quantify the trophic overlap among groups. Probabilities were estimated by Bayesian inference indicating uncertainty and central tendency measures based on permutations presenting 50%, 75% e 95% credibility intervals.
Results
The marine species, exhibited broad ranges of δ13C (− 16.6 to − 10.6‰) and δ15N (6.6 to 16.0‰) values (Table 1). Significantly higher δ13C values (t-test, t = 16.0, p < 0.0001), but similar δ15N values (t-test, t = 1.9, p = 0.06) were observed in marine species compared to species from lowland and estuarine areas.
Sotalia guianensis from NC presented similar δ15N values (t-test, t = 0.80, p = 0.43) but higher mean δ13C values than individuals from AE (t-test, t = − 3.39, p < 0.001). Excluding Sgui_AE from the analysis, a pattern of decreasing δ13C values was observed from inshore (i.e., S. bredanensis, T. truncatus) to offshore (G. macrorhynchus, G. griseus, L. hosei, P. electra, S. attenuata) species. The most 13C-depleted values of all marine species were observed for Physeter macrocephalus (− 14.4 ± 1.9‰, range − 16.6 to − 12.9‰) and the most 15N-enriched δ15N values. The only P. crassidens specimen analyzed herein presented high δ13C and δ15N values (Table 1; Fig. 2).
Mean (dots) and standard deviation (bars) carbon and nitrogen isotopic values in bone-collagen of aquatic mammals collected from the Amazon Estuary and adjacent coastal zones (AE) and Northeastern coast of Brazil (NC). Isotope ratios for marine species presented a spatial trend of decreasing δ13C values towards offshore habitats for the following delphinids: Pseudorca crassidens (Pcr), Tursiops truncatus (Ttr), Delphinus sp. (Dsp), Steno bredanensis (Sbr), Grampus griseus (Ggr), Peponocephala electra (Pel), Lagenodelphis hosei (Lho), Globicephala macrorhynchus (Gma), Stenella attenuata (Sat) and the most depleted in δ13C and most enriched δ15N of all cetaceans, Physeter macrocephalus (Pma). Species drawings are not to scale.
A wide range of δ13C (− 25.5 to − 12.5‰) and δ15N (6.0 to 15.3‰) isotope values was observed for species that inhabit both AL and AE regions (T. inunguis and Inia spp.). These species did not differ in δ13C values (t-test, t = 0.86, p = 0.40) but differed significantly in δ15N values (t-test, t = 10.4, p < 0.0001).
T. inunguis specimens from AL exhibited a large dispersion of δ13C values (− 25.5 to − 15.8‰), whereas those from AE presented a narrow range (− 16.2 to − 12.9‰). T. inunguis occupied the lowest isospace position, with δ15N values of 7.6 ± 1.2‰ (range 6.0 to 10.3‰) (Fig. 3).
Mean (dots) and standard deviation (bars) carbon and nitrogen isotopic values in bone-collagen for Inia geoffrensis (Igeo), I. araguaiaensis (Iarag) and Trichechus inunguis (Tinung). Species showed significant differences in δ15N values but did not differ in δ13C values. Specimens were collected along the Amazon lowland (AL), and Amazon Estuary and adjacent coastal zone (AE) in Northern Brazil. Species drawings are not to scale.
Regarding Inia spp., non-significant differences in mean δ13C and δ15N values were observed between species (Fig. 3, t-tests, t = 0.77, p = 0.46 and t = − 0.09, p = 0.92, for δ13C and δ15N, respectively).
Isotopic niche variation
The isotopic niches were highly distinct among the aquatic mammals that inhabit the AE region (T. inunguis, S. guianensis, I. geoffrensis and I. araguaiaensis) with no overlaps (standard ellipse areas, SEAc). In addition, no overlap among the most representative species of the study area (Sotalia guianensis, Inia spp. and Trichechus inunguis) was observed, while a significant overlap among Delphinids and S. guianensis populations was noted, expressing isotope niche partitioning.
Moreover, T. inunguis and Inia spp. displayed larger SEAc areas compared to that of S. guianensis from AE (Table 2, Figs. 4, 5).
Bayesian standard ellipse areas (solid lines represent SEAc, standard ellipse areas adjusted for small sample sizes), containing c. 40% of data67 for Inia_spp (n = 10, black line), T_inung (n = 11, red line), Sgui_AE (n = 188, dark blue line), Sgui_NC (n = 32, green line) and Delph (n = 26, light blue line). Species drawings are not to scale.
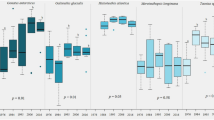
Bayesian results of the variation in δ13C and δ15N values for Delphinids (Delph), Inia geoffrensis and I. araguaiaensis (Inia_spp), Sotalia guianensis from Amazon estuarine complex (Sgui_AE) and Northeast (Sgui_NC) and Trichechus inunguis (T_inung) from Northern and Northeastern Brazil. Measures of Bayesian standard ellipse areas: black circles represent the mode and boxes indicate 50%, 75% and 95% credible intervals from dark grey to light grey, respectively. Red squares indicate the SEAc (SEA corrected for small samples sizes).
The smallest isotopic niche area was occupied by Sgui_NC, followed by Sgui_AE. S. guianensis populations presented a niche area overlap of 68.98% (Fig. 6).
Bayesian results of the variation in δ13C and δ15N values for the Guiana dolphin Sotalia guianensis from the Northeastern coast (NC) and Amazon Estuary and adjacent coastal zone (AE), respectively (Sgui_NC and Sgui_AE). Black circles represent the SEA mode. Boxes indicate 50%, 75% and 95% credible intervals from dark grey to light grey, respectively. Red squares indicate the SEAc (SEA corrected for small samples sizes) of Bayesian standard ellipse areas.
Discussion
The isotopic composition in bone/teeth that integrate a long-term diet were evaluated for the first time in aquatic mammals from the Amazon estuarine complex and Northeastern Brazil. No isotopic overlap was observed among coexisting species within the Amazon estuarine complex and adjacent coastal areas (AE). Indeed, isotopic niches were highly distinct among S. guianensis, Inia spp. and Trichechus inunguis, indicating strong niche segregation among these sympatric species, at least in Marajó Bay. Among marine delphinids, the carbon isotopic composition in the bone/tooth collagen in offshore species (i.e. Grampus griseus, Globicephala macrorhynchus) mirrored that of nearshore species (i.e. Sotalia guianensis), contradicting the pattern of decreasing δ13C values characteristic of many areas around the world56 including areas in Southeastern68 and Southern Brazil69. This pattern can be explained by the huge freshwater discharge into the adjacent oceanic areas that contributes with13C-depleted organic material derived from terrestrial and mangrove sources to their dissolved and particulate organic pool31,70,71. Therefore, carbon isotopic values in offshore pelagic areas under the influence of the Amazon plume exhibit low δ13C values, while higher values are reported for high salinity oceanic waters72. The similar carbon isotopic values found in the collagen of estuarine and oceanic species, suggest that the low basal δ13C values are transferred up along the food chain resulting in a lower predicting power of this isotope to track the foraging habitat of these species in this peculiar tropical region. Collagen δ15N values in the cetacean species analyzed, on the other hand, were generally correspondent to the known trophic habits of the species, where mostly teutophagous delphinid species (i.e. Grampus griseus, Globicephala macrorhynchus) showed lower values than those with more piscivorous feeding habits (i.e. Tursiops truncatus). Among delphinid species, the bottlenose dolphin Tursiops truncatus and Pseudorca crassidens presented 13C- and 15N-enriched collagen reflecting their high trophic position in this tropical food web. Both species are considered mainly piscivorous, and as such, they exhibit high trophic levels within pelagic food webs73,74,−75. Steno bredanensis is considered an offshore species in Southeastern Brazil76, although regularly sighted in shallow bays and other coastal areas77. Increased stranding events and fishermen reports on T. truncatus and S. bredanensis reinforces their regular presence off Northern Brazil39,48, mostly associated with negative interactions with artisanal fisheries. Indeed, stranding events are considered an important source to access ecology data of marine mammals. The study area is influenced by the water and wind regime of the North Brazil Current (NBC), defined as a major low latitude western boundary current in the Atlantic that transports upper-ocean waters northward across the Equator. NBC does not show significant seasonal variation in the lower-frequency fluctuations in the thermocline layer throughout the year78. As such, carcasses are expected to be transported westwards on a regular basis, following trade winds, drifting in a water mass of warm (> 24°C) temperatures. Teutophagous species (e.g., Globicephala macrorhynchus, Peponocephala electra and Grampus griseus) and species that feed on small pelagic fish and squid (e.g., Lagenodelphis hosei and Stenella attenuata) displayed similar δ13C and δ15N values, coinciding with their low trophic level predation in offshore areas79. So far, no previous isotopic data for G. macrorhynchus, P. electra and Physeter macrocephalus have been reported for Brazilian waters. P. macrocephalus samples were the most 15N-enriched and 13C-depleted compared to the other assessed taxa. Due to the long-time interval integrated in the bone collagen of this long-lived cetacean, these values are possibly averaging their movement between isotopically distinct foodwebs throughout their lives, thus limiting the interpretation of their trophic position within the studied cetacean community. Nevertheless, it is expected that the species occupy high trophic levels within offshore foraging grounds80,81 which is supported by the isotopic values in the two mature females and the immature specimens analyzed in this study. Higher nitrogen isotopic values were expected for the coastal Sotalia guianensis, regardless of its trophic position, as higher basal nitrogen values were observed in the estuarine waters82. Basal nitrogen isotopic values seem to track the main nitrogen sources used by the producers83. Indeed, higher δ15N values are found within estuarine and nearshore areas dominated by diatoms while lower δ15N values, typical of diatom-diazotroph associations and oceanic diazotroph producers, which are reported within the Amazon plume and the adjacent oceanic areas, respectively32,82,83. In this context, a higher relative importance of low trophic level prey such as shrimps and squids in the diet of the species84 may account for the low nitrogen isotopic values found in this species.
Niche partitioning
The largest isotopic niche areas were found for Inia spp. and T. inunguis, mainly resulting from a wide range of δ13C values, reflecting higher habitat plasticity, i.e., foraging along a gradient encompassing lowland freshwaters and AE estuarine habitats. Variable carbon isotope values in these aquatic mammals may be the result of a diversity of basal sources found mainly in the Amazon estuarine complex (AE), characterized by the presence of both 13C-enriched C4 plants (e.g., seagrasses) and low δ13C plants, such as mangrove leaves (δ13C = − 28.4 ± 0.5‰85). Ontogenetic variation were found in manatee diets at the Tapajós and Negro rivers, in the Amazon basin, with a proportional consumption of C4 and C3 plants for lactating females and other adults, respectively86. Some potential diet items were identified for both manatees, Amazonian and Antillean, in estuarine Marajó Bay areas (i.e., Blutaparon portulacoides, Eleocharis geniculata, Crenea maritima), including both C3 and C4 plants87.
Vegetation composition and rainfall season could influence the biomass and exposure of seagrass and macroalgae and, consequently, manatee foraging habits88. In the Amazon estuarine complex, the local tidal regime probably causes changes in vegetation availability, such as substrate and flood level point differences in size, shape and plant appearance87. Manatee movements inside this dynamic habitat can be influenced by food availability, which should vary according to the dry and rainy seasons. The rainy season has a strong influence on the presence of manatees in Marajó Bay89.
Until recently, the Amazon River dolphin, Inia geoffrensis, was considered endemic to the Amazon and Orinoco basins90 while the newly described I. araguaiaensis was restricted to the Tocantins-Araguaia River basin52. However, Costa et al.91 described the presence of Inia spp. in estuarine areas of Marajó Island and the eastern Pará coast. Further molecular analyses confirmed the occurrence of both Inia species around Marajó Is. and the Curuçá Estuary, extending I. araguaiaensis distribution area in nearly 500 km42. The small sample size in the present study did not allow for comparisons between the isotopic niche of these two species. Data on one I. araguaiaensis collected at Curuçá Estuary was 13C-depleted in δ13C values, similar to samples from the AL sector, suggesting that it probably fed in a 13C-depleted food web (e.g., mangrove)85 and moved to estuarine areas. Prey availability could determine this species movements and might affect species distribution92,93. Although I. geoffrensis and I. araguaiaensis are considered exclusively freshwater species52,90, the findings reported herein reinforce that the use of estuarine areas is not circumstantial but rather reflects their occupancy in these environments for active foraging.
Although S. guianensis and Inia spp. are considered piscivorous species90,94,95 no isotopic niche overlap was observed, indicating spatial and trophic partitioning, at least in Marajó Bay. Studies on the trophic ecology of I. geoffrensis are scarce, although the analysis of stomach contents revealed at least forty-three fish species90. However, no studies describing the species’ diet in flooded areas of the eastern Amazon or in the Amazon estuarine complex are available to date.
Isotopic values indicate that Inia spp. forage at higher trophic levels than S. guianensis. Morphological Inia characters (e.g., lateral mobility) probably allow individuals to explore areas with dense vegetation in floodplains (i.e., várzea) and flooded forests (i.e., igapó)96. In the Central Amazon I. geoffrensis and S. fluviatilis have been observed foraging in extensive floodplain areas around main rivers (Solimões and Amazonas). In this region, the flood cycle impact can determine both habitat and prey availability92. Differences in habitat use, foraging strategies and prey availability, as well as consumption of prey belonging to different trophic guilds, could reduce inter-specific competition between Inia and Sotalia in sympatric areas.
Ecological Sotalia guianensis stocks
At least six management units for S. guianensis have been suggested alongshore Brazil (Pará, Ceará, Rio Grande do Norte, Bahia, Espírito Santo and South-Southeastern area) through the use of molecular markers, evidencing a strong population structure51. Other ecological parameters have been used to differentiate ecological stocks. Different geographic areas (Espírito Santo, north and south Rio de Janeiro and São Paulo) have been recognized mainly for cranial and feeding apparatuses variables97. Cranial morphometry exhibited a latitudinal growth pattern from North to South, i.e., specimens from São Paulo had smaller skulls. Skull analyses from the north, northeast and southeast have been demonstrated a complete separation between Pará and Rio de Janeiro populations, and a partial separation between Pará, Maranhão and Piauí98. Analyses of the periotic-tympanic bone complex of three Brazilian regions also reported geographic variations99.
In order to evaluate ecological S. guianensis stocks, Botta100 analyzed stable isotopes in Guiana dolphin teeth from the Amazon River estuary, Ceará, Espírito Santo, northern Rio de Janeiro, southern São Paulo and northern Paraná and northern Santa Catarina. Four groups were recognized, indicating that SIA is a powerful tool to confirm ecological stock differences.
Significant differences in isotopic carbon composition were found for S. guianensis from AE and NC in the present study, although with similar nitrogen isotope values. Sgui_AE bone collagen samples were more depleted in 13C than those from Sgui_NC. Dolphins from AE occupy a broader isotopic niche area, indicating higher trophic plasticity. Groups in this sector probably have a larger availability of more diverse prey associated to freshwater habitats101,102 and carbon source variability due to the huge influence of the Amazon River and Pará River estuary (i.e. Marajó Bay). Individuals from Sgui_NC occupy a narrower isotopic niche area, probably resulting from the association of a greater consumption of marine fishes, as identified in stomach contents of individuals from that population102.
In summary, this study provided a first attempt to evidence trophic relationships and resource partitioning among aquatic mammals from the Amazon estuarine complex and its adjacent coastal zones. The obtained SIA results allowed for insights on habitat use and the trophic ecology of marine mammals inhabiting different oceanographic regions off Northern/Northeastern Brazil. Furthermore, δ13C and δ15N values in S. guianensis inhabiting two coastal regions (AE and NC) indicated an ecological distinction between the populations. These findings provide novel data on the stable isotope composition for these threatened cetaceans and sirenian species in this large section of Brazilian coast.
References
Mèndez-Fernandez, P. et al. Foraging ecology of five toothed whale species in the Northwest Iberian Peninsula, inferred using carbon and nitrogen isotope ratios. J. Exp. Mar. Bio. Ecol. 413, 150–158 (2012).
Browning, N. E., Cockcroft, V. G. & Worthy, G. A. J. Resource partitioning among South African delphinids. J. Exp. Mar. Bio. Ecol. 457, 15–21 (2014).
Loizaga de Castro, R., Saporiti, F., Vales, D. G., Cardona, L. & Crespo, E. A. Using stable isotopes to assess whether two sympatric dolphin species share trophic resources. Mar. Mammal Sci. 33, 1235–1244 (2017).
Franco-Trecu, V., Drago, M., Costa, P., Dimitriadis, C. & Passadore, C. Trophic relationships in apex predators in an estuary system: a multiple-method approximation. J. Exp. Mar. Bio. Ecol. 486, 230–236 (2017).
Newsome, S. D., Clementz, M. T. & Koch, P. L. Using stable isotope biogeochemistry to study marine mammal ecology. Mar. Mammal Sci. 26, 509–572 (2010).
DeNiro, M. J. & Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506 (1978).
DeNiro, M. J. & Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geo-Marine Lett. 45, 341–351 (1981).
Minagawa, M. & Wada, E. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 48, 1135–1140 (1984).
Post, D. M. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718 (2002).
McCutchan, J. H., Lewis, W. M., Kendall, C. & Mcgrath, C. C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. OIKOS 102, 378–390 (2003).
Vanderklift, M. A. & Ponsard, S. Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia 136, 169–182, https://doi.org/10.1007/s00442-003-1270-z (2003).
Fry, B. Stable Isotope Ecology (Springer, Berlin, 2006).
Bearhop, S. et al. Determining trophic niche width: a novel approach using stable isotope analysis. J. Anim. Ecol. 73, 1007–1012 (2004).
Newsome, S. D., Martinez del Rio, C., Bearhop, S. & Phillips, D. L. A niche for isotopic ecology. Front Ecol Env. 5, 429–436 (2007).
Hutchinson, G. E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 22, 415–427 (1957).
Layman, C. A., Arrington, D. A., Montana, C. G. & Post, D. M. Can stable isotope ratio provide for community-wide measures of trophic structure?. Ecology 88, 42–48 (2007).
Ryan, C. et al. Stable isotope analysis of baleen reveals resource partitioning among sympatric rorquals and population structure in fin whales. Mar. Ecol. Prog. Ser. 479, 251–261 (2013).
Staudinger, M. D., McAlarney, R. J., McLellan, W. A. & Ann Pabst, D. Foraging ecology and niche overlap in pygmy (Kogia breviceps) and dwarf (Kogia sima) sperm whales from waters of the U.S. mid-Atlantic coast. Mar. Mammal Sci. 30, 626–655 (2014).
Gallagher, A. J., Shiffman, D. S., Byrnes, E. E., Hammerschlag-Peyer, C. M. & Hammerschlag, N. Patterns of resource use and isotopic niche overlap among three species of sharks occurring within a protected subtropical estuary. Aquat. Ecol. 51, 435–448 (2017).
Graham, B. S., Koch, P. L., Newsome, S. D., Mcmahon, K. W. & Aurioles, D. Isoscapes. in (ed. J.B.West) 299–318 (Springer Science, London 2010). https://doi.org/10.1007/978-90-481-3354-3
Jaeger, A., Lecomte, V. J., Weimerskirch, H., Richard, P. & Cherel, Y. Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators ’ foraging areas in the Southern Ocean. RAPID Commun. MASS Spectrom. 24, 3456–3460 (2010).
McMahon, K. W., Hamady, L. L. & Thorrold, S. R. A review of ecogeochemistry approaches to estimating movements of marine animals. Limnol. Ocean. 58, 697–714 (2013).
Brault, E. K., Koch, P. L., Mcmahon, K. W. & Broach, K. H. Carbon and nitrogen zooplankton isoscapes in West Antarctica reflect oceanographic transitions. Mar. Ecol. Prog. Ser. 593, 29–45 (2018).
Troina, C. G. et al. Deep-sea research part I Zooplankton-based δ13C and δ15N isoscapes from the outer continental shelf and slope in the subtropical western South Atlantic. Deep. Res. Part I https://doi.org/10.1016/j.dsr.2020.103235 (2020).
Montoya, J. P. Abundance as indicators of forest nitrogen status and soil carbon dynamics stable isotopes in ecology and environmental science. In Stable Isotopes in Ecology and Environmental Science, 176–201 (ed. Lajtha, R. M. K.) (Blackwell Publishing, London, 2007). https://doi.org/10.1002/9780470691854.ch3.
Sigman, D. M., Karsh, K. L. & Casciotti, K. L. Ocean Process Tracers: Nitrogen Isotopes in the Ocean. in Encyclopedia of Ocean Sciences (ed. Steele, J.H., Turekian, K.K., Thorpe, S. A.) 4138–4153 (Elsevier Ltd, 2009).
Laws, E. A., Popp, B. N., Bidigare, J. R. R., Kennicutt, M. C. & Macko, S. A. Dependence of phytoplankton carbon isotopic composition on growth rate and [CO2]aq: Theoretical considerations and experimental results. Geochim. Cosmochim. Acta 59, 1131–1138 (1995).
Trueman, C. N. & St John Glew, K. Chapter 6—Isotopic Tracking of Marine Animal Movement Tracking Animal Migration with Stable Isotopes (Elsevier Inc., London, 2019). https://doi.org/10.1016/B978-0-12-814723-8.00006-4.
France, R. L. Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar. Ecol. Prog. Ser. 124, 307–312 (1995).
Mayer, M., Joye, B. & Aller, C. Importance of suspended participates in riverine delivery of bioavailable nitrogen to coastal zones. Glob. Biochem. Cycles 12, 573–579 (1998).
Druffel, E. R. M., Bauer, J. E. & Griffin, S. Input of particulate organic and dissolved inorganic carbon from the Amazon to the Atlantic Ocean. Geochem. Geophys. Geosyst 6, 1–7 (2005).
Weber, S. C. et al. Amazon River influence on nitrogen fixation and export production in the western tropical North Atlantic. Limnol. Ocean. https://doi.org/10.1002/lno.10448 (2016).
Cai, D. L. & Edmond, J. M. Sources and transport of particulate organic carbon in the Amazon River and Estuary. Estuar. Coast. Shelf Sci. 26, 1–14 (1988).
Weber, S. C. et al. Habitat delineation in highly variable marine environments. Front. Mar. Sci. 6, 1–16 (2019).
De Menezes, M. P. M., Berger, U. & Mehlig, U. Mangrove vegetation in Amazonia: a review of studies from the coast of Pará and Maranhão States, north Brazil. Acta Amaz. 38, 403–420 (2008).
Hayashi, S. N., Souza-filho, P. W. M., Nascimento, W. R. Jr. & Fernandes, M. E. B. The effect of anthropogenic drivers on spatial patterns of mangrove land use on the Amazon coast. PLoS ONE 14, 1–20 (2019).
Barthem, R. B. Ocorrência, distribuição e biologia dos peixes da Baía de Marajó, Estuário Amazônico. Bol. do Mus. Para. Emílio Goeldi. Série Zool. 2, 49–69 (1985).
Camargo, M. & Isaac, V. Os peixes estuarinos da região Norte do Brasil: Lista de espécie e consideração sobre sua distribuição geográfica. Bol. do Mus. Para. Emílio Goeldi, Ser. Zool. 17, 135 – 147 (2002).
Siciliano, S. et al. Revisão do conhecimento sobre os mamíferos aquáticos da costa norte do Brasil. Arq. do Mus. Nac. 66, 381–401 (2008).
Meirelles, A. C. O. et al. Cetacean strandings on the coast of Ceará, north-eastern Brazil (1992–2005). J. Mar. Biol. Assoc. United Kingdom 89, 1–8 (2009).
Emin-Lima, R. et al. Os mamíferos aquáticos associados aos manguezais da costa norte brasileira. in Mamíferos de Restingas e Manguezais do Brasil 45–57 (Sociedade Brasileira de Mastozoologia, 2010).
Siciliano, S. et al. New genetic data extend the range of river dolphins Inia in the Amazon Delta. Hydrobiologia 777. https://doi.org/10.1007/s10750-016-2794-7 (2016).
Siciliano, S. et al. Confirmed sightings of the Antillean manatee (Trichechus manatus) on the coast of Ilha de Marajó, northern Brazilian coast. JMBA Glob. Mar. Environ. 34–35 (2007).
Alves, M. D. et al. First abundance estimate of the Antillean manatee (Trichechus manatus) in Brazil by aerial survey. J. Mar. Biol. Assoc. UK https://doi.org/10.1017/S0025315415000855 (2015).
Monteiro-Neto, C. et al. Impact of fisheries on the tucuxi (Sotalia fluviatilis) and rough-toothed dolphin (Steno bredanensis) populations off Ceará state, northeastern Brazil. Aquat. Mamm. 26, 49–56 (2000).
Barreto, A. S. et al. Plano de Ação nacional para Conservação dos Mamíferos Aquáticos - Pequenos Cetáceos. (2010).
Moura, J. F., Hauser-Davis, R. A., Lemos, L., Emin-Lima, R. & Siciliano, S. Guiana Dolphins (Sotalia guianensis) as marine ecosystem sentinels: ecotoxicology and emerging diseases. in Reviews of Environmental Contamination and Toxicology, Vol. 228 (ed Whitacre, D. M.) 1–29, https://doi.org/10.1007/978-1-4419-6260-7 (2014).
Costa, A. F. et al. Stranding survey as a framework to investigate rare cetacean records of the north and north-eastern Brazilian coasts. Zookeys https://doi.org/10.3897/zookeys.688.12636 (2017).
Domning, D. P. Distribution and Status of manatees Trichechus spp. near the mouth of the Amazon River, Brazil. Biol. Conserv. 19, 85–97 (1981).
Siciliano, S. et al. Going back to my roots: Confirmed sightings of the Antillean manatee (Trichechus manatus) on the coast of Ilha de Marajó, northern Brazilian coast. JMBA Glob. Mar. Environ. 1, 34–35 (2007).
Cunha, H. A., da Silva, V. M. F. & Solé-Cava, A. M. Molecular Ecology and Systematics of Sotalia Dolphins. in Biology, Evolution and Conservation of Rivers Dolphins within South America and Asia (eds. Ruiz-Garcia, M. & Shostell, J.) 261–283 (Nova Science Publishers, Inc., 2010).
Hrbek, T. et al. A new species of river dolphin from Brazil or: how little do we know our biodiversity. PLoS ONE 9, https://doi.org/10.1371/journal.pone.0083623 (2014).
Niño-Torres, C. A., Gallo-Reynoso, J. P., Galván-Magaña, F., Escobar-Briones, E. & Macko, S. A. Isotopic analysis of δ13C, δ15N, and δ34S ‘a feeding tale’ in teeth of the longbeaked common dolphin, Delphinus capensis. Mar. Mammal Sci. 22, 831–846, https://doi.org/10.1111/j.1748-7692.2006.00065.x (2006).
Pinela, A. M., Borrell, A., Cardona, L. & Aguilar, A. Stable isotope analysis reveals habitat partitioning among marine mammals off the NW African coast and unique trophic niches for two globally threatened species. Mar. Ecol. Prog. Ser. 416, 295–306, https://doi.org/10.3354/meps08790 (2010).
Walker, J. L. & Macko, S. A. Dietary studies of marine mammals using stable carbon and nitrogen isotopic ratios of teeth. Mar. Mammal Sci. 15, 314–334 (1999).
Riccialdelli, L., Newsome, S. D., Fogel, M. L. & Goodall, R. N. P. Isotopic assessment of prey and habitat preferences of a cetacean community in the southwestern South Atlantic Ocean. Mar. Ecol. Prog. Ser. 418, 235–248 (2010).
Vighi, M. et al. Stable isotopes indicate population structuring in the Southwest Atlantic population of right whales (Eubalaena australis). PLoS ONE 9, https://doi.org/10.1371/journal.pone.0090489 (2014).
Junk, W. J. et al. A classification of major naturally-occurring amazonian lowland wetlands. Wetlands 31, 623–640 (2011).
Rosário, R. P., Borba, T. A. C., Santos, A. S. & Rollnic, M. Variability of Salinity in Pará River Estuary: 2D Analysis with Flexible Mesh Model. J. Coast. Res. 75, 128–132 (2016).
Bezerra, M. O. & Rollnic, M. Physical oceanographic behavior at the Guamá/Acará-Moju and the Paracauari river mouths, Amazon Coast (Brazil). in Journal of Coastal Research 1448–1452 (Proceedings of The 11th International Coastal Synposium, 2011).
Rosário, R. P. & Santos, A. S. Contribution to understanding the surface seawater intrusion in the Pará River estuary during low discharge. Proceedings of the 17th Physics of Estuaries and Coastal Seas (PECS) (2014).
Nittrouer, C. a. & DeMaster, D. J. The Amazon shelf setting: tropical, energetic, and influenced by a large river. Cont. Shelf Res. 16, 553–573 (1996).
Moreira, A. M. & Mavigner, D. S. Conhecendo história e geografia do Piauí. (Gráfica Ferraz, 2007).
Szczygielski, A. et al. Evolution of the Parnaíba Delta (NE Brazil) during the late Holocene. Geo-Marine Lett. 35. 105–117, https://doi.org/10.1007/s00367-014-0395-x (2014).
Foote, A. D. et al. Tracking niche variation over millennial timescales in sympatric killer whale lineages. Proc. Biol. Sci. 280, 2–9 (2013).
Newsome, S. D., Koch, P. L., Etnier, M. A. & Aurioles-Gamboa, D. Using Carbon and Nitrogen Isotope Values To Investigate Maternal Strategies in Northeast Pacific Otariids. Mar. Mammal Sci. 22, 556–572 (2006).
Jackson, A. L., Inger, R., Parnell, A. C. & Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER - Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602 (2011).
Bisi, T. L. et al. Trophic relationships and habitat preferences of Delphinids from the Southeastern Brazilian Coast determined by carbon and nitrogen stable isotope composition. PLoS ONE 8, e82205 (2013).
Botta, S., Hohn, A. A., Macko, S. A. & Secchi, E. R. Isotopic variation in delphinids from the subtropical western South Atlantic. J. Mar. Biol. Assoc. United Kingdom https://doi.org/10.1017/S0025315411000610 (2012).
Medeiros, P. et al. Fate of the Amazon River dissolved organic matter in the tropical Atlantic Ocean. Global Biogeochem. Cycles 29, 677–690 (2015).
Moura, R. L. et al. An extensive reef system at the Amazon River mouth. Sci. Adv. https://doi.org/10.1126/sciadv.1501252 (2016).
Loick-Wilde, N. et al. Nitrogen sources and net growth efficiency of zooplankton in three Amazon River plume food webs. Limnol. Oceanogr. 61, 460–481 (2016).
Fernández, R. et al. Stable isotope analysis in two sympatric populations of bottlenose dolphins Tursiops truncatus: Evidence of resource partitioning?. Mar. Biol. 158, 1043–1055. https://doi.org/10.1007/s00227-011-1629-3 (2011).
Riccialdelli, L. & Goodall, N. Intra-specific trophic variation in false killer whales (Pseudorca crassidens) from the southwestern South Atlantic Ocean through stable isotopes analysis. Mamm. Biol. 80, 298–302 (2015).
Haro, D., Riccialdelli, L., Blank, O., Matus, R. & Sabat, P. Estimating the isotopic niche of males and females of false killer whales (Pseudorca crassidens) from Magellan Strait. Chile. Mar. Mammal Sci. https://doi.org/10.1111/mms.12564 (2018).
Bastida, R., Rodríguez, D. & Secchi, E. Mamiferos Acuaticos de Sudamerica y Antartida. (Vázquez Manzini Editores, 2007).
Cardoso, J., Francisco, A., de Souza, S. P. & Siciliano, S. Rough-toothed dolphins (Steno bredanensis) Along Southeastern Brazil: report of an anomalous pigmented juvenile and description of social and feeding behaviors. Aquat. Mamm. 45, 30–36 (2019).
Johns, W. E. et al. Annual cycle and variability of the North Brazil current. J. Phys. Oceanogr. 28, 103–128 (1998).
Gross, A., Kiszka, J., Canneyt, O. V., Richard, P. & Ridoux, V. A preliminary study of habitat and resource partitioning among co-occurring tropical dolphins around Mayotte, southwest Indian Ocean. Estuar. Coast. Shelf Sci. 84, 367–374 (2009).
Trites, A. W. W. Marine mammal trophic levels and interactions. In Encyclopedia of Ocean Sciences (eds Steele, J. H. et al.) 1628–1633 (Academic Press, Berlin, 2001). https://doi.org/10.1029/2002EO000342.
Ruiz-Cooley, R. I., Engelhaupt, D. T. & Ortega-Ortiz, J. G. Contrasting C and N isotope ratios from sperm whale skin and squid between the Gulf of Mexico and Gulf of California: Effect of habitat. Mar. Biol. 159, 151–164 (2012).
Goes, J. I. et al. Influence of the Amazon River discharge on the biogeography of phytoplankton communities in the western tropical north Atlantic. Prog. Oceanogr. 120, 29–40 (2014).
Montoya, J. P., Landrum, J. P. & Weber, S. C. Amazon River influence on nitrogen fixation in the western tropical North Atlantic. J. Mar. Res. 77, 191–213 (2019).
Beltrán-Pedreros, S. & Pantoja, T. M. A. Feeding habits of Sotalia fluviatilis in the Amazonian Estuary. Acta Sci. - Biol. Sci. 28, 389–393 (2006).
Giarrizzo, T., Schwamborn, R. & Saint-Paul, U. Utilization of carbon sources in a northern Brazilian mangrove ecosystem. Estuar. Coast. Shelf Sci. 95, 447–457. https://doi.org/10.1016/j.ecss.2011.10.018 (2011).
Crema, L. C., da Silva, V. M. F., Botta, S., Trumbore, S. & Piedade, M. T. F. Does water type influence diet composition in Amazonian manatee (Trichechus inunguis)? A case study comparing black and clearwater rivers. Hydrobiologia https://doi.org/10.1007/s10750-019-3900-4 (2019).
Lins, A. L. F. A., Gurgel, E. S. C., Bastos, M. N. C., Sousa, M. E. M. & Emin-Lima, R. Which aquatic plants of the intertidal zone do manatees of the Amazon estuary eat? Sirenews 11–12 (2014).
Ciotti, L. L., Luna, F. O. & Secchi, E. Intra- and interindividual variation in D13C and D15N composition in the Antillean manatee Trichechus manatus manatus from northeastern Brazil. Mar. Mammal Sci. https://doi.org/10.1111/mms.12102 (2014).
Sousa, M. E. M., Martins, B. M. L. & Fernandes, M. E. B. Meeting the giants: the need for local ecological knowledge (LEK) as a tool for the participative management of manatees on Marajó Island, Brazilian Amazonian coast. Ocean Coast. Manag. 86, 53–60 (2013).
Best, R. C. & Da Silva, V. M. F. Inia geoffrensis. Mamm. Species 1–8 (1993).
Costa, A. F. et al. How far does it go along the coast? Distribution and first genetic analyses of the boto (Inia geoffrensis) along the coast of Pará, Amazon, Brazil. In 2013 SC65a Meeting of The International Whaling Commission 1–12 (IWC/Scientific Commitee, Jeju Island, South Korea, Small Cetaceans, Cambridge, 2013).
Martin, A. R. R. & Da Silva, V. M. F. River dolphins and flooded forest: seasonal habitat use and sexual segregation of botos (Inia geoffrensis) in an extreme cetacean environment. J. Zool. Lond. 263, 295–305 (2004).
Da Silva, V. M. F., Goulding, M. & Barthem, R. Golfinhos da Amazônia. (INPA, 2008).
Di Beneditto, A. P. M. & Ramos, R. M. A. Biology of the marine tucuxi dolphin (Sotalia fluviatilis) in south-eastern Brazil. J. Mar. Biol. Assoc. United Kingdom 84, 1245–1250 (2004).
Pansard, K. C. A., Gurgel, H. D. C. B., Andrade, L. C. D. A. & Yamamoto, M. E. Feeding ecology of the estuarine dolphin (Sotalia guianensis) on the coast of Rio Grande do Norte, Brazil. Mar. Mammal Sci. 27, 673–687 (2011).
Smith, T. D. & Burrows, A. M. Mobility of the axial regions in a captive Amazon river dolphin (Inia geoffrensis). in Biology, Evolution, and Conservation of river dolphins within South America and Asia (eds. Ruiz-Garcia, M. & Shostell, J. M.) 71–81 (Nova Science Publishers, Inc., 2009).
Ramos, R. M. A. et al. Morphology of the Guiana dolphin (Sotalia guianensis) off southeastern Brazil: growth and geographic variation. Lat. Am. J. Aquat. Mamm. 8, 137–149 (2010).
Emin-Lima, R. Preenchendo Lacunas em Saúde de Ecossistemas: Estudo Morfológico e de Contaminantes nos Botos-cinza (Sotalia guianensis) da Costa Norte do Brasil. (Tese de Doutorado. Programa de Pós-Graduação em Saúde Pública e Meio Ambiente. Escola Nacional e Saúde Pública, FIOCRUZ, 147p., 2012).
Arcoverde, D. L. et al. Evaluation of periotic–timpanic bone complex of Sotalia guianensis (Cetacea: Delphinidae) as tool in identification of geographic variations. J. Mar. Biol. Assoc. United Kingdom 94, 1127–1132. https://doi.org/10.1017/S0025315413001689 (2013).
Botta, S. Caracterização do uso do habitat e identificação de Unidades Populacionais de pequenos cetáceos do Atlântico Sul-Ocidental através de métodos químicos. (Tese de Doutorado. PÓS-GRADUAÇÃO EM OCEANOGRAFIA BIOLÓGICA. UNIVERSIDADE FEDERAL DO RIO GRANDE - FURG, 238p., 2011).
Camargo, M. & Isaac, V. Os peixes estuarinos da região norte do Brasil: lista de espécies e considerações sobre sua distribuição geográfica. Bol. do Mus. Para. Emílio Goeldi, série Antropol. 17, 133–157 (2002).
Vieira, J. O. Diferenças alimentares em populações de boto-cinza Sotalia guianensis (Van Benédén, 1864) (Cetacea, Delphinidae) nas costas Norte e Nordeste brasileira. (Dissertação de Mestrado. Programa de Pós-Graduação em Zoologia. Universidade Federal do Pará, 2014).
Acknowledgements
The authors would like to thank the Grupo de Estudos de Mamíferos Aquáticos da Amazônia/MPEG staff of logistical and technical support of our research activities during all these years. A.F. Costa is indebted to Herbert Freitas (Quadrado) and Mário Serejo (Maíca) for their assistance in fieldworks at the Parnaíba Delta. Special thanks are due to Ingrid Clark for all support during activities at Projeto Cetáceos do Maranhão (PROCEMA). Thanks are also due to Rory Romero for assistance with isotope processing, and Allan Jameson for his immense help in the R analysis. We would also like to thank Seth Newsome for his valuable suggestions and advice on the stable isotope analysis and Viorel Atudorei from University of New Mexico. Financial support was provided by VALE/FAPESP/FAPESPA/FAPEMIG (Grant No. 038/2011), Projeto Bicho D’água and PROCEMA were sponsored by Petrobras. This paper was part of A.F.C.’s Ph.D. thesis in Aquatic Ecology and Fisheries from the Universidade Federal do Pará (Programa de Pós-Graduação em Ecologia Aquática e Pesca/PPGEAP/UFPA). A.F. Costa was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) during Ph.D. course and CNPq/Programa de Capacitação Institucional (MPEG/MCTI, Process No. 313522/20154) research Grant. S. Siciliano is supported by CNPq (Produtividade em Pesquisa, No. 306076/2019-5) and INOVA Fiocruz. T. Giarrizzo receives a productivity Grant from CNPq (No. 311078/2019-2). This publication was supported by Pró-Reitoria de Pesquisa e Pós-Graduação - PROPESP/UFPA (No. 01/2020 PAPQ).
Author information
Authors and Affiliations
Contributions
A.F.C. and T.G. performed data collection and specimen processing; A.F.C. designed and performed laboratory processing; A.F.C., S.B. and T.G. designed isotope analyses; A.F.C, SS and T.G. wrote and reviewed the manuscript; A.F.C., S.B., S.S. and T.G. All authors read, reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Costa, A.F., Botta, S., Siciliano, S. et al. Resource partitioning among stranded aquatic mammals from Amazon and Northeastern coast of Brazil revealed through Carbon and Nitrogen Stable Isotopes. Sci Rep 10, 12897 (2020). https://doi.org/10.1038/s41598-020-69516-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69516-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.