Abstract
Most of snakes exhibit a ZZ/ZW sex chromosome system, with different stages of degeneration. However, undifferentiated sex chromosomes and unique Y sex-linked markers, suggest that an XY system has also evolved in ancestral lineages. Comparative cytogenetic mappings revealed that several genes share ancestry among X, Y and Z chromosomes, implying that XY and ZW may have undergone transitions during serpent’s evolution. In this study, we performed a comparative cytogenetic analysis to identify homologies of sex chromosomes across ancestral (Henophidia) and more recent (Caenophidia) snakes. Our analysis suggests that, despite ~ 85 myr of independent evolution, henophidians and caenophidians retained conserved synteny over much of their genomes. However, our findings allowed us to discover that ancestral and recent lineages of snakes do not share the same sex chromosome and followed distinct pathways for sex chromosomes evolution.
Similar content being viewed by others
Introduction
Non-avian reptiles’ evolution is dynamic and remarkably diverse, especially in their sex-determining strategies, making them an interesting group to investigate the evolutionary trends of sex chromosome evolution1,2,3,4,5. An array of different strategies of sex determination have been reported in different vertebrate lineages6,7,8,9. However, non-avian reptiles display the most diversities, including TSD (Temperature-dependent Sex Determination), GSD (Genotypic Sex Determination) and GSD with the influence of temperature (reviewed in3, 5). In addition, variants of major mode of sex chromosome systems (XY, ZW and multiple chromosome systems) are frequently present among reptile clades, even within sister clades as well as within allopatric populations of the same species10,11,12,13,14,15,16. Moreover, frequent transitions between modes of sex-determining mechanisms (e.g. TSD–GSD–TSD) are also evident1,12,15,17,18. Such variabilities highlight the complexity of sex chromosome evolutionary history in this group. Besides, non-avian reptiles are the sole vertebrate group where master sex-determining genes are yet to be discovered, although some candidates have been suggested for few groups such as geckos, agamids, varanids and testudines19,20,21,22. Due to these enormous diversities in modes of sex determination and sex chromosomes across reptilian lineages, it is therefore not surprising that sex determination and sex chromosome evolution in non-avian reptile remains as a matter of much discussion and debate over decades.
Among non-avian reptiles, the majority of the snakes karyotyped so far exhibit a ZZ/ZW sex chromosome system, with different stages of evolutionary degeneration or amplification of W chromosomes23,24,25. However, undifferentiated sex chromosomes (and more recently unique Y sex-linked markers, suggesting an XY male heterogametic system) have also been reported in some genera such as in Python and Boa26. This implies that at least two transitions involving XY and ZW and independent turnovers of these sex chromosome systems may have occurred in Pythonoidea and Booidea superfamilies.
The gene content of both the Z and the W chromosomes are thought to be relatively conserved in snakes24,27,28. Still, the high variability of W chromosome (regarding morphology and/or gene content) in major clades suggests a remarkable role of repetitive sequences accumulation in their architecture and evolution24,25,29. Unlike the W, the Z chromosomes are thought to be more stable among Serpentes lineages, and non-drastic shifts in morphology have been reported in different snake clades23,24,25,30.
Although suggested as having an independent origin, the homomorphic XX/XY chromosomes in Boa and Python are yet to be characterized by molecular cytogenetic techniques. Thus, their relationships and transitions among both homomorphic and heteromorphic ZZ/ZW in Pythonoidea and Booidea superfamilies31,32,33 still remain unanswered. In the amazonian red-tailed Boa constrictor (formally Boa constrictor constrictor), which has 2n = 36 chromosomes, the fourth chromosomal pair is thought to represent the putative sex pair, which would be a typical feature for henophidians (i.e. a former superfamily of the suborder Serpentes, which harbors boas, pythons and other old lineages of snakes, usually referred as "Primitive Snakes")31. In addition, two different classes of sequences, the PBI-MspI and EQU-BamHI-4 (EQU-BamHI-4 being putatively reported as sex-linked27,35) have been identified on the 4th homomorphic pair of Boa constrictor females, therefore, being identified as the sex pair in these studies34,35. Similarly, in most of the pit vipers, rattlesnakes and colubrids, the 4th chromosomal pair also represent the sex chromosomes24,25,36,37,38, even when homomorphic, as already identified through accumulation of Bkm repeats in some species39,40, suggesting a conserved trend for sex chromosome evolution in Snakes.
Homomorphic sex chromosomes are frequently observed among non-avian reptiles. In the Serpentes suborder, for instance, they are found in major clades of henophidian and caenophidian species (i.e. Caenophidia is a monophyletic group that contains over 80% of all the extant species of snakes, commonly referred as “Advanced Snakes”)30,31,39,41. On the other hand, well-differentiated sex chromosomes are more common in the more recently diversified groups of snakes, the advanced lineages25, but also present in the former groups of snakes as Typhlopoidea and Booidea32,42. The molecular and cytogenetic mechanisms of evolution of homomorphic sex chromosomes in snakes have not been the subject of rigorous studies compared to well-differentiated ones. Therefore, many homomorphic or micro W or Y sex chromosomes in the ancestral lineages of snakes remained undetected. Serpentes was thought to have a well-stable sex chromosome system and, despite different levels of W degeneration, as well the occurrence of multiple sex chromosomes (e.g. Z1Z2/Z1Z2W and Z/W1W2), only ZW system had been described until recently43,44. Indeed, the arise of a putative and independently evolved XY sex chromosome system in Boidae and Phytonidae raised questions regarding the cytogenetic and molecular mechanisms involving the evolution of sex chromosomes in snakes. Why did snakes independently evolve a new and homomorphic sex chromosome system solely in ancestral lineages? Did snakes retain homology of XY and ZW chromosomes along the Serpentes’ evolution owing to some evolutionary advantage conferred by the shift and transitions between these systems? Why has the XY sex chromosome system been lost in the more advanced lineages?
Application of molecular cytogenetic tools, such as chromosomal mapping using Bacterial Artificial Chromosomes (BAC-FISH) and comparative genomic hybridization (CGH) have been instrumental in overcoming limitations in identification of undifferentiated or cryptic sex chromosomes in several vertebrate groups such as fishes45,46,47, amphibians48,49, and reptiles20,50,51. In this study, we aimed toward understanding the relationship between the homomorphic XY and heteromorphic ZW chromosomes found in some ancestral (henophidians) and more recent (caenophidians) snakes. For that, we performed an extensive comparative analysis of the amazonian red-tailed boa (Boa constrictor constrictor) chromosomes (homomorphic XY) through cross-species comparisons using whole genomic DNA (gDNA) from several caenophidian species with varying degrees of the ZW sex chromosomes differentiation. We also performed WCP (Whole Chromosomal Painting) of a highly degenerated W sex chromosome and mapped BACs specific for several genes on Boa constrictor chromosomes. We identified chromosome homologies of sequences for all analyzed species, however, with different patterns of accumulation, which enabled us to infer the relationship and landscape of snake’ sex chromosomes evolution spanning 85MYR.
Results
Chromosome painting with amazonian pit-viper (Bothrops atrox) W paints and cross-species chromosome painting to Boa constrictor chromosomes
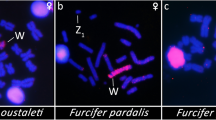
The isolated W chromosome probe of B. atrox (BaW) was amplified and the homology was tested onto metaphase spreads of the same species (Fig. 1). We carried out cross-species chromosome painting using BaW probe to metaphase spreads of male and female of B. constrictor in order to test for the homology of the sex chromosomes between Henophidia and Caenophidia. The BaW probe hybridized completely on a small metacentric (W chromosome/pair 4) of Bothrops atrox (Fig. 1). However, in male and female B. constrictor metaphase spreads the BaW probe showed faint hybridization signals on microchromosomes and on the centromeric position of the 7th pair (Fig. 1), with no differences between males and females.
Chromosomal painting using derived probes of a highly heterochromatic and degenerated W chromosome from the amazonian pit viper (Bothrops atrox). The W probes (BaW) was used on the B. atrox chromosome spreads as control, showing large hybridized segments on the W chromosome. In the Boa constrictor, the BaW probe showed signals on the 7th pair. ISIS software was used for microphotography and analyzing images.
Comparative genomic hybridization
Comparison between male and female gDNAs of Boa constrictor (Fig. 2), produced intense and faint hybridization signals on macrochromosomes and microchromosomes, respectively (Fig. 3), co-located with C-banded regions as previously reported31. Small-shared signals were observed on the pericentric regions of the p arms of chromosome pair 1, whereas strong bright signals were observed on the centromere of chromosome pairs 2 and 4 and on the telomeric position of chromosome pair 7. Male or female-specific hybridization signals were neither detected in B. constrictor male and female nor on the homomorphic sex chromosomes (putatively the chromosome pair 4) (Fig. 3).
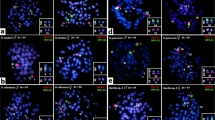
Interspecific hybridization among henophidian (Boa constrictor—red-tailed boa) (ancestral lineage XY) and caenophidian (advanced lineages ZW) (pitvipers—Bothrops, bushmaster—Lachesis, rattle snakes—Crotalus and puffer snake—Spilotes) species (Fig. 4a, b), revealed that all species share conserved sequences to that of Boa constrictor chromosomes, particularly with the macrochromosomes pairs 1, 4, 7 (Figs. 5a–c, 6a–d). The cross-species hybridization (Boa constrictor/Bothrops bilineatus) revealed shared sequences on the centromeric position of the 4th and 7th pairs (Fig. 5a). However, the Boa constrictor/Bothrops taeniatus pitviper comparisons showed hybridization signals only on the centromeric position of the 4th pair (Fig. 5b). The Boa constrictor/Bothrops atrox—amazonian pitviper comparisons, on the other hand, showed hybridization signals only on the centromeric position of the 7th pair (Fig. 5c).
The second set of experiments, (A) male- and female-derived gDNAs of Bothrops bilineatus (Bb); Bothrops taeniatus (Bt) Bothrops atrox (Ba); Lachesis muta = (Lm); Crotalus terrificus (Ct); Crotalus ruruima (Cr) and Spilotes pullatus (Sp) were used for hybridization against male and female chromosomal background of Boa constrictor (Bc). In the (B) an example of how the second set of experiments was conducted, where the gDNA of male and female of caenophidians were used together the gDNA of boas male and females and hybridized on the chromosomes of Boa male and female. Pictures of the caenophidians by Ayrton Costa.
Cross-species comparisons between Bc (Boa constrictor) chromosomes and caenophidians gDNA. The cross-species hybridization (A–C) the Bc gDNA is in green and Bothrops bilineatus, Bothrops taeniatus and Bothrops atrox (Bb, Bt, and Ba) in red. ISIS software was used for microphotography and analyzing images, Bar = 20 µm.
Cross-species comparisons among Bc (Boa constrictor) chromosomes (green) and caenophidians gDNA (red). (A) Lachesis muta, (B) Crotalus terrificus, (C) Crotalus ruruima, (D) Spilotes pullatus (Lm, Ct, Cr, and Sp respectively). ISIS software was used for microphotography and analyzing images, Bar = 20 µm.
Comparisons between Boa constrictor/Lachesis muta revealed shared hybridization signals on the 1st and 7th chromosomal pairs. However, Lachesis hybridization signals on the 1st pair were more intense than Boa constrictor signals (Fig. 6a). The Boa constrictor/Crotalus terrificus showed shared sequences on the 4th and 7th pairs, similar to that of the Boa constrictor/Bothrops bilineatus (Fig. 6b). The Boa constrictor/Crotalus ruruima comparisons showed the same hybridization pattern to that of Boa / Lachesis, with signals only on the 1st and 7th pairs, likewise with more intense signals on the 1st pair (Fig. 6c). Unlike most patterns, Boa constrictor/Spilotes pullatus showed hybridization signals on 3 chromosomal pairs: near the pericentromeric region of the 1st pair (similar to that of the Boa / Lachesis and Boa/C. ruruima) and on the centromere of both 4th and 7th pairs (similar to those of the Boa/B. bilineatus and Boa/C. terrificus) (Fig. 6d). The 2nd pair was the sole representative with strong Boa constrictor specific hybridization signals, but with no shared regions of gDNA with all caenophidian snakes.
For all interspecific comparisons (Fig. 4a,b), we used pooled male and female gDNA from the seven species of caenophidian snakes (advanced lineages ZW) against chromosome spreads of Boa constrictor male and female. Once the hybridization patterns from all Caenophidian snakes were exactly the same on the chromosomes of red-tailed boa males and females, for convenience, representative metaphase was selected for illustrating the above results among the genomic Henophidia and Caenophidian comparisons.
BAC mapping on Boa constrictor chromosomes
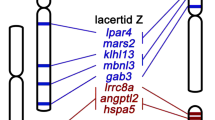
All Pogona vitticeps derived BACs used showed hybridized signals on B. constrictor chromosomes (Fig. 7a–h). Six BAC clones (APTX; CHD1; CTNNB1; TAX1BP1; KLF6; WAC1) were mapped to the centromeric position of the 4th pair of Boa constrictor, however, the KLF6 was also mapped on the centromeric position of the 2nd pair. The 3L7 and NR5A1 were mapped to the terminal position of the 2nd pair and in the centromeric position of the 7th pair respectively. The ATPX and CHD1 are located on the 2nd pair of Pogona vitticeps, CTNNB1; TAX1BP; KLF6; WAC1 on the 6th pair whereas OPRD1/RCC1 and NR5A1 on the sex chromosomes19,52,53.
Discussion
In reptiles, independent turnovers and transitions among sex chromosomes systems (XY and ZW) and sex-determining mechanisms (TSD and GSD) within closely related species are more common than previously thought, being thus, a widespread feature among non-avian reptiles1,3,4,54,55. Unlike most snakes, Boa imperator was reported to have XY homomorphic sex chromosomes26, and its sister species Boa constrictor, shares ancestry presenting also an XY system. Therefore, it is plausible that transitions between homomorphic ZW and XY have occurred in the Boidae family without much substantial genotypic innovation (e.g. considering the 4th pair of boas as the putative sex chromosomes, the XY and ZW are morphologically similar), as reported in the Japanese frog Glandirana rugosa56. In our study, we did not detect any sex-specific pattern using intra- and interspecific CGH experiments (Figs. 3, 5, 6), suggesting that only minute sequence differences exist between sex chromosomes (putatively the pair 4). A similar pattern was observed in the Sanziniidae family, a sister group to Boidae (Fig. 8)32, but the Z and W chromosomes of Acrantophis sp. cf. dumerili are morphologically well-differentiated nevertheless.
Whilst CGH has been applied for the identification of undifferentiated or cryptic sex chromosomes across a range of vertebrates ranging from fish to reptiles45,46,50,51,57,58, this technique, in some cases, may not be efficient in detecting specific sex domains (even in the heteromorphic sex chromosomes) as already seen in amphibians59 and in the well-differentiated ZW present in Acrantophis sp. cf. dumerili (Booidea)32. Perhaps some ancestral lineages still need more time to achieve sex-specific signatures (e.g. morphological changes, accumulation of sequences, heterochromatinization), or simply use alternative mechanisms for sex chromosome evolution, which makes it difficult to detect, especially when they retain huge traits of homology, as here observed in Boa constrictor.
Our comparative cytogenetic analysis suggests that henophidian and caenophidians indeed followed different evolutionary pathways regarding the origin of their sex chromosomes. Several genes share ancestry between putative homomorphic X and Y chromosomes of Python (at that time considered to be Z and W) and the Z chromosomes of caenophidians27, suggesting that X, Y and Z chromosomes can easily undergo transitions in ancestral lineages conferred by the similarity of morphology and gene content. For instance, even though located in different positions regarding other snakes’ lineages, the genes linked to the putative sex pair of Boa constrictor male and female points homology with the independently evolved putative pair of burmese python (Python bivitattus XY), with the sex pair of habu pit viper (Protobothrops flavoriridis ZW) and the four-lined ratsnake (Elaphe quadrivirgata ZW) (Fig. 9). Interestingly but not surprisingly, once sex chromosomes evolve fastly and independently across lineages4, the XY of Python bivittatus seems to share more similarities with caenophidians than with the other sole representative XY system existing in Serpentes, the XY present in Boa26. Regardless, this shared ancestry, in spite of some fine adjustments in the gene position on the sex pair, indicates that henophidian and caenophidian snakes do not share the same set of sex-determining genes, since other genes located on the putative XY of Boa also share homology with the second pair of Elaphe quadrivirgata (Caenophidia), which partially correspond to the Z chromosome of chicken. Furthermore, the mapping of BaW chromosome probe also provided strong evidence that caenophidian and henophidian snakes do not share the same sex chromosomes, because the W of Bothrops atrox (Caenophidia) has homology with the 7th autosomal pair of Boa constrictor and not with the putative homomorphic sex chromosomes (4th pair) (Fig. 1), which correspond to a well-differentiated ZW system in the sister group (Sanziniidae) 32. CGH also revealed that, among all the 7 caenophidians snakes involved in our comparative study, six of them shared ancestry with the 7th pair of Boa constrictor, that somehow share some degree of homology with the W sex chromosome of B. atrox. Perhaps this 7th pair represent a large conserved segment of the henophidians and caenophidians ancestor. To fully understand the real status of ZW–XY–ZW transitions and homology of sequences, combined whole genome sequencing and refined cytogenetic approaches will be required, especially in representatives from the four major clades of Serpentes suborder (Typhlopoidea ZW, Pythonoidea XY, Booidea ZW/XY, and Colubroidea ZW) (Fig. 8), where the XY sex chromosome system arose only twice and remained morphologically undifferentiated.
Notably, our study revealed that the 4th pair of Boa also shares homology with the Z, W and 2nd chromosome pair of chicken (Fig. 9). While chicken’s Z partially correspond to the Squamata chromosome 22,29,60, however, at least 2 genes (CHD1, APTX) located on the 4th pair of Boa constrictor also share homology with the second pair of Elaphe quadrivirgata and the bearded dragon (Pogona vitticeps). As hypothesized by Ezaz and colleagues, this synteny among different squamate clades and chicken Z chromosome could represent part of an ancestral super-sex chromosome for Aminiotes2. Interestingly, two sex-linked genes in Pogona vitticeps also share homology with the Boa constrictor 2 (BAC containing genes OPRD1 and RCC1 ) and 7 (NR5A2) chromosome pairs (Fig. 10). These genes correspond to the chicken chromosomes 17 and 2319. Concordantly, OPRD1 / RCC1 and NR5A2 genes, also mapped in the yellow and green anacondas (Eunectes notaeus and Eunectes murinus), cerrado rainbow boa (Epicrates crassus) and in the amazonian puffer snake (Spilotes pullatus), showed a similar scenario (Viana personal communication). Although the homology of Squamates 2 and chicken Z is considered a conserved trait across lineages2,12,29, 61,62,63,76, the Boa constrictor 2 shares homology to the chicken chromosome 17 and 23, whereas the chicken Z, W and 2 with the putative XY of amazonian red-tailed boa (4th pair) (Figs. 9, 10), which highlights the homology and ancestry of sequences among close and distantly related lineages, possibly remnants of a common evolutionary history among avian and non-avian reptiles.
It is intriguing that after the divergence of Henophidia and Caenophidia in the Upper Cretaceous (~ 85 MYR)64,65 snakes still share conserved sequences across lineages even after such long period of independent evolution (Fig. 11). Even more puzzling is that some closely related lineages (e.g. C. terrificus and C. ruruima) show a divergent pattern of gDNA hybridization on the Boa constrictor chromosomes (Figs. 6b,c, 11), perhaps unique particularities at species level. For the two rattle snakes, only C. terrificus shared sequences on the putative homomorphic sex chromosomes of Boa constrictor. Likewise, the Bothrops species (B. bilineatus, B. taeniatus and B. atrox), showed divergent patterns of hybridization to Boa constrictor chromosomes (Figs. 5a–c, 11). This evolutionary landscape might be product of the mechanisms that shape the processes of chromosomal differentiation during the evolution, as for example the association with TEs (Transposable Elements) and SSRs (Simple Short Repeats) sequences, that triggers an important role on the genome architecture leading to independent evolution processes (e.g. silencing, deleting, or increasing genomic regions)63,66,67,68. This seems to be also the case for the snakes here analyzed.

The phylogenetic tree was adapted from Figueroa et al.75.
Relationships among Henophidia and Caenophidia species highlighting the reciprocal mapping male and female gDNA of Caenophidia species in Bc chromosomes.
Nevertheless, all caenophidians used in our study shared sequences with B. constrictor chromosomes, representing a possible inheritance of ancestry, being the assortment of hybridization patterns due to the tempo of sequence divergence and transient evolutionary mechanisms linked to their evolution spanning ~ 85 my of independent evolution. However, we were not able to identify any sex-specific sequence from all caenophidians gDNA derived probes and W chromosome probes (BaW), that somehow showed the same hybridization pattern in both male and females of Boa constrictor. In fact, this is not surprising because hybridization has not even detected within Boa comparisons, such sex-specific patterns. The shared sequences and different patterns could simply be the result of the convergent accumulation of repetitive sequences during Snakes’ evolution. However, all caenophidian species used here share the same W sex chromosome (Viana personal communication).
This lack of sex-specific signals in Boa (XY) from caenophidian (ZW) gDNA derived probe is likely that the sex-linked sequences in advanced snakes are different and, therefore, do not share any similarity with those sex-linked sequences in Boa. This suggests an independent evolution of sex chromosome sequences in snakes, but in caveats, given the similarity of morphologies and gene content of putative sex pair of henophidian and the sex chromosomes of caenophidian snakes we cannot conclusively infer which homomorphic system, XY or ZW really occurs in ancestral lineages (Boidae and Pythonidae). Regardless, our study provides first evidence that caenophidian and henophidian snakes have a common evolutionary history but likely evolving a different set of sex-determining sequences, where the sex chromosomes followed divergent evolutionary pathways. However, in henophidians, the real status of homology with the cryptic sex chromosomes of Boa and the heteromorphic ZW present in the sister group (Sanziniidae) is yet to be investigated, which will require developing probes from Y sex-linked markers of Boa imperator for cross species chromosome mapping. Such combined methods of genomics and cytogenetics will enable us to unreveal the dynamic evolutionary history and transitions between XY and ZW sex chromosomes system in the major clades of Serpentes. This study is part of a series of further cytogenetic and genomic studies, focusing on Neotropical reptiles and their hidden evolutionary diversity.
Material and methods
Sampling, mitotic chromosomes preparation, and DNA extraction
Snakes were collected from natural populations across Amazon region under permission granted by Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) number 45275. We analyzed chromosomes of six males and seven females of Boa constrictor and the genomic DNA (gDNA) of several caenophidian snakes with differentiated ZW chromosomes (Bothrops bilineatus, B. taeniatus, B. atrox, Lachesis muta, Crotalus terrificus, C. ruruima and Spilotes pullatus) in the cross-species mapping. Chromosomal preparations were obtained following69. The gDNA of males and females for all species were extracted from blood using the Wizard Genomic Purification Kit (Promega), according to the manufacturer’s recommendations. We also highlight that in our present study, no animal needed to be euthanized.
Microdissection of W sex chromosome of Bothrops atrox and preparation of the BaW chromosome paints
We performed microdissection using an inverted phase-contrast microscope Zeiss Axiovert.A1 (Zeiss, Oberkochen, Germany) equipped with Eppendorf TransferMan NK 2 micromanipulator (Eppendorf, Hamburg, Germany). We prepared glass needles from 1.0 mm diameter capillary glass using a glass capillary puller, Sutter P-30 Micropipette Puller (Sutter Instrument, Novato, Calif., USA) and sterilized using ultraviolet irradiation. We microdissected a W chromosome from freshly prepared slides of a female B. atrox using a glass needle and the micromanipulation system, subsequently transferring the W chromosome into 0.2 ml PCR tubes. The W chromosome DNA (BaW) was amplified using GenomePlex Single Cell Whole Genome Amplification Kit (Sigma-Aldrich, St. Louis, Mo., USA) according to the manufacturer’s protocol with slight modifications according to70. The volume of the reactions was scaled down to half, and the PCR amplification step was increased to 30 cycles. The W chromosome paint of B. atrox was labeled by nick translation means incorporating SpectrumGreen-dUTP (Abbott, North Chicago, Ill., USA). The hybridization was carried out for 1 day in the B. atrox chromosomes (control) and 3 days in cross-species chromosome painting (Boa constrictor male and female).
Preparation of probes for CGH
The gDNA of males and females of all species was used for comparative approaches focused on an intraspecific comparison between males and females of Bc (Boa constrictor), with special emphasis on the homomorphic sex chromosomes in this species and in an interspecific genomic comparison among henophidian and caenophidian species. For intraspecific comparisons, male and female-derived gDNA of Boa constrictor were hybridized against male and female metaphase chromosomes of the species (Fig. 2). The female-derived gDNA was labeled with biotin-16-dUTP and male gDNAs with digoxigenin-11-dUTP by Nick translation means (Roche, Mannheim, Germany). Interspecific comparisons gDNA of male and female of all caenophidian species were hybridized against metaphase chromosomes and gDNA of male and female of Boa constrictor (Bc) (Fig. 4a,b). For this purpose, the gDNA of caenophidians male and female (green pit viper / Bothrops bilineatus = Bb; forest pit viper Bothrops taeniatus = Bt; amazonian pit viper / Bothrops atrox = Ba; bushmaster/Lachesis muta = Lm; common rattle snake/Crotalus terrificus = Ct; north rattle snake/Crotalus ruruima = Cr and puffer snake/Spilotes pullatus = Sp) were labeled with digoxigenin-11-dUTP (red), whereas male and female-derived gDNA of Boa constrictor (Bc) were labeled with biotin-16-dUTP (green) by Nick translation means above mentioned. For both intra and interspecific purposes, the final hybridization mixture for each slide was composed of gDNAs of the species (500 ng each), 20 μg of male-derived C0t-1 DNA (i.e. fraction of genomic DNA enriched for highly and moderately repetitive sequences, prepared according to71) and 20 μl of the hybridization buffer containing 50% formamide, 2 × SSC, 10% SDS, 10% dextran sulfate and Denhardt´s solution, pH 7.0.
FISH used for CGH
The FISH experiments were performed primarily according to72 and subtle modifications according to our previous studies. The slides were incubated at 37 °C in a dark humid chamber for three days and the hybridization signals were detected with Anti-digoxigenin-Rhodamin (Roche) diluted in 0.5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), and avidin-FITC (fluorescein isothiocyanate, Sigma) diluted in PBS containing 10% normal goat serum (NGS). The chromosomes were counterstained with DAPI (1.2 µg/ml) and mounted in an antifade solution (Vector, Burlingame, CA, USA).
Bacterial artificial chromosome (BAC) preparation and FISH
We mapped Pogona vitticeps derived 8 BAC clones containing 8 chromosome-linked genes (WAC; KLF6; APTX; CHD1; CTNNB1; TAX1BP1; OPRD1/RCC1 and NR5A1)12,19,52,53 to B. constrictor male and female metaphase chromosomes. The clones were selected from Pogona vitticeps genomic BAC library as previously described in Ezaz et al.12,52, Young et al.53 and Deakin et al.19. All 8 BACs were anchored to P. vitticeps metaphase chromosomes as control (data not shown). BAC DNA was extracted using the Promega Wizard Plus SV Minipreps DNA Purification System following the manufacturer’s protocol, with volumes scaled up for 15 ml cultures. The BACs were labeled with SpectrumOrange-dUTP or SpectrumGreen-dUTP (Abbott, North Chicago, Ill., USA) and hybridized for 2 days. The slides were then washed twice in 0.4 × SSC, 0.3% IGEPAL (Sigma-Aldrich) at 55 °C for 5 min each and after air-dried, counterstained using DAPI (1.2 µg/ml) and mounted in an antifade solution (Vector, Burlingame, CA, USA).
Microscopy and image analyses
Images were captured using an Olympus BX51 microscope (Olympus Corporation, Ishikawa, Japan) with CoolSNAP. For W painting and BAC-FISH, images were captured using a Zeiss Axioplan epifluorescence microscope equipped with a CCD camera (Zeiss). ISIS software was used for microphotography and analyzing images.
Ethics statement
We declare that all procedures and experimental protocols were approved and performed under the rules of the Ethics Committee of the National Institute of Amazonian Research (Permission number: 018/2017).
References
Gamble, T. et al. Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining systems. Mol. Biol. Evol. 32(5), 1296–1309 (2015).
Ezaz, T., Srikulnath, K. & Graves, J. A. M. Origin of amniote sex chromosomes: An ancestral super-sex chromosome, or common requirements?. J. Hered. 108(1), 94–105 (2016).
Alam, S. M. I. et al. Did lizards follow unique pathways in sex chromosome evolution?. Genes 9(5), 239 (2018).
Pennell, M. W., Mank, J. E. & Peichel, C. L. Transitions in sex determination and sex chromosomes across vertebrate species. Mol. Ecol. 27(19), 3950–3963 (2018).
Deakin, J. E. & Ezaz, T. Understanding the evolution of reptile chromosomes through applications of combined cytogenetics and genomics approaches. Cytogenet. Genome Res. 157(1–2), 7–20 (2019).
Bull, J. J. Sex determination in reptiles. Q. Rev. Biol. 55(1), 3–21 (1980).
Bull, J. J. Evolution of sex determining mechanisms (The Benjamin/Cummings Press. Company, Menlo Park, 1983).
Janzen, F. J. & Paukstis, G. L. Environmental sex determination in reptiles: ecology, evolution, and experimental design. Q. Rev. Biol. 66(2), 149–179 (1991).
Bachtrog, D. et al. Sex determination: Why so many ways of doing it?. Plos Biol. 12(7), e1001899 (2014).
Moritz, C. The evolution of a highly variable sex chromosome in Gehyra purpurascens (Gekkonidae). Chromosoma 90(2), 111–119 (1984).
Ezaz, T. et al. Relationships between vertebrate ZW and XY sex chromosome systems. Curr. Biol. 16(17), R736–R743 (2006).
Ezaz, T. et al. The ZW sex microchromosomes of an australian dragon lizard share no homology with those of other reptiles or birds. Chromosome Res. 17(8), 965 (2009).
Pokorná, M. & Kratochvíl, L. Phylogeny of sex-determining mechanisms in squamate reptiles: Are sex chromosomes an evolutionary trap?. Zool. J. Linn. Soc. 156(1), 168–183 (2009).
Sarre, S. D., Ezaz, T. & Georges, A. Transitions between sex-determining systems in reptiles and amphibians. Annu. Rev. Genomics Hum. Genet. 12, 391–406 (2011).
Holleley, C. E. et al. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 523(7558), 79 (2015).
Rovatsos, M. et al. Mixed-up sex chromosomes: Identification of sex chromosomes in the X1X1X2X2/X1X2Y system of the legless lizards of the genus Lialis (Squamata: Gekkota: Pygopodidae). Cytogenet. Genome Res. 149(4), 282–289 (2016).
Radder, R. S. et al. Genetic evidence for co-occurrence of chromosomal and thermal sex-determining systems in a lizard. Biol. Lett. 4(2), 176–178 (2007).
Quinn, A. E. et al. Evolutionary transitions between mechanisms of sex determination in vertebrates. Biol. Lett. 7(3), 443–448 (2011).
Deakin, J. E. et al. Anchoring genome sequence to chromosomes of the central bearded dragon (Pogona vitticeps) enables reconstruction of ancestral squamate macrochromosomes and identifies sequence content of the Z chromosome. BMC Genomics. 17(1), 447 (2016).
Montiel, E. E. et al. Discovery of the youngest sex chromosomes reveals first case of convergent co-option of ancestral autosomes in turtles. Chromosoma 126(1), 105–113 (2017).
Rovatsos, M. et al. Shared ancient sex chromosomes in varanids, beaded lizards and alligator lizards. Mol. Biol. Evol. 36(6), 1113–1120 (2019).
Rovatsos, M. et al. The rise and fall of differentiated sex chromosomes in geckos. Mol. Ecol. 28(12), 3042–3052 (2019).
Beçak, W. & Beçak, M. L. Cytotaxonomy and chromosomal evolution in Serpentes. Cytogenet. Genome Res. 8(4), 247–262 (1969).
Augstenová, B. et al. Evolutionary dynamics of the W chromosome in caenophidian snakes. Genes 9(1), 5 (2017).
Viana, P. F. et al. Evolutionary insights of the ZW sex chromosomes in snakes: A new chapter added by the amazonian puffing snakes of the genus Spilotes. Genes 10(4), 288 (2019).
Gamble, T. et al. The discovery of XY sex chromosomes in a boa and python. Curr. Biol. 27(14), 2148–2153 (2017).
Matsubara, K. et al. Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes. P. Natl. Acad. Sci. 103(48), 18190–18195 (2006).
Rovatsos, M. et al. Evolutionary stability of sex chromosomes in snakes. P. R. Soc. B. 282(1821), 20151992 (2015).
Singchat, W. et al. Chromosome map of the siamese cobra: Did partial synteny of sex chromosomes in the amniote represent “a hypothetical ancestral super-sex chromosome” or random distribution?. BMC Genomics. 19(1), 939 (2018).
Singh, L. Evolution of karyotypes in snakes. Chromosoma 38(2), 185–236 (1972).
Viana, P. F. et al. Is the karyotype of neotropical boid snakes really conserved? Cytotaxonomy, chromosomal rearrangements and karyotype organization in the Boidae family. PLoS ONE 11(8), e0160274 (2016).
Augstenová, B. et al. ZW, XY, and yet ZW: Sex chromosome evolution in snakes even more complicated. Evolution 72(8), 1701–1707 (2018).
Augstenová, B. et al. Cytogenetic analysis did not reveal differentiated sex chromosomes in ten species of boas and pythons (Reptilia: Serpentes). Genes. 10(11), 934 (2019).
Matsubara, K. et al. Molecular cloning and characterization of satellite DNA sequences from constitutive heterochromatin of the habu snake (Protobothrops flavoviridis, Viperidae) and the burmese python (Python bivittatus, Pythonidae). Chromosoma 124(4), 529–539 (2015).
Matsubara, K. et al. Sex chromosome evolution in snakes inferred from divergence patterns of two gametologous genes and chromosome distribution of sex chromosome-linked repetitive sequences. Zool. Lett. 2(1), 19 (2016).
Beçak, W. Constituição cromossômica e mecanismo de determinação do sexo em ofidios sulamericanos. I. Aspectos cariotipicos. Mem. Inst. Butantan. 32, 37–78 (1965).
Nery, M. D. A. et al. Karyotype of Philodryas nattereri and Philodryas olfersii with a comparative analysis of the Dipsadidae family. Genet. Mol. Res. 14(2), 6297–6302 (2015).
Falcione, C., Hernando, A. & Bressa, M. J. Comparative cytogenetic analysis in Erythrolamprus snakes (Serpentes: Dipsadidae) from Argentina. An. Acad. Bras. Cienc. 90(2), 1417–1429 (2018).
De Smet, W. H. The chromosomes of 23 species of snakes. Acta Zool. Pathol. Antverp. 70, 85–118 (1978).
Singh, L., Purdom, I. F. & Jones, K. W. Sex chromosome associated satellite DNA: Evolution and conservation. Chromosoma 79(2), 137–157 (1980).
Aprea, G. et al. The karyology of Vipera aspis, V. atra, V. hugyi, and Cerastes vipera. Amphibia-Reptilia. 27(1), 113–119 (2006).
Matsubara, K. et al. Karyotype analysis of four blind snake species (Reptilia: Squamata: Scolecophidia) and karyotypic changes in Serpentes. Cytogenet. Genome Res. 157(1–2), 98–106 (2019).
Singh, L., Sharma, T. & Ray-Chaudhuri, S. P. Multiple sex-chromosomes in the common Indian krait, Bungarus caeruleus. Schneider. Chromosoma. 31(4), 386–391 (1970).
Singh, L. Multiple W chromosome in a sea snake, Enhydrina schistosa daudin. Experientia 28(1), 95–97 (1972).
Freitas, N. L. et al. Early stages of XY sex chromosomes differentiation in the fish Hoplias malabaricus (Characiformes, Erythrinidae) revealed by DNA repeats accumulation. Curr. Genomics. 19(3), 216–226 (2018).
Sember, A. et al. Sex chromosome evolution and genomic divergence in the fish Hoplias malabaricus (Characiformes, Erythrinidae). Front. Genet. 9, 71 (2018).
Shams, F. et al. Karyotypes and sex chromosomes in two australian native freshwater fishes, golden perch (Macquaria ambigua) and murray cod (Maccullochella peelii) (Percichthyidae). Int. J. Mol. Sci. 20(17), 4244 (2019).
Abramyan, J. et al. Z and W sex chromosomes in the cane toad (Bufo marinus). Chromosome Res. 17(8), 1015 (2009).
Keinath, M. C. et al. Miniscule differences between sex chromosomes in the giant genome of a salamander. Sci. Rep-UK 8(1), 17882 (2018).
Ezaz, T. et al. The dragon lizard Pogona vitticeps has ZZ/ZW micro-sex chromosomes. Chromosome Res. 13(8), 763–776 (2005).
Ezaz, T. et al. An XX/XY sex microchromosome system in a freshwater turtle, Chelodina longicollis (Testudines: Chelidae) with genetic sex determination. Chromosome Res. 14(2), 139–150 (2006).
Ezaz, T. et al. Sequence and gene content of a large fragment of a lizard sex chromosome and evaluation of candidate sex differentiating gene R-spondin 1. BMC Genom. 14(1), 899 (2013).
Young, M. J. et al. Molecular cytogenetic map of the central bearded dragon, Pogona vitticeps (Squamata: Agamidae). Chromosome Res. 21(4), 361–374 (2013).
Ezaz, T. et al. Sex chromosome evolution in lizards: independent origins and rapid transitions. Cytogenet. Genome Res. 127(2–4), 249–260 (2009).
Rovatsos, M. et al. Little evidence for switches to environmental sex determination and turnover of sex chromosomes in lacertid lizards. Sci. Rep. 9(1), 7832 (2019).
Ogata, M. et al. Reconstruction of female heterogamety from admixture of XX–XY and ZZ–ZW sex-chromosome systems within a frog species. Mol. Ecol. 27(20), 4078–4089 (2018).
Traut, W. & Winking, H. Meiotic chromosomes and stages of sex chromosome evolution in fish: Zebrafish, platyfish and guppy. Chromosome Res. 9(8), 659–672 (2001).
de Moraes, R. L. R. et al. Comparative cytogenetics and neo-Y formation in small-sized fish species of the genus Pyrrhulina (Characiformes, Lebiasinidae). Front. Genet. 10, 678 (2019).
Gazoni, T. et al. More sex chromosomes than autosomes in the amazonian frog Leptodactylus pentadactylus. Chromosoma 127(2), 269–278 (2018).
O’Meally, D. et al. Non-homologous sex chromosomes of birds and snakes share repetitive sequences. Chromosome Res. 18(7), 787–800 (2010).
Srikulnath, K. et al. Karyotypic evolution in squamate reptiles: comparative gene mapping revealed highly conserved linkage homology between the butterfly lizard (Leiolepis reevesii rubritaeniata, Agamidae, Lacertilia) and the japanese four-striped rat snake (Elaphe quadrivirgata, Colubridae, Serpentes). Chromosome Res. 17(8), 975 (2009).
Pokorná, M. et al. Strong conservation of the bird Z chromosome in reptilian genomes is revealed by comparative painting despite 275 million years divergence. Chromosoma 120(5), 455 (2011).
Ezaz, T. & Deakin, J. E. Repetitive sequence and sex chromosome evolution in vertebrates. Adv. Evol. Biol. 2014, 1–9 (2014).
Hsiang, A. Y. et al. The origin of snakes: Revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record. BMC Evol. Biol. 15(1), 87 (2015).
Harrington, S. M. & Reeder, T. W. Phylogenetic inference and divergence dating of snakes using molecules, morphology and fossils: new insights into convergent evolution of feeding morphology and limb reduction. Biol. J. Linn. Soc. 121(2), 379–394 (2017).
Lippman, Z. et al. Role of transposable elements in heterochromatin and epigenetic control. Nature 430, 471–476 (2004).
Chalopin, D. Transposable elements and early evolution of sex chromosomes in fish. Chromosome Res. 23, 545–560 (2015).
Li, S. F. et al. Repetitive sequences and epigenetic modification: inseparable partners play important roles in the evolution of plant sex chromosomes. Planta 243, 1083–1095 (2016).
Viana, P. F. et al. An optimized protocol for obtaining mitotic chromosomes from cultured reptilian lymphocytes. Nucleus 59(3), 191–195 (2016).
Matsubara, K. et al. Non-homologous sex chromosomes in two geckos (Gekkonidae: Gekkota) with female heterogamety. Cytogenet. Genome Res. 143(4), 251–258 (2014).
Zwick, M. S. et al. A rapid procedure for the isolation of C0t–1 DNA from plants. Genome 40(1), 138–142 (1997).
Symonová, R. et al. Characterization of fish genomes by GISH and CGH. In Fish Cytogenetic Techniques Ray-Fin Fishes and Chondrichthyans (eds Ozouf-Costaz, C. et al.) 118–131 (CCR Press, Boca Raton, 2015).
Pyron, R. A., Reynolds, R. G. & Burbrink, F. T. A taxonomic revision of boas (Serpentes: Boidae). Zootaxa 3846(2), 249–260 (2014).
Reynolds, R. G., Niemiller, M. L. & Revell, L. J. Toward a Tree-of-Life for the boas and pythons: Multilocus species-level phylogeny with unprecedented taxon sampling. Mol. Phylogenet. Evol. 71, 201–213 (2014).
Figueroa, A. et al. A species-level phylogeny of extant snakes with description of a new colubrid subfamily and genus. PLoS ONE 11(9), e0161070 (2016).
Singchat, W. et al. Do sex chromosomes of snakes, monitor lizards, and iguanian lizards result from multiple fission of an “ancestral amniote super-sex chromosome”?. Chromosome Res. 28, 209–228 (2020).
Acknowledgements
This work was supported by Fundação de Amparo a Pesquisa do Amazonas (FAPEAM), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Grant number: 88881.190036/2018-01), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant number: 302449/2018-3), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CAPES/Alexander von Humboldt (Grant number: 88881.136128/2017-01), Center for Studies on Adaptations of Aquatic Biota of the Amazon (ADAPTA), Projects (Grant number: Pronex/FAPEAM/CNPq 003/2009), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CAPES—Pro-Amazon Program: Biodiversity and Sustainability (Grant number: Public Notice No. 047/2012). We are grateful to the Milena Miranda and Leonardo Matos from the Animal Husbandry of the National Institute of Amazonian Research (Biotério Central—INPA); Institute of Environmental Protection of Amazonas (IPAAM); Isaías José dos Reis (ICMBio); Marcos Flávio (Corpo de Bombeiros do Amazonas); Milena and Breno Almeida from the Amazonian Center for Herpetology (Centro Amazônico de Herpetologia) for all valuable support provided. We are also grateful to Shayer M. Alam for assistance in the laboratory activities.
Author information
Authors and Affiliations
Contributions
Designed the study and initial structure, P.V.; Project administration, P.V.; Investigation, P.V.; T.E.; M.B.C.; E.F.; Methodology and laboratory experiments, P.V.; T.E.; M.B.C.; T.L.; A.A.; L.G.G. and A.M.R. All authors analyzed and interpreted the data. P.V wrote the manuscript with contributions from all coauthors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Viana, P.F., Ezaz, T., de Bello Cioffi, M. et al. Landscape of snake’ sex chromosomes evolution spanning 85 MYR reveals ancestry of sequences despite distinct evolutionary trajectories. Sci Rep 10, 12499 (2020). https://doi.org/10.1038/s41598-020-69349-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69349-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.