Abstract
To reveal the self-coordination mechanism of the fragile ecosystem of alpine tundra, we explored the relationship between soil microorganisms and other elements. On the alpine tundra of the Changbai Mountain, different vegetation types, altitudes and soil properties were selected as driving factors of soil microbial community. Soil microbial community, C- and N-cycling functional microbial and fungal biomass were analyzed. Structural equation model was used to study the control of biotic and abiotic factors in rhizosphere soil microbial community. The results showed that the pH value of soil had the strongest direct impact on the diversity and community structure of soil microorganisms, and had significant correlation with most of the C- and N-cycling functional microbial; organic carbon and vegetation also have strongest direct effect on fungal biomass, but all of them were not main factors influence soil microbial community structure, the elevation was the main controlling factor. In addition, the elevation mainly through indirect action affects the soil microbial community by driving distribution of plant species, soil organic carbon and pH value. This finding highlighted that elevation was the main predictor to determine rhizosphere microbial community structure but not vegetation in alpine tundra of Changbai Mountain.
Similar content being viewed by others
Introduction
Soil microorganism as one of the most important links in the biochemical cycle, can affect many important cycling processes of soil ecosystem1, such as carbon and nitrogen cycling in the ecosystem, transformation and cycling of soil nutrients (nitrogen, phosphorus, etc.). Therefore, changes in soil microbial community structure are often used as important sensitive indicators of changes in soil environment and nutrients2. Given that the influence of environmental factors on microorganisms can reveal fundamental problems in microbial ecology3,4, research on it will be of great significance for the function of soil ecosystem5.
Vegetation plays an important role in shaping the rhizosphere microbiome6,7. It has been found that narrow areas of soil around plant roots are affected by root activity and/or root exudates8, and that each vegetation type supports a rhizosphere-specific microbial community9,10. Thus, specific soil conditions under different vegetation types coexist with specific soil microbial communities11. As a frontier ecological topic in the field of microbial–environmental interaction12,13, a deeper understanding of the relationship between vegetation types and soil microbial communities may help us develop better strategies to protect the harsh environment of alpine tundra14.
Alpine tundra elevation gradients have also proven to be important when people try to understand the effects of environmental factors on the structure and distribution of microbial communities. A short elevation gradient on a high mountain can provide a serious environmental gradient. Therefore, the species number and richness of which decrease with the elevation rising15, and the composition and metabolic rate of soil microbial communities can also be regulated16. To date, some is known regarding the shift in microbial community structure and functional genes along an alpine climosequence17,18,19. Although the assessment of effects such as high turnover of aboveground vegetation, local soil conditions, and climate regimes on spatial patterns of microbial communities along elevation gradients20 has been performed, most experimental studies have focused on large-scale natural ecosystems or microbial communities along latitudinal gradients21,22 rather than across the range and scale of elevation gradients23,24.
Besides vegetation and elevation gradient, soil microbial is also sensitive to soil properties such as pH, moisture, nutrient amounts, and nitrogen/carbon (C/N) ratio25,26,27. Uroz et al. observed that bacterial community composition was strongly correlated with soil characteristics under different vegetation types27. Similarly, Shi et al. have recently observed that bacterial, fungal, and eukaryotic communities were correlated with ammonium concentration, dissolved organic nitrogen (DON) content, and C/N ratio in the Arctic tundra28. In addition, as important component of the soil microbial community, some studies have suggested that the biomass of fungi is significantly correlated with soil nutrients, because of relatively high amounts of chitin and protein in fungal cell walls and their biopolymers, thus representing an important source of both carbon (C) and nitrogen (N)29,30,31. The relationships between soil physicochemical characteristics and the development of plant and microbial successions have been subject of investigation for a long time27.
The above relationships are all interrelated. Vegetation along the mountainside elevation distribution may vary, when mirroring the latitudinal vegetation gradient from temperate to frigid zones on the Eurasian continent32,33. Soil properties such as pH and nutrient availability are also important drivers of structure, diversity, and functioning of both plant and microbial communities34,35,36. However, little studies taking all of them into account.
The objectives of this study were (1) to explore the relationship among vegetation, elevation, soil physicochemical properties and soil microbial communities, (2) to determine the main driving factor controlling the microbial community. Considering that climate change and the change of vegetation type which caused by alien species invasion can pose an important challenge to the environmental protection of tundra, our study should provide scientific basis for predicting the change trend of microbial community under different environmental conditions. Moreover, in view of the important position of soil microorganisms in the terrestrial ecosystem, to explore the relationship between soil microorganisms and other elements of the tundra ecosystem will help us to understand the self-coordination mechanism of the whole ecosystem and provide theoretical basis for environmental protection or disaster recovery.
In this study, the soil bacterial communities in the alpine tundra of Changbai Mountain were studied at different elevations (2,000–2,600 m) and under different conditions of vegetation rhizospheres and soil environments. Due to the plant rhizosphere soil as our research object which is specifically influenced by plant root activities and/or root exudates, we further hypothesized that vegetation may be the main controlling factors for microbial community composition in rhizosphere soil. Therefore, we set up two research groups along the elevation gradient to study the relationship between soil biological factors, abiotic factors and microbial communities under different dominant vegetation types and different mixed types of the same species (see “Experimental design” method). We used the sequencing method (Illumina MiSeq) to analyze the soil microbial community structure, real-time PCR to quantitatively analyze the nutrient cycling functional genes, and high-performance liquid chromatography (HPLC) to quantify the fungal biomass, and related it to the elevation, species investigation and measurement of soil variables to reveal the potential interaction.
Results
Rhizosphere soil bacterial community along the elevation gradient
Soil nutrients significantly varied across the elevation gradient (Supplementary Table S1); soil enzyme activity did not significantly vary, with the exception of urease activity. Ergosterol levels were the highest at the elevation 2,200 m and the lowest at 2,600 m (Supplementary Fig. S1), but fungal biomass did not differ significantly (P = 0.079). Correlation analyses (Supplementary Fig. S2) showed that fungal biomass was significantly positively correlated with TN (total nitrogen), TOC (total organic carbon), MBC (microbial biomass carbon), and sucrase (P ≤ 0.001). Assessment of the abundance of C- and N-cycling functional groups (bacterial aomA ammonia oxidizing bacteria functional gene, archaeal aomA ammonia oxidizing archaea functional gene, nifH nitrogen-fixing bacteria functional gene, nosZ denitrifying bacteria functional gene, cbbl carbon-fixing bacteria functional gene) revealed that only the abundances of archaeal aomA and nosZ significantly differed (P ≤ 0.05) among the seven elevations (Fig. 1A). Pearson´s correlation coefficient (r) for the abundances of C- and N-cycling function genes and soil properties showed that the functional genes were significantly (P ≤ 0.05) correlated with pH values, except for archaeal aomA genes (Fig. 1B–F). Among them, the abundance of archaeal aomA was correlated with elevation, soil moisture, and urease, the abundance of cbbl was positively correlated with SOC, C/N ratio, urease, and soil moisture and negatively correlated with NH4+. The abundance of nosZ was positively correlated with phosphatase and bacterial aomA was positively correlated with soil moisture (Supplementary Table S2).
(A) Real-time PCR quantification of the C- and N-cycling function genes (aomA bacterial amoA, Arch-amoA archaeal amoA, nifH nitrogen-fixing bacteria functional gene, nosZ denitrifying bacteria functional gene, cbbl carbon-fixing bacteria functional gene) in the bulk soil under seven elevations. The copy number of genes in each gram of dry soil was estimated based on the results of real-time PCR (copies per ng DNA). The standard curve of all these genes was > 0.99. Each sample was measured in triplicate. (B–F) Pearson correlations between C- and N-cycling function genes abundance and pH-value. (B) was bacterial amoA; (C) was archaeal amoA; (D) was nifH; (E) was nosZ and (F) was cbbl correlation with pH. Pearson correlations between C- and N-cycling function genes abundance and other indexes see Supplementary Table S2.
16S rRNA gene sequencing showed that alpha diversity was significantly different between the seven elevations, except in terms of the Simpson index (Table 1). The OTU richness exhibited a unimodal pattern with elevation, albeit with no significant differences (P = 0.255), and showed higher values for samples from lower or higher elevations than for samples from medium elevations (Supplementary Fig. S3).
In terms of the response of the bacterial community to the different elevations, Acidobacteria (33%) was the most dominant phylum, while Proteobacteria was the second most abundant phylum, accounting for 28% of all sequences; Actinobacteria were accounting for 17% (Fig. 2A). Testing by ANOSIM revealed that three dominant bacterial phyla (Bacteroidetes, Gemmatimonadetes, and Nitrospirae) showed significantly different abundances between different elevations (Supplementary Table S3). Nitrospirae was most abundant at 2,600 m, but non-existent at elevations of 2,500, 2,400, and 2,100. Non-metric multidimensional scaling (NMDS) ordination revealed microbial communities were significantly different (P = 0.011) between elevations (Fig. 3A). The microbial community at 2,600 m was separate from that of other elevations, but communities at 2,500 and 2,400 m were more similar. The microbial community at lower elevations (below 2,300 m) was not obviously different (Fig. 3A).
Relative abundances of the dominant bacterial phyla in soils separated according to elevation and species categories. Relative abundances are based on the proportional frequencies of those DNA sequences that could be classified at the phylum level. (A) was separated according to elevation categories; (B) was separated according to species categories at seven elevations.
Non-metric multidimensional scaling (NMDS) plot of community composition based on pyrosequencing of (A) microbial communities of seven elevations and (B) microbial communities of Da (the independent community of R. aureum Georgi), Da + S (R. aureum Georgi lives with other shrub), Da + H (R. aureum Georgi lives with herbaceous). Distances for (A,B) are based on weighted Unifrac scores. Bray-Stress for (A,B) are 0.134 and 0.082.
Different rhizosphere soil bacterial communities at similar elevations
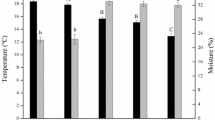
The results of the ANOVA showed the rhizosphere soil properties of different plants at the same altitude were different significantly. At 2,600, 2,500, 2,200, and 2,000 m, total nutrient levels at Da (the independent community of R. aureum Georgi) sites were higher than at other sites. However, soil pH was not significantly different among the three plant soils at same elevations of 2,600, 2,500, and 2,100 m. Soil enzymatic activities at Da sites were highest at 2,600 and 2,100 m (Supplementary Table S1). At the same elevations, the content of ergosterol was significantly different between the three plant soil samples (Supplementary Table S7). Among them, Pc, Rc, and Do had the highest ergosterol contents, respectively, at 2,500, 2,200, and 2,100 m; ergosterol contents of Da sites were higher than others at the remaining three elevations (Fig. 4A).
(A) Ergosterol (mg g−1) in rhizosphere soil for different species on the seven elevations. (B–F) Real-time PCR quantification of the C- and N-cycling function genes in the rhizosphere soil under different species on the seven elevations. (B) is bacterial amoA, (C) is archaeal amoA; (C) is nifH; (D) is nosZ and (F) is cbbl.
Abundance of C- and N-cycling functional groups in the rhizosphere soil, for bacterial amoA, was highest at 2,500 and 2,100 for Pc. At other elevations, highest abundance was found for Da sites. The abundance of archaeal amoA (Fig. 4B–F) was highest in Pc and Do sites at 2,500 and 2,300 m respectively; Da sites showed higher values than the two other sites at the remaining elevations. For nifH abundance, there was no significant difference among the three plant species at 2,600 m; Vs had the highest abundance at 2,200 m, at other three elevations, the highest abundance of nifH was found in Da sites. For nosZ abundance, values at 2,200 m were not significantly different between plant species. The rhizosphere soil of Da had the highest abundance of cbbl at elevations of 2,600, 2,500 and 2,200 m.
Taxonomic summary of the relative abundance of bacteria (Fig. 2B) showed that rhizosphere soil microbial communities were different between the three plant species at the same elevation. The rhizosphere soil of Da had a higher relative abundance of Proteobacteria (phyla) than other plant species at the same elevation, except at 2,400 m. The heatmap shows pairwise comparisons of the bacterial community structures between all samples (Fig. 5A) showed that the sites at 2,600 m were clustered into one clade. At 2,500, 2,400, and 2,300 m, the Da sites were separated from the other two sampling sites. Soil communities were clustered into different clades at lower altitudes. Hierarchical clustering analysis (Fig. 5B) for Da at seven elevations showed that 2,600 m and 2,400 m were clustered into one clade, Da at other elevations formed clustered into the other clade.
The rhizosphere soil bacterial community in different plant communities formed by the same vegetation
The rhizosphere soil properties of different plant communities formed by the same plant in the alpine tundra were not significantly different (Supplementary Table S4). The highest content of ergosterol in the soil was found for Da; Da + S (R. aureum Georgi lives with other shrub) had lower content and Da + H (R. aureum Georgi lives with herbaceous) the lowest (Supplementary Fig. S4).
The abundance of C- and N-cycling functional groups was assessed for three treatments. Treatment Da + H had the lowest abundance of nifH and cbbl genes, but the abundance of other functional genes was not significantly different between the three treatments (Supplementary Fig. S5). There was no significant difference in OTU richness among the three plant communities (Supplementary Fig. S6). Pearson correlation analysis indicated that OTU richness and fugal biomass were significantly intercorrelated and both were significant positively correlated with TOC and species degree (P < 0.05), but negative correlated with pH (P < 0.05) (Supplementary Table S5). Fugal biomass was also negatively correlated with elevation (P < 0.05). NMDS ordination revealed that there was no significant difference (P = 0.145) in microbial communities among Da, Da + S and Da + H (Fig. 3B). However, microbial communities at Da + S and Da + H sites were more similar than at Da. In the redundancy analysis (RDA) ordination biplot (Fig. 6A), fungal biomass and cover degree showed a significant correlation with community composition (Supplementary Table S6). Other factors, such as moisture, pH, elevation, and TOC also showed a high correlation with bacterial community composition (Supplementary Table S6).
(A) Redundancy analysis (RDA) triplots of 16S rDNA fingerprint patterns, showing the contribution of 14 environmental parameters to variability. Arrows indicate environmental factors and their relative effects on microbial community structure. The red triangles indicate Da; yellow triangles indicate Da + S; green triangles indicate Da + H. Eigenvalues of RDA1 and RDA2 are 0.336 and 0.2436. (B) A structural equation model (SEM) showing the causal influences of soil TOC, pH, species cover degree, elevation, OTU richness, fungal biomass and microbial communities in the soil. The width of arrows indicates the strength of the causal effect. The numbers above the arrows indicate path coefficients (*indicate significant (P < 0.05) effects, **indicate significant (P < 0.01) effects, ***indicate significant (P < 0.001) effects). Bold and dashed lines indicate positive and negative effects respectively. R2 values represent the proportion of the variance explained for each variable.
In order to further analyze the relationship between soil biological factors and non-biological factors, the structural equation model (SEM) was constructed. The path model on the controls of soil microbial community structure by dominant site factors passed all the statistical tests on adequacy (λ2 = 0.980, P = 0.806, CMIN /df = 0.327; GFI = 0.990; CFI = 1.000; RMSEA < 0.001) and explained 63%, 83%, and 77%, respectively, of the variance in the abundance of OTU richness, bacterial communities and fungal biomass. The path coefficients (λ) for direct effects are displayed in Fig. 6B. The direct, indirect, and total effects on bacterial communities and intermediate explanatory variables are shown in Table 2. Regarding the total effects, elevation (λ = 0.603) was the strongest predictor for microbial community composition in rhizosphere soil compared TOC, pH and vegetation coverage (Table 2). Regarding the direct effects, pH (λ = 0.850, P < 0.001) was the strongest direct predictor for microbial communities in rhizosphere soil, followed by fungal biomass (λ = − 0.704, P < 0.001) and OTU richness (λ = 0.441, P = 0.008). The direct effects of TOC (λ = 0.057, P > 0.05), elevation (λ = − 0.066, P > 0.05), and vegetation cover degree (λ = 0.060, P > 0.05) were relatively weak (Fig. 6B); these factors only influenced microbial communities mainly through the indirect path. Elevation was the strongest indirect predictor (mediated through pH, TOC, fungal biomass, and OTU richness), followed by pH (mediated through OTU richness and fungal biomass), vegetation cover degree (mediated through fungal biomass, pH, and OTU richness), and TOC (mediated through cover degree, fungal biomass, and OTU richness), whereas the effect of elevation, pH, vegetation cover degree, and TOC on bacterial communities was through a combination of both direct and indirect paths. Among them, soil TOC and the vegetation cover degree for fungal biomass had the largest direct effect (λ = 0.504, P < 0.05) of all parameters. Soil pH had a positive direct effect on microbial community structure, but a negative indirect one.
Discussion
Effects of vegetation on the microbial community
The interaction between plants and soil microbial is one of the forefront topics of international ecological research12,13. In our research, soil nutrients, fungal biomass, and nutrient cycling gene abundance of different plant rhizosphere soils were significantly different at similar altitudes. Host specificity to a species or group of related species is also well documented in plant-associated fungi37,38. Some studies have shown strong impacts of vegetation on the abundance of C- and N-cycling soil bacteria and archaea39. In addition, our result showed that N-fixing gene (nifH) and C-fixing gene (cbbl) abundance were significant different. Aureum plant community lives with other species. Further said that the variation of species have an impact on functional microbial abundance.
In-depth analysis showed that R. aureum Georgi rhizosphere soil had higher soil nutrient, fungal biomass, and C- and N-fixing gene abundance at most elevations compared to other species, as well as higher nutrient cycling rates. Plants and their microbiomes can be considered as “superorganisms”, partly due to their reliance on soil microbiota for specific functions and traits13,40. Plant species appear to be an important factor for soil N transformation in the alpine tundra, and, consequently, may influence plant community structures and ecosystem functions41,42. This might be one of the reasons why R. aureum was abundant at each altitude.
The microbial community structure in different vegetation rhizosphere soils at similar elevations was different. This is in agreement with the findings of previous studies that different vegetation types significantly affect the microbial community structure and functional diversity in rhizosphere soil43,44. Moreover, NMDS ordination revealed that mixing with herbaceous species resulted in higher dissimilarly than mixing with shrub species. Different plant types have different effects on the soil43,45, and soil microbial community structure over altitudinal gradients is mainly governed by changes in vegetation cover type46.
Effects of elevation gradients on the microbial community
Elevation strongly affects rhizosphere soil properties. The contents of TN, TOC, water content, C/N, urease decreased with the increase of elevation which is consistent with the previous research results45. There was no significant correlation between fungal biomass and elevation gradient. Similarly, previous studies have shown no significant difference in fungal biomass at different altitudes38. Our study found that with the decrease of altitude, the fungal biomass related to the same shrub root system increased. Margesin et al. also suggested that fungal biomass decreased with altitude. In the high altitude area, the environment condition is harsh, and the fungi associate with roots of the host plants, thereby increasing absorptive surface area and allowing enhanced uptake of essential nutrients and water47. Similarly, soil fungal biomass has been reported to vary along elevation gradients48,49. Although different fungal groups produce different levels of ergosterol (which may not exist in some fungal groups)50,51, few fungi (< 5% of OTUs) are found lacking ergosterol, and all communities have similar order-level composition38, which allows for comparison of biomass concentrations. Contrary to fungal biomass, C- and N-cycling gene abundances did not vary along elevation gradients, but there were significant differences in different vegetation types. Shen et al. suggest that soil microbial functional gene richness dramatically increases at the treeline ecotone from temperate forest to alpine tundra in the Changbai Mountains52. Different vegetation types with different litter input which can lead to difference in the composition of soil organic matter53. Tian et al. using the Biolog method found that labile organic carbon contribute to variation in functional microbial diversity54. This indicates that vegetation types drive the spatial distribution of functional gene richness along elevation gradients in alpine tundra. To the best of our knowledge, this finding has not been reported in a small-scale elevation gradient in alpine tundra studies.
Taxonomic richness does not follow the classic decreasing or unimodal elevation diversity patterns, which agrees with most studies finding non-significant elevation patterns52,55,56. Shen et al. observed that taxonomic richness linearly decreased with increased elevation and phylogenetic diversity exhibited a unimodal pattern with elevation57. This observation is not in agreement with our results, possibly because these authors were studying the bulk soil, while we focused on rhizosphere soil influenced by plant roots and a zone of high microbial activity, clearly distinct from bulk soil58. Here, we found similar bacterial communities at higher elevations (2,600, 2,500, and 2,400 m), but dissimilarities at lower elevations. Based on the results of the RDA, the bacterial community composition was significantly affected by altitude and three phyla (Bacteroidetes, Gemmatimonadetes, and Nitrospirae). Abundance of Nitrospirae, belonging to the nitrite-oxidizers, was highest at 2,600 m. Nitrifying bacteria appear significantly impacted by elevation and may be drivers of living at high altitude. Many researchers have found that bacterial or micro-eukaryotic composition varied with elevation55,5659,60. As a consequence, elevation is a main factor contributing to the overall bacterial community, but different vegetation types also interfere with the composition of the rhizosphere soil microbial community structure.
Effects of soil properties on the microbial community
There is accumulated evidence that soil physical and chemical properties are usually the best predictors of variations in microbial diversity42,61,62. Fungal biomass was significantly correlated with soil TOC, TN, MBC, and PH. José et al. stated that fungal abundance at higher altitudes was significantly and positively related to the high amounts of C (TOC and humus), EC, N, and mineral nutrients in subalpine and alpine environments63. The higher C, N, and P levels with increasing altitudes may contribute to enhanced microbial growth63. As previously observed, the bacterial diversity/structure and function was strongly influenced by soil properties34,52,64,65. In our research, correlation analyses demonstrated that C- and N-cycling gene (except ammonia-oxidizing archaea) abundance is associated with pH, some of them also correlated with soil moisture, TOC, C/N, and acid phosphatase. Previous studies have suggested that N-cycling is usually associated with NH4+ or NO3−, but we did not observe this in our study. This result was to be expected, since gene presence is not necessarily related to gene activity66. Similar studies have reported that N-cycling functional genes were contingent on local site characteristics such as soil nutrients, soil temperatures, and site latitude66,67,68.
Similar to N-cycling genes, soil pH also has a stronger influence on soil microbial community structure than some other parameters. This agrees with other studies which found that the dominant bacterial taxonomic diversity/structure was strongly influenced by soil pH62,69. Here, pH was significantly correlated with OTU richness, fungal biomass, and other soil properties, especially soil TOC, which strongly influenced microbial community.
In summary, our study reveals complex impacts of the microenvironment by various elevation, species cover degree, and soil properties and their interactive effects, using the SEM. Rhizosphere soil microbial community composition was most strongly influenced by fungal biomass, followed by elevation. Elevation, species cover degree, and soil TOC had little direct effect on the microbial composition, while TOC and species cover degree were most strongly affected by fungal biomass. Elevation also had a strong effect on fungal biomass. Previous studies have demonstrated no significant variation in fungal biomass between elevation zones; instead, ergosterol varied according to host species type70 and fungal richness was primarily affected by host species identity71. Moreover, fungal richness was governed by host-associated factors such as soil nutrients3849. Our results indicate that the soil TOC, species cover degree, and elevation indirectly and significantly impact the microbial community structure. Regarding the effect of pH, it had the strongest impact on the microbial community, acting significantly, albeit indirectly. This is in agreement with previous studies in which soil pH did not alter the microbial community itself, but directly or indirectly interacted with other soil parameters36,63.
Soil properties (TOC and pH) have the greatest impact on the abundance of OTU, which directly affects the microbial community structure. Overall, soil variables often indirectly affect microbial diversity/community. Although rhizosphere soil fungi have a strong and direct impact on microbial community structure, the impact is controlled by host species and soil properties. The overall effect of plant species coverage on microbial diversity/community was significant, indicating that plant species can shape rhizosphere microenvironment. In addition to microbial factors, altitude, vegetation and soil properties affect each other. The altitude and species coverage have the greatest influence on soil pH. Root exudates mainly include mucilage, ecotoenzymes, organic substances, sugars, and various kinds of amino acids. They can significantly improve soil structure, cause soil mineral weathering, increase soil cation exchange capacity, and affect soil pH value, soil surface adsorption characteristics and soil biological characteristics72. Therefore, different plant communities have different effects on pH value of rhizosphere soil73.
Although vegetation has a strong impact on microbial community structure, altitude as an abiotic factor has the greatest impact on microbial community structure. This is contrary to our initial hypothesis that species may be the most important factor affecting the microbial community structure of rhizosphere soil. The altitude pattern of plant species in Changbai Mountain has been reported previously33. With the increase of altitude, the environment becomes harsher and harsher, which, among other things, results in nutritional stress, which leads to great changes in plant community structure74. As pH value, soil nutrients and vegetation are widely considered as the main driving factors of soil bacteria. The results showed that the elevation was the main factor affecting the microorganism, and the distribution of plant species, soil organic carbon and pH value was driven indirectly.
Conclusion
Our results revealed the relationship between vegetation types, elevation, soil properties and soil microbial communities on Changbai Mountain, and found that elevation was the main controlling factor. To the best of our knowledge, this is the first study to reveal that elevation is the main predictor of rhizosphere microbial community structure rather than vegetation in the alpine tundra. It should provide a basis for understanding and predicting species succession and stability of alpine tundra ecosystem.
Material and methods
Study area
We performed our sampling in the Changbai Mountains National Nature Reserve (41° 41′ 49″–42° 25′ 18″ N, 127° 42′ 55″–128° 16′ 48″ E). Precisely, the study area is on the north slope of the alpine tundra (42° 01′–42° 05′ N, 128° 03′–128° 07′ E) at an elevation between 2,000 and 2,600 m. Such area is characterized by short growing season (June–September), low temperature (3 °C–7 °C in the growing season) and heavy precipitation (exceeds 1,400 mm annually)75. Common plants are dominated by shrubs such as D. octopetala L. var. asiatica, Rhododendron aureum Georgi, Vaccinium uliginosum Linn, and tussocks such as Trollius chinensis, Sanguisorba tenuifolia var. alba, Rhodiola cretinii (Hamet) H. Ohba subsp. sino-alpina (Frod.) H. Ohba, Ligularia jamesii (Hemsl.) Kom, etc76,77. The soil is mountain tundra soil, the development of which is very low. The soil layer is thin, and not connected into pieces. Soil profile: 1–5 cm is peat layer, 5–10 cm is peat gravel layer, and 10–30 cm is volcanic rock layer.
Experimental design
The experimental design was set up two research groups. The first group was used to analyze the effects of altitude and vegetation types on soil microbial community. We set 7 altitudes and at each altitude we selected 3 dominant species communities as research object. So we selected 21 sample locations in all. The coverage of each dominant species in the community was higher than 85%. We selected R. aureum Georgi communities at each altitude, because it is the only woody plant with a distribution at each elevation. The other two dominant species plant communities are different at the seven elevations. Detailed distributions can be seen in Supplementary Table S7.
The second research group was used to analyze the direct or indirect effects of altitude, species coverage, and soil physicochemical characteristics on soil microbial community. We set three treatments: The independent community of R. aureum Georgi (Da), R. aureum Georgi lives with other shrub (Da + S) and R. aureum Georgi lives with herbaceous (Da + H). Da + S sites had less than 5% coverage of herbaceous plants, and Da + H sites less than 5% coverage of other shrub. In the three treatments, R. aureum Georgi had different cover degree from 40 to 95%, the detailed see Supplementary Table S8. We selected 8 locations of Da + S, 4 locations of Da + H, and the sits of Da in the first and second group are the same locations. Detailed distributions can also be seen in Supplementary Table S8. Therefore, the total sample location number of this research is 33.
Soil sampling
Soil samples were collected in July of 2014. We randomly selected three 1 × 1 m plots in each location and extracted approximately 500 g soil. As a result, the study included 99 plots. In each plot, we removed the litter layer of the soil and collected plant affected soil samples with five replicates (5 cm diameter × 10 cm deep). As most the plants in the sampling areas grew at relatively high densities (especially for Rhododendron aureum Georgi, Sanguisorba tenuifolia var, Ligularia jamesii), the collected soil was originally only a few millimeters away from the root axis surface, hence they seem to have great impacts on the surrounding soil. Therefore, the collected soil samples were defined as “rhizosphere soil samples”78. Except for the 16rDNA sequencing of microbial communities, all the results in this paper are based on the analysis of those 99 plots. By mixing all samples collected from the same location, we sequenced 33 composite soil samples. The collected samples were refrigerated at 4 °C immediately and have been transported to the laboratory within 4 h. They were further sieved by 2 mm mesh sieve and have been assigned into two groups. Half of the samples were stored at 4 °C for chemical analysis and the other half at − 80 °C for microbial community analyses.
Analysis of soil characteristics and fungal biomass
Soil moisture was measured by the gravimetrical mass loss after drying samples to a constant weight (105 °C for at least 12 h). We measured pH by using a pH meter on a 1:10 (w/v) ratio in distilled water. Soil organic matter was determined by dichromate oxidation with external heat and titration with ferrous ammonium sulphate79. TN was determined by semimicro-Kjeldahl (KDY-9820) digestion80. Soil total phosphorus (TP) was determined colorimetrically using the molybdate method81. MBC and microbial biomass nitrogen (MBN) were determined using the chloroform fumigation extraction method82. Soil nitrate nitrogen and ammonium nitrogen were analyzed using a flow-injection autoanalyzer (SKALAR SAN+ +, Netherlands). Phosphatase activity was measured using the modified method of Schinner and von Mersi83. Soil catalase activity was measured using the 0.1 N KMnO4 titration method84. Urease activity was determined according to Klose and Tabatabai85. Invertase activity was assayed using the 3,5-dinitrosalicylic acid technique86.
Fungal biomass was determined by HPLC analyzing ergosterol content in the soil87. Briefly, ergosterol was extracted by KOH, methanol, and ethanol from 3 g of fresh soil (70 °C water bath for 30 min). After n-hexane extraction, concentration, and solvent replacement, ergosterol content was quantified by HPLC (AGILENT 1,200, USA; reverse C18 column; mobile phase, 95% methanol; flow rate, 1 min/ml; detection wavelength, 282 nm).
Real-time PCR
In order to eliminate the internal variability within each site, we performed 10 replications in the mixed soil samples. Each DNA sample was extracted using 0.3 g freeze-dried soil with the Power Soil DNA Isolation Kit (MOBIO), according to the manufacturer’s protocol. Due to the qPCR is extremely sensitive and vulnerable to interference by humic material. Extracted DNA was purified using the GV-High-Efficiency Agarose Gel DNA Purification Kit (BEI JING DINGGUO CHANGSHENG BIOTECHNOLOGY CO.LTD., China), DNA concentrations were determined using the Qubit quantification platform with QUANI-IT ESDNA BR Assay Kit (INVITROGEN). DNA was diluted to 10 ng/μl and stored at − 80 °C prior to molecular analysis.
The abundances of genes (cbbl, nifH, archaeal amoA, bacterial amoA, nosZ) encoding the key enzymes for biological C- and N-cycling were quantified by real-time PCR (IBA7500) for all soil samples. The primers N-cycling functional genes were reference by Mao et al. and Levy-Booth et al.34,88, and C-cycling functional genes were reference by Yuan et al.89 (details see Supplementary Table S9). Standards for the qPCR assays were generated by PCR products from a common DNA mixture (equal amounts of DNA from all samples)90. PCR product was diluted to 100 ng/μl and set five concentration gradients in 10 times diluted. For each sample-derived standard, copy number concentration was calculated based on the length of the PCR product and the mass concentration (measured by Qubit). The 20 μl reaction mixture contained 0.2 μl of each primer (20 μM), 10 μl of 2 × Trans Start Green qPCR Super Mix (TRANSGEN BIOTECH, China), 0.4 μl Passive Reference Dye (50 ×), 0.3 μl of BSA (10 mg/ml), and 10 ng of DNA template.
Bioinformatics analysis
The 16SrDNA high-throughput sequencing was performed by the REALBIO Genomics Institute (Shanghai, China) using the ILLUMINA MiSeq platform. The 16S V3–V4 region was amplified using the primers U341F (ACT CCT ACG GGA GGC AGC AG) and U806R (GGA CTA CHV GGG TWT CTA AT). The raw data were then subjected to a quality control procedure using UPARSE91. The qualified reads were clustered to generate OTUs at the 97% similarity level using USEARCH92. A representative sequence for each OTU was assigned to a taxonomic level in the RDP database by the RDP classifier93.
A total of 1.8 million high-quality 16S rRNA gene sequence reads were obtained from 33 samples. There were a total of 29,607 distinct OTUs (operational taxonomic units) across samples, with a total of 87,156 unique reads that were assigned to these OTUs. In QIIME, sequences were subsampled to an even depth of 20,067 reads prior to estimating relative taxon abundances and running diversity analyses. Alpha diversity was measured using Chao1, observed species, Shannon, Simpson, goods coverage, and PD whole tree.
Statistical analysis
All analyses were performed in the corrplot package in R94. For soil parameters, one-way analysis of variance (ANOVA) was used to determine differences among elevation treatments and different vegetation treatments. Two-way analysis of variance (ANOVA) was used to the determine differences among elevation × vegetation treatments. The correlations between the abundance of N-cycling functional genes, diversity metrics, and OTU richness and fungal biomass were correlated with soil characteristics using Pearson correlations. Statistical significance was set at P < 0.05.
NMDS was performed via the vegan package of R v.3.1.1 project95 (Weighted Unifrac scores). We also used analysis of similarities (ANOSIM, 999 permutations) to evaluate the null hypothesis. Heatmaps and clustering tree were constructed using ggplot2 package in R. RDA was performed to determine the environmental factors that significantly correlated with community composition (abundance of OTUs), via the function envfit (999 permutations) in the vegan package of R v.3.1.1 project96,97. SEM was constructed via AMOS 17.098 and the ‘vegan’ package in R. Only abiotic factors variables that were both significant correlated (P < 0.05) with OTU richness, fungal biomass, elevation and vegetation coverage were included in the model construction, namely soil TOC and pH. We tested the fitness of the model with the data using the maximum likelihood (λ2) goodness-of-fit test, Jöreskog’s GFI, the Bollen–Stine bootstrap test and RMSEA.
References
Ruan, H., Zou, X., Scatena, F. & Zimmerman, J. Asynchronous fluctuation of soil microbial biomass and plant litterfall in a tropical wet forest. Plant Soil 260, 147–154 (2004).
Ibekwe, A. M. et al. Impact of fumigants on soil microbial communities. Appl. Environ. Microbiol. 67, 3245–3257 (2001).
Reiss, J., Bridle, J. R., Montoya, J. M. & Woodward, G. Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 24, 505–514 (2009).
Fuhrman, J. A. & Fuhrman, J. A. Microbial community structure and its functional implications. Nature 459, 193–199 (2009).
Lanzen, A. et al. Multi-targeted metagenetic analysis of the influence of climate and environmental parameters on soil microbial communities along an elevational gradient. Sci. Rep. 6, 28257. https://doi.org/10.1038/srep28257 (2016).
Berendsen, R. L., Pieterse, C. M. J. & Bakker, P. A. H. M. The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486 (2012).
Oh, Y. M. et al. Distinctive bacterial communities in the rhizoplane of four tropical tree species. Microb. Ecol. 64, 1018–1027 (2012).
Walker, T. S., Bais, H. P., Grotewold, E. & Vivanco, J. M. Root exudation and rhizosphere biology. Plant Physiol. 132, 44–51 (2003).
Herre, E. A. Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466, 752–755 (2010).
Philippot, L., Raaijmakers, J. M., Lemanceau, P. & Wh, V. D. P. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11, 789–799 (2013).
Hackl, E., Pfeffer, M., Donat, C., Bachmann, G. & Zechmeister-Boltenstern, S. Composition of the microbial communities in the mineral soil under different types of natural forest. Soil Biol. Biochem. 37, 661–671 (2005).
Park, S. et al. Principal component analysis and discriminant analysis (PCA–DA) for discriminating profiles of terminal restriction fragment length polymorphism (T-RFLP) in soil bacterial communities. Soil Biol. Biochem. 38, 2344–2349 (2006).
Mendes, R. et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332, 1097–1100 (2011).
Klimeš, L. Alpine plant life. Functional plant ecology of high mountain ecosystems by C. Körner. Folia Geobot. 41, 454–455 (2006).
Diaz, H. F., Grosjean, M. & Graumlich, L. Climate variability and change in high elevation regions: Past, present and future. Clim. Change 59, 1–4 (2003).
Schinner, F. & Gstraunthaler, G. Adaptation of microbial activities to the environmental conditions in alpine soils. Oecologia 50, 113–116 (1981).
Cui, H.-J. et al. Soil microbial community composition and its driving factors in alpine grasslands along a mountain elevational gradient. J. Mt. Sci. 13, 1013–1023. https://doi.org/10.1007/s11629-015-3614-7 (2016).
Zhang, B., Liang, C., He, H. & Zhang, X. Variations in soil microbial communities and residues along an altitude gradient on the northern slope of changbai mountain, china. PLoS ONE 8, e66184 (2013).
Collins, C. G., Carey, C. J., Aronson, E. L., Kopp, C. W. & Diez, J. M. Direct and indirect effects of native range expansion on soil microbial community structure and function. J. Ecol. 104, 1271–1283 (2016).
Margesin, R., Jud, M., Tscherko, D. & Schinner, F. Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol. Ecol. 67, 208–218 (2009).
Fierer, N. & Mcculley, R. L. Reconstructing the microbial diversity and function of pre-agricultural tallgrass prairie soils in the United States. Science 342, 621–624 (2013).
Shi, Y. et al. Multi-scale variability analysis reveals the importance of spatial distance in shaping Arctic soil microbial functional communities. Soil Biol. Biochem. 86, 126–134 (2015).
Yang, Y. et al. The microbial gene diversity along an elevation gradient of the Tibetan grassland. Isme J. Multidiscipl. J. Microb. Ecol. 8, 430–440 (2013).
Ding, J. et al. Integrated metagenomics and network analysis of soil microbial community of the forest timberline. Sci. Rep. 5, 7994 (2015).
Liu, J. et al. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 70, 113–122 (2014).
Brockett, B. F. T., Prescott, C. E. & Grayston, S. J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 44, 9–20 (2012).
Uroz, S., Tech, J. J., Sawaya, N. A., Frey-Klett, P. & Leveau, J. H. J. Structure and function of bacterial communities in ageing soils: Insights from the Mendocino ecological staircase. Soil Biol. Biochem. 69, 265–274 (2014).
Shi, Y. et al. Vegetation-associated impacts on arctic tundra bacterial and microeukaryotic communities. Appl. Environ. Microbiol. 81, 492–501 (2014).
Zeglin, L. H. & Myrold, D. D. Fate of decomposed fungal cell wall material in organic horizons of old-growth douglas-fir forest soils. Soil Sci. Soc. Am. J. 77, 489–500 (2013).
Wallander, H., Göransson, H. & Rosengren, U. Production, standing biomass and natural abundance of 15N and 13C in ectomycorrhizal mycelia collected at different soil depths in two forest types. Oecologia 139, 89–97 (2004).
Colpaert, J. V., Laere, A. V. & Assche, J. A. V. Carbon and nitrogen allocation in ectomycorrhizal and non-mycorrhizal Pinus sylvestris L. seedlings. Tree Physiol. 16, 787–793 (1996).
Zhang, M. et al. Distribution of soil organic carbon fractions along the altitudinal gradient in Changbai mountain, China. Pedosphere 21, 615–620 (2011).
Wenduo, X. U., Xingyuan, H. E., Chen, W. & Liu, C. Characteristics and succession rules of vegetation types in Changbai mountain. Chin. J. Ecol. 23, 162–174 (2004).
Mao, Y., Yannarell, A. C., Davis, S. C. & Mackie, R. I. Impact of different bioenergy crops on N-cycling bacterial and archaeal communities in soil. Environ. Microbiol. 15, 928–942 (2012).
Grayston, S. J. et al. Assessing shifts in microbial community structure across a range of grasslands of differing management intensity using CLPP, PLFA and community DNA techniques. Appl. Soil. Ecol. 25, 63–84 (2004).
Lauber, C. L., Hamady, M., Knight, R. & Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 75, 5111–5120 (2009).
Tedersoo, L. et al. Towards global patterns in the diversity and community structure of ectomycorrhizal fungi. Mol. Ecol. 21, 4160–4170 (2012).
Davey, M. L., Heegaard, E., Halvorsen, R., Kauserud, H. & Ohlson, M. Amplicon-pyrosequencing-based detection of compositional shifts in bryophyte-associated fungal communities along an elevation gradient. Mol. Ecol. 22, 368–383 (2013).
Mao, Y., Yannarell, A. C. & Mackie, R. I. Changes in N-transforming archaea and bacteria in soil during the establishment of bioenergy crops. PLoS ONE 6, e24750 (2011).
Heijden, M. G. A. V. D., Bardgett, R. D. & Straalen, N. M. V. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310 (2008).
Steltzer, H. & Bowman, W. D. Original articles: Differential influence of plant species on soil nitrogen transformations within moist meadow alpine Tundra. Ecosystems 1, 464–474 (1998).
Shen, C., Ni, Y., Liang, W., Wang, J. & Chu, H. Distinct soil bacterial communities along a small-scale elevational gradient in alpine tundra. Front. Microbiol. 6, 538–541 (2014).
Zhang, C., Liu, G. B., Xue, S. & Xiao, L. Effect of different vegetation types on the rhizosphere soil microbial community structure in the loess plateau of China. J. Integr. Agric. 12, 2103–2113 (2013).
Weand, M. P., Arthur, M. A., Lovett, G. M., Mcculley, R. L. & Weathers, K. C. Effects of tree species and N additions on forest floor microbial communities and extracellular enzyme activities. Soil Biol. Biochem. 42, 2161–2173 (2010).
Wu, Z. et al. Terminal restriction fragment length polymorphism analysis of soil bacterial communities under different vegetation types in subtropical area. PLoS ONE https://doi.org/10.1371/journal.pone.0129397 (2015).
Singh, D. et al. Strong elevational trends in soil bacterial community composition on Mt. Halla, South Korea. Soil Biol. Biochem. 68, 140–149 (2014).
Fallen, M. Linking water and nutrients through the vadose zone: A fungal interface between the soil and plant systems. J. Arid Land 206, 155–163 (2011).
Grayston, S. J., Griffith, G. S., Mawdsley, J. L., Campbell, C. D. & Bardgett, R. D. Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biol. Biochem. 33, 533–551 (2001).
Studies, R. Forest floor properties across sharp compositional boundaries separating trembling aspen and jack pine stands in the southern boreal forest. Plant Soil 345, 353–364 (2011).
Ruzicka, S., Edgerton, D., Norman, M. & Hill, T. The utility of ergosterol as a bioindicator of fungi in temperate soils. Soil Biol. Biochem. 32, 989–1005 (2000).
Weete, J. D., Abril, M. & Blackwell, M. Phylogenetic distribution of fungal sterols. PLoS ONE 5, e10899 (2010).
Shen, C. et al. Dramatic increases of soil microbial functional gene diversity at the treeline ecotone of Changbai mountain. Front. Microbiol. https://doi.org/10.3389/fmicb.2016.01184 (2016).
Quideau, S. A., Chadwick, O. A., Benesi, A., Graham, R. C. & Anderson, M. A. A direct link between forest vegetation type and soil organic matter composition. Geoderma 104, 41–60 (2001).
Tian, J. et al. Linkages between the soil organic matter fractions and the microbial metabolic functional diversity within a broad-leaved Korean pine forest. Eur. J. Soil Biol. 66, 57–64 (2015).
Yuan, Y., Si, G., Jian, W., Luo, T. & Zhang, G. Bacterial community in alpine grasslands along an altitudinal gradient on the Tibetan plateau. FEMS Microbiol. Ecol. 87, 121–132 (2013).
Fierer, N. et al. Microbes do not follow the elevational diversity patterns of plants and animals. Ecology 92, 797–804 (2011).
Shen, C., Ni, Y., Liang, W., Wang, J. & Chu, H. Distinct soil bacterial communities along a small-scale elevational gradient in alpine tundra. Front. Microbiol. 6, 582. https://doi.org/10.3389/fmicb.2015.00582 (2015).
Hinsinger, P., Bengough, A. G., Vetterlein, D. & Young, I. M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 321, 117–152 (2009).
Shen, C. et al. Contrasting elevational diversity patterns between eukaryotic soil microbes and plants. Ecology 95, 3190–3202 (2014).
Jarvis, S. G., Woodward, S. & Taylor, A. F. S. Strong altitudinal partitioning in the distributions of ectomycorrhizal fungi along a short (300 m) elevation gradient. New Phytol. 206, 1145–1155 (2015).
Lanzén, A. et al. Multi-targeted metagenetic analysis of the influence of climate and environmental parameters on soil microbial communities along an elevational gradient. Sci. Rep. 6, 28257 (2016).
Shen, C. et al. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai mountain. Soil Biol. Biochem. 57, 204–211 (2013).
Siles, J. A. & Margesin, R. Abundance and diversity of bacterial, archaeal, and fungal communities along an altitudinal gradient in alpine forest soils: What are the driving factors?. Microb. Ecol. 72, 207–220 (2016).
Sagovamareckova, M., Cermak, L., Omelka, M., Kyselkova, M. & Kopecky, J. Bacterial diversity and abundance of a creek valleysites reflected soil pH and season. Open Life Sci. https://doi.org/10.1515/biol-2015-0007 (2015).
Smith, J. L., Halvorson, J. J. & Bolton, H. Soil properties and microbial activity across a 500m elevation gradient in a semi-arid environment. Soil Biol. Biochem. 34, 1749–1757 (2002).
Yergeau, E., Kang, S., He, Z., Zhou, J. & Kowalchuk, G. A. Functional microarray analysis of nitrogen and carbon cycling genes across an Antarctic latitudinal transect. ISME J. 1, 163–179 (2007).
Kai, X. et al. Warming alters expressions of microbial functional genes important to ecosystem functioning. Front. Microbiol. 7, 668 (2016).
Jing, C. et al. Available nitrogen is the key factor influencing soil microbial functional gene diversity in tropical rainforest. BMC Microbiol. 15, 397–398 (2015).
Griffiths, R. I. et al. The bacterial biogeography of British soils. Environ. Microbiol. 13, 1642–1654 (2011).
Davey, M. L., Nybakken, L., Kauserud, H. & Ohlson, M. Fungal biomass associated with the phyllosphere of bryophytes and vascular plants. Mycol. Res. 113, 1254–1260 (2009).
Meier, C. L., Rapp, J., Bowers, R. M., Silman, M. & Fierer, N. Fungal growth on a common wood substrate across a tropical elevation gradient: Temperature sensitivity, community composition, and potential for above-ground decomposition. Soil Biol. Biochem. 42, 1083–1090 (2010).
Tu, S., Sun, J., Guo, Z. & Gu, F. On relationship between root exudates and plant nutrition in rhizosphere. Soil Environ. 9, 64–67 (2000).
Zhang, C., Liu, G., Xue, S. & Song, Z. Rhizosphere soil microbial activity under different vegetation types on the Loess Plateau, China. Geoderma 161, 115–125 (2011).
Zeng, S., Zhiyao, S. U., Chen, B. & Yuanchun, Y. U. A review on the rhizosphere nutrition ecology research. J. Nanjing For. Univ. 27, 79 (2003).
Wei, Z., Xiaojuan, Q. I., Jianwei, L., Zhengxiang, Y. U. & Xia, C. Characterization of microbial community structure in rhizosphere soils of cowskin Azalea (Rhododendron aureum Georgi) on northern slope of Changbai mountains, China. Chin. Geogr. Sci. 26, 78–89 (2016).
Yang, X. & Wu, G. The strategy for conservation and sustainable utilization of biodiversity in Changbaishan biosphere reserve. J. For. Res. 9, 217–222 (1998).
Zong, S. et al. Analysis of the process and impacts of Deyeuxia angustifolia invasion on the Alpine Tundra, Changbai mountain. Acta Ecol. Sin. 34, 87–104 (2014).
Batten, K. M., Scow, K. M., Davies, K. F. & Harrison, S. P. Two invasive plants alter soil microbial community composition in serpentine grasslands. Biol. Invas. 8, 217–230 (2006).
Mebius, L. J. A rapid method for the determination of organic carbon in soil. Anal. Chim. Acta 22, 120–124 (1960).
Industry, D. I. Design in industry. Electr. Power 28, 228–228 (1982).
Murphy, J. & Riley, J. P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 27, 31–36 (1962).
Brookes, P. C., Landman, A., Pruden, G. & Jenkinson, D. S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 17, 837–842 (1985).
Schinner, F. & Mersi, W. V. Xylanase-, CM-cellulase- and invertase activity in soil: An improved method. Soil Biol. Biochem. 22, 511–515 (1990).
Johnson, J. L. & Temple, K. L. Some variables affecting the measurement of “catalase activity” in Soil1. Soil Sci. Soc. Am. J. 28, 207–209 (1964).
Klose, S. & Tabatabai, M. A. Urease activity of microbial biomass in soils as affected by cropping systems. Biol. Fertil. Soils 31, 191–199 (2000).
Vaughan, D. & Ord, B. G. An effect of soil organic matter on invertase activity in soil. Soil Biol. Biochem. 12, 449–450 (1980).
Djajakirana, G., Joergensen, R. G. & Meyer, B. Ergosterol and microbial biomass relationship in soil. Biol. Fertil. Soils 22, 299–304 (1996).
Levy-Booth, D. J., Prescott, C. E. & Grayston, S. J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 75, 11–25 (2014).
Yuan, H. et al. Abundance and composition of CO_2 fixating bacteria in relation to long-term fertilization of paddy soils. Acta Ecol. Sin. 32, 183–189 (2012).
Chen, J., Yu, Z., Michel, F. C. Jr., Wittum, T. & Morrison, M. Development and application of real-time PCR assays for quantification of erm genes conferring resistance to macrolides-lincosamides-streptogramin B in livestock manure and manure management systems. Appl. Environ. Microbiol. 73, 4407–4416. https://doi.org/10.1128/AEM.02799-06 (2007).
Edgar, R. C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998 (2013).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Maidak, B. L. et al. The ribosomal database project (RDP). Nucleic Acids Res. 24, 82–85 (1996).
CoreTeam, R. R: A language and environment for statistical computing. (2013).
Oksanen, B. J., Kindt, R., Legendre, P. & O’Hara, B. vegan: Community Ecology Package. R package version 1.8-6 (accessed 10 December 2019); https://CRAN.R-project.org/package=vegan.
Oksanen, J. et al. vegan: Community ecology package. R package version 1.17-3. J. Stat. Softw. 48, 103–132 (2010).
Nd, S. A., Potvin, L. R. & Lilleskov, E. A. Fertility-dependent effects of ectomycorrhizal fungal communities on white spruce seedling nutrition. Mycorrhiza 25, 649–662 (2015).
Arbuckle, J. L. Amos 7.0 User's Guide. (SPSS, 2006).
Acknowledgements
We thank Wei Zhao, Yangyang Jin, Jiaxin Zhao for their help with soil sampling.
Author information
Authors and Affiliations
Contributions
C.X. and. L.L. conceived the study. L.L., W.X.L., T.M.Z., Y.J. and L.J.N. performed the experiments. T.M.Z., L.L. and C.X. interpreted the results and T.M.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, M., Li, L., Wang, X. et al. Elevational is the main factor controlling the soil microbial community structure in alpine tundra of the Changbai Mountain. Sci Rep 10, 12442 (2020). https://doi.org/10.1038/s41598-020-69441-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69441-w
This article is cited by
-
Construction and assessment of an indicator system of stream conditions in a typical mountain landscape in Northeast China
Environmental Science and Pollution Research (2024)
-
Altitude rather than season and slope aspect has the greatest effect on the bacterial communities in subtropical forests in Yunnan, China
Plant and Soil (2023)
-
Plant invasion shifts soil microbiome and physico-chemical attributes along an elevational gradient in Kashmir Himalaya
Environmental Science and Pollution Research (2023)
-
Distinct soil bacterial patterns along narrow and broad elevational gradients in the grassland of Mt. Tianshan, China
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.