Abstract
Cold agglutinin disease (CAD) is a rare form of autoimmune haemolytic anaemia, and because of its rareness, there is no standard treatment for CAD patients. We retrospectively analysed the response to rituximab-containing therapy in CAD patients at our hospital. All patients received rituximab-containing therapy for at least 1 month. A total of 16 patients (11 males and 5 females) were included. The median age at the onset of the disease was 63.5 years (range 41–79). Most patients had manifestations including anaemia (81.3%) or cold-induced circulatory symptoms (75.0%). The median haemoglobin level was 72 g/L (range 29–101), and the median cold agglutinin titre was 1,024 (range 64–2,048). Thirteen of 16 patients (81%) responded to the therapy. Responders achieved a median increase in haemoglobin levels of 45 g/L. Grade 3–4 neutropenia occurred in 3 patients (19%), but only 1 (6%) of them experienced infection. Anaphylaxis related to rituximab occurred in 1 patient. During follow-up, five patients experienced relapse, and two patients died. The estimated median progression-free survival was 36 months, and median overall survival was not yet reached. In conclusion, A rituximab-based therapy in accordance with individual patient characteristics may be a reasonable choice for CAD patients.
Similar content being viewed by others
Introduction
Cold agglutinin disease (CAD) is an autoimmune haemolytic anaemia without other underlying diseases such as aggressive lymphoma, overt malignancies, or specific infections1,2. CAD is associated with a clonal lymphoproliferative disorder (LPD), producing monoclonal cold agglutinins (CA), usually immunoglobin (Ig) M autoantibodies2,3. CAs bind to type I carbohydrate antigen on the erythrocyte surface below normal central body temperatures, resulting in complement-mediated, classical pathway-dependent autoimmune haemolysis1,4,5. Extravascular destruction of C3b-coated erythrocytes is considered to be the predominant mechanism of erythrocyte breakdown in CAD patients6. As a rare disease, CAD accounts for approximately 15% of autoimmune haemolytic anaemias2. The most common clinical manifestations include anaemia, cold-induced acrocyanosis and Raynaud phenomena1.
At present, there is no approved treatment for CAD, and conducting randomized studies with sufficient statistical power will be difficult given the rareness of CAD. Non-pharmacological management, such as keeping warm, warm liquid infusions, and erythrocyte transfusions, is still a choice7,8,9. Reasonable criteria for beginning drug therapy should include symptomatic anaemia, transfusion dependence, and disabling circulatory symptoms1. Corticosteroids and other unspecific immune suppression have failed to demonstrate an acceptable effect3,8,10,11,12. The first breakthrough, rituximab monotherapy, 375 mg/m2 once a week for four weeks, resulted in a response rate of 50–60%, but with a response duration of only 11 months9,13,14,15. Subsequently, a combination of rituximab with fludarabine had a satisfactory response rate of 76%, with a complete remission rate of 21% and a long response duration, but toxicity is a concern16. In 2017, Berentsen et al. published their results in the use of bendamustine-rituximab therapy, which resulted in a similar response rate of 71%, with an even higher complete remission rate of 40% and sustained remissions, and the safety profile appeared more amenable than the fludarabine-rituximab combination17. Until recently, most effective therapies have been directed at pathogenic B-cells, and the response rate and response duration need further improvement; bendamustine-rituximab therapy or rituximab monotherapy should be considered first-line treatments15,18. However, none of these studies have been confirmed sufficiently.
Given the limitations in therapy choices for CAD, we have retrospectively analysed the efficacy of rituximab-containing therapies in the treatment of CAD patients at our hospital.
Methods
Patients
Patients diagnosed with CAD and who received any rituximab-containing therapy for at least 1 month between January 2012 and September 2019 at the Peking Union Medical College Hospital, were included in this retrospective study. The diagnosis of CAD was as previously described1: (i) Chronic haemolysis; (ii) Coombs testing strongly positive, especially for C3d; (iii) CA titre ≥ 64 at 4 °C. Patients associated with an aggressive lymphoma or infection were ineligible for the study. Clinical data, including age, sex, disease duration, clinical manifestations, physical examination results, laboratory test results, treatment and survival, were collected for all patients.
We detected MYD88L265P and CXCR4S338X mutations by real-time allele-specific oligonucleotide polymerase chain reaction using unsorted bone marrow samples before January 2018, as previously described19. Since January 2018, a next‐generation sequencing assay covering 69 genes was used to investigate recurrently mutated genes. Read pairs were aligned to Refseq hg19 (downloaded from UCSC Genome Browser, URLs) by Burrows–Wheeler Aligner (BWA) version 0.7.13-r1126. Samtools version 1.3 was used to generate chromosomal coordinate-sorted bam files. The mean depth of each sample was 1,000 ×, with an average of 5% of the target sequence being covered sufficiently deeply for variant calling. Samtools mpileup was applied for SNV/indel calling and filter workflow.
Waldenström macroglobulinemia (WM), splenic marginal zone lymphoma, and chronic lymphocytic lymphoma were diagnosed according to the World Health Organization classification20.
Treatment
All patients received one of the following rituximab-containing regimens: rituximab monotherapy (with or without prednisone, 375 mg/m2 once a week for four weeks), R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone), DRC (dexamethasone, rituximab and cyclophosphamide), RFC (rituximab, fludarabine and cyclophosphamide).
Response criteria
The response criteria were referenced from previously published papers1. The criteria for complete response (CR) were a disappearance of clinical symptoms of CAD, an absence of anaemia, no signs of haemolysis, and no monoclonal serum protein and no signs of clonal lymphoproliferation as assessed by bone marrow histology, immunohistochemistry, and flow cytometry. Partial response (PR) was defined as a stable increase in haemoglobin (Hgb) levels by at least 20 g/L or to the normal range, a reduction of serum IgM levels by at least 50% of the initial level or to the normal range, improvement of clinical symptoms and transfusion independence. Any patient not meeting the criteria for CR or PR was labelled no response (NR).
Overall survival (OS) was calculated from the date of initiation of treatment to the date of death or last follow-up. Progression-free survival (PFS) was defined as initiation of treatment until relapse, death or last follow-up. Relapse was defined as the need for re-treatment, as mentioned above. The final follow-up date was Sep 30, 2019.
Statistics
Median values were calculated for variables with non-normal distributions. The statistical significance of differences between paired continuous data was calculated with the Wilcoxon signed-rank test. OS and PFS were estimated according to Kaplan–Meier survival analysis. Statistical calculations were performed on a personal computer using SPSS version 24.0.0.0 software (SPSS Inc., Chicago, IL, USA). All figures were performed on a personal computer using GraphPad Prism version 8.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com.
Ethical approval and informed consent
Written informed consent was obtained from all individual participants included in the study. The protocol was approved by the Peking Union Medical College Hospital Ethics Committee. This study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Results
Baseline characteristics
Sixteen patients were included, 11 men and 5 women. The median age at the onset of clinical symptoms or anaemia was 63.5 years (range 41–79), and the median duration from the onset of clinical symptoms or anaemia until the time of diagnosis was 7.5 months (range 1–129). Detailed information for the patients included in this study is shown in Table 1. Cold-induced circulatory symptoms were found in 12 patients (75.0%), 13 patients (81.3%) had symptoms related to anaemia such as fatigue and weakness, and 5 patients (31.3%) had symptoms pertinent to haemolysis such as jaundice and haemoglobinuria. Five patients (31.3%) received erythrocyte transfusions before therapy. Twelve patients were diagnosed with indolent lymphoma, including 7 WM, 2 splenic marginal zone lymphoma, 1 chronic lymphocytic lymphoma and 2 indolent B-cell lymphomas that were unclassifiable according to the World Health Organization classification. At baseline, the median Hgb level was 72 g/L (range 29–101), the median reticulocyte percentage was 5.29% (range 1.02–11.38), the median total bilirubin was 30.4 μmol/L (range 6.2–154.2), the median indirect bilirubin was 21.0 μmol/L (range 2.2–85.0), and the median lactate dehydrogenase (LDH) was 280 U/L (range 124–517, normal range 0–250). The serum monoclonal Ig class was IgMκ in 15 patients (93.8%) and IgMλ in 1 patient. The median monoclonal immunoglobulin of 14 patients was 2.7 g/L (range 0.1–38.0), and the remaining 2 patients did not have monoclonal immunoglobulin tested at baseline. The median IgM level was 7.11 g/L (range 1.58–55.8). The results of Coombs testing were all positive; 13 patients had strong positive anti-C3 and anti-C3d antibodies, 1 was positive for anti-IgM antibodies, and the remaining 2 patients had unspecific results. All CA titres were ≥ 64 by definition, and the median CA titre was 1,024 (range 64–2,048). The MYD88L265P and CXCR4S338X mutations were tested for 4 patients by real-time allele-specific oligonucleotide polymerase chain reaction, and a next-generation sequencing assay was performed for the other 4 patients using their bone marrow. The MYD88L265P mutation was shown in 2 patients (25%), and both were diagnosed with WM simultaneously. None of the patients showed the CXCR4S338X mutation. Patient 5 and patient 16 showed mutations in TP53 and TET2, respectively, by the next‐generation sequencing assay, and patient 5 was diagnosed with unclassifiable indolent B-cell lymphoma simultaneously.
Treatment and response
Six patients received single-agent rituximab with or without prednisone, while the other 10 patients were treated with one of the rituximab-containing therapies: 1 R-CHOP, 2 R-CVP, 3 DRC/RFC combination and 4 DRC. The treatments resulted in 13 PRs (81%), of which 3 (19%) fulfilled all CR criteria except for the bone marrow examination (patients 4, 9, 14), and 3 NR (19%). The difference in the response rate between single-agent rituximab and the rituximab-containing therapies was not significant (P > 0.05).
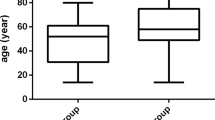
The response data are shown in Fig. 1. Peripheral circulatory symptoms improved by definition in all responders and in 1 of 3 non-responders. Hgb levels increased by a median of 45 g/L among the responders. In the responders, median total bilirubin levels decreased from 30.4 μmol/L (range 6.2–154.2) to 17.1 μmol/L (range 6.4–31.7), median indirect bilirubin levels from 21.0 μmol/L (range 2.2–85.0) to 11.6 μmol/L (range 4.7–19.6), and median LDH levels from 280 U/L (range 124–517) to 242 U/L (range 147–517). One of the responders had a normal baseline IgM concentration and did not have IgM retested after treatment; the median IgM decreased from 7.11/L (range 1.58–55.8) to 2.10 g/L (range 0.48–13.27) among responders.
Laboratory response data in the responders. Hgb, haemoglobin; IgM, immunoglobulin M. ※n = 12; one responder did not have immunoglobulin M retested during follow-up. (Figure was performed on a personal computer using GraphPad Prism version 8.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com.)
Among the non-responders, patients 6 and 16 were under treatment for 1 month and 2 months, respectively; patient 6 showed an improvement of anaemia-related symptoms, but the laboratory characteristics do not yet fulfil the criteria of PR. Patient 13 received supportive measures instead of second-line therapy during the study period, and his disease is now stable.
Adverse events
The most common adverse event was neutropenia grade 3–4 (n = 3, 19%): patient 7 had a fever requiring treatment, and the other 2 patients had no evidence of infection (patients 12, 13). For infusion-related events with rituximab, anaphylaxis occurred in 1 patient presenting as hypotension, receiving DRC treatment (dexamethasone, rituximab and cyclophosphamide), and he responded to saline infusion and finished the rituximab infusion (patient 14).
Follow-up
Median follow-up was 16 months (range 1–82), and the estimated median PFS was 36 months (Fig. 2). Five patients (31%; patients 1, 2, 7, 14, 15) experienced relapses, and 3 of them (patients 2, 7, 14) were treated with rituximab-containing therapy again. All 3 re-treatment patients underwent PR, and 1 of them fulfilled all CR criteria except the bone marrow examination (patient 14). Patient 1 died before further therapy could be administered. Patient 15 changed to Zanubrutinib, a novel inhibitor of Bruton tyrosine kinase, resulting in PR during the follow-up period.
Overall survival and progression-free survival among all patients. (Figure was performed on a personal computer using GraphPad Prism version 8.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com.)
Two patients died during follow-up. One responder (patient 1) died of disease progression 5 months after the initiation of treatment, and he did not have the chance to receive any further treatment. Patient 12 died of unknown reasons. The median OS was not yet reached.
Discussion
CAD is a rare form of haemolytic anaemia, and only a few studies have been conducted regarding its treatment. The pathogenesis of CAD is recognized as a clonal lymphoproliferative B-cell disorder and a complement-mediated immune haemolytic anaemia15. In a Norwegian study, CAD-associated LPD was found to be distinct from LPL, MZL, and other previously recognized lymphoma entities, with the absence of an MYD88L265P mutation21. However, the histological, immunophenotypic, and flow cytometric characteristics they described have not yet been confirmed by other studies. Our results show that some of the CAD patients had a clear diagnosis of WM or other indolent B-cell lymphoma, and the response was similar regardless of the concomitant LPD. The frequency of the MYD88L265P mutation was quite low among the WM patients in our group. However, whether CAD is an independent LPD is still inconclusive.
As for the treatment of CAD, we have to adopt expert opinions rather than results from standardized clinical trials. According to the studies Berentsen’s group conducted, the rituximab plus bendamustine combination therapy and rituximab monotherapy may be used as a first-line treatment15,17. Bortezomib-based therapy and rituximab plus fludarabine therapy were proven efficacious in prospective trials and case reports16,22,23,24. In this study, we analysed any rituximab-containing therapy among CAD patients in one institution. The response rate was 81% altogether in 16 patients who had not previously received rituximab, which confirmed previous findings that rituximab either as a monotherapy or in combination is effective in treating CAD13,14,16,17. Two NRs were recently treated with a short follow-up time, and their treatment may need more time to take effect. Remissions lasted longer than 12 months, and the expected response duration was 36 months. Furthermore, although the number of patients included was limited, rituximab-based treatment would probably take effect when re-treatment is required.
When comparing our study with the bendamustine-rituximab combination trial17, our patients’ baseline Hgb and LDH levels were lower, and other baseline characteristics, inclusion criteria and response criteria were similar. The overall response rates were similar, although the CR rate of our study was remarkably lower and is even lower in reality, and the observed response duration was shorter mainly because of our shorter observation time. Regarding the safety of the therapy, our study shows acceptable outcomes. Thus, we think it reasonable to choose a rituximab-based therapy in accordance with individual patient characteristics, weighing the efficacy and tolerance of the treatment.
Inhibition of the classical complement pathway is another approach to treat CAD patients. Complement inhibitors, such as eculizumab, sutimlimab, ANX005 and APL-2, are under study, and the most approved option is sutimlimab15. Recently, it was reported that sutimlimab can rapidly stop C1s complement–mediated haemolysis, increase Hgb levels and preclude the need for transfusions25. Complement modulations have limitations that it will not improve cold-induced symptoms and require continuous administration indefinitely to maintain their effect25. However, novel complement-directed therapies can still be very promising in the future, especially in severe cases and acute exacerbations18,26.
This study has several limitations. First, it is a single-institution retrospective study, and the number of patients included in the study was limited, which might limit the generalizability of our results. An expanded sample size is required for further analysis. Second, the choice of treatment mainly depends on the experience of the clinicians, and the rituximab-based treatment used in our study had marked heterogeneity. Therefore, it is difficult to determine which specific regimen is better, and further prospective trials are required to optimize the therapy.
Conclusion
In conclusion, our study shows a favourable effect of rituximab-containing therapy in patients with CAD.
References
Berentsen, S. & Tjonnfjord, G. E. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 26, 107–115. https://doi.org/10.1016/j.blre.2012.01.002 (2012).
Berentsen, S., Randen, U. & Tjonnfjord, G. E. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol. Oncol. Clin. N. Am. 29, 455–471. https://doi.org/10.1016/j.hoc.2015.01.002 (2015).
Berentsen, S. et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica 91, 460–466 (2006).
Gehrs, B. C. & Friedberg, R. C. Autoimmune hemolytic anemia. Am. J. Hematol. 69, 258–271. https://doi.org/10.1002/ajh.10062 (2002).
Shi, J. et al. TNT003, an inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinins. Blood 123, 4015–4022. https://doi.org/10.1182/blood-2014-02-556027 (2014).
Jaffe, C. J., Atkinson, J. P. & Frank, M. M. The role of complement in the clearance of cold agglutinin-sensitized erythrocytes in man. J. Clin. Invest. 58, 942–949. https://doi.org/10.1172/JCI108547 (1976).
Hill, Q. A. et al. The diagnosis and management of primary autoimmune haemolytic anaemia. Br. J. Haematol. 176, 395–411. https://doi.org/10.1111/bjh.14478 (2017).
Berentsen, S. How I manage patients with cold agglutinin disease. Br. J. Haematol. 181, 320–330. https://doi.org/10.1111/bjh.15109 (2018).
Ladogana, S. et al. Diagnosis and management of newly diagnosed childhood autoimmune haemolytic anaemia. Recommendations from the Red Cell Study Group of the Paediatric Haemato-Oncology Italian Association. Blood Transfus 15, 259–267. https://doi.org/10.2450/2016.0072-16 (2017).
Swiecicki, P. L., Hegerova, L. T. & Gertz, M. A. Cold agglutinin disease. Blood 122, 1114–1121. https://doi.org/10.1182/blood-2013-02-474437 (2013).
Barcellini, W. et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood 124, 2930–2936. https://doi.org/10.1182/blood-2014-06-583021 (2014).
Wu, Y. Y. et al. Clinical features and prognosis of 17 patients with primary cold agglutinin disease. Zhonghua Xue Ye Xue Za Zhi 38, 789–793. https://doi.org/10.3760/cma.j.issn.0253-2727.2017.09.011 (2017).
Berentsen, S. et al. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood 103, 2925–2928. https://doi.org/10.1182/blood-2003-10-3597 (2004).
Schollkopf, C. et al. Rituximab in chronic cold agglutinin disease: a prospective study of 20 patients. Leuk. Lymphoma 47, 253–260. https://doi.org/10.1080/10428190500286481 (2006).
Berentsen, S., Roth, A., Randen, U., Jilma, B. & Tjonnfjord, G. E. Cold agglutinin disease: current challenges and future prospects. J. Blood Med. 10, 93–103. https://doi.org/10.2147/JBM.S177621 (2019).
Berentsen, S. et al. High response rate and durable remissions following fludarabine and rituximab combination therapy for chronic cold agglutinin disease. Blood 116, 3180–3184. https://doi.org/10.1182/blood-2010-06-288647 (2010).
Berentsen, S. et al. Bendamustine plus rituximab for chronic cold agglutinin disease: results of a Nordic prospective multicenter trial. Blood 130, 537–541. https://doi.org/10.1182/blood-2017-04-778175 (2017).
Berentsen, S. Cold agglutinins: fending off the attack. Blood 133, 885–886. https://doi.org/10.1182/blood-2019-01-894303 (2019).
Cao, X. X. et al. Detection of MYD88 L265P and WHIM-like CXCR4 mutation in patients with IgM monoclonal gammopathy related disease. Ann. Hematol. 96, 971–976. https://doi.org/10.1007/s00277-017-2968-z (2017).
Swerdlow, S. H. et al. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues Revised 4. (IARC, Lyon, France, 2017).
Randen, U. et al. Primary cold agglutinin-associated lymphoproliferative disease: a B-cell lymphoma of the bone marrow distinct from lymphoplasmacytic lymphoma. Haematologica 99, 497–504. https://doi.org/10.3324/haematol.2013.091702 (2014).
Jacobs, A. Cold agglutinin hemolysis responding to fludarabine therapy. Am. J. Hematol. 53, 279–280. https://doi.org/10.1002/(SICI)1096-8652(199612)53:4<279::AID-AJH17>3.0.CO;2-7 (1996).
Carson, K. R., Beckwith, L. G. & Mehta, J. Successful treatment of IgM-mediated autoimmune hemolytic anemia with bortezomib. Blood 115, 915. https://doi.org/10.1182/blood-2009-09-242917 (2010).
Rossi, G. et al. Short course of bortezomib in anemic patients with relapsed cold agglutinin disease: a phase 2 prospective GIMEMA study. Blood 132, 547–550. https://doi.org/10.1182/blood-2018-03-835413 (2018).
Jager, U. et al. Inhibition of complement C1s improves severe hemolytic anemia in cold agglutinin disease: a first-in-human trial. Blood 133, 893–901. https://doi.org/10.1182/blood-2018-06-856930 (2019).
Shapiro, R., Chin-Yee, I. & Lam, S. Eculizumab as a bridge to immunosuppressive therapy in severe cold agglutinin disease of anti-Pr specificity. Clin. Case Rep. 3, 942–944. https://doi.org/10.1002/ccr3.399 (2015).
Acknowledgements
The authors would like to thank all the patients and their families who participated in this study. Institutional research funding was provided by Beijing Natural Science Foundation (Grant No.7202160 for X.X.C.).
Author information
Authors and Affiliations
Contributions
D.B.Z., X.X.C. and J.L. contributed to the conception and design of the study. M.N.J., Y.Q. and Y.Y.W. contributed to data collection. Y.Y.W. contributed to follow up patients. Y.Q. and H.C. did the technical work. M.N.J. wrote the paper. All authors revised the paper and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, Mn., Qiu, Y., Wu, Yy. et al. Rituximab-containing therapy for cold agglutinin disease: a retrospective study of 16 patients. Sci Rep 10, 12694 (2020). https://doi.org/10.1038/s41598-020-69465-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69465-2
This article is cited by
-
Practical therapy for primary autoimmune hemolytic anemia in adults
Clinical and Experimental Medicine (2022)
-
Rituximab
Reactions Weekly (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.