Abstract
Serum cardiac troponin I (cTnI) is often elevated in patients with ischemic stroke, and is associated with their prognosis. Since cTnI is also closely related to atrial fibrillation (AF), cTnI may be a sensitive prognostic indicator in patients with AF-related stroke. This study aimed to evaluate the association between serum cTnI and early neurological deterioration (END) in patients with AF-related stroke. We included consecutive AF-related stroke patients between 2013 and 2015. END was defined as an increase ≥ 2 in the total NIHSS score or ≥ 1 in the motor NIHSS score within the first 72 h of admission. A total of 1,133 patients with AF-related stroke were evaluated. In multivariable analysis, cTnI [adjusted odds ratio (aOR) = 1.16, 95% confidence interval (CI) 1.00–1.34; P = 0.047] remained significant after adjusting for confounders. Initial NIHSS score (aOR = 1.03, 95% CI 1.00–1.06; P = 0.043) was also positively associated with END; meanwhile, the use of anticoagulants was negatively associated in both vitamin K antagonists (aOR = 0.35, 95% CI 0.23–0.54; P < 0.001) and new oral anticoagulants (aOR = 0.41, 95% CI 0.19–0.89; P = 0.024). In conclusion, higher serum cTnI was associated with END in patients with AF-related stroke.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is a well-known risk factor for ischemic stroke1,2. AF not only increases the risk of ischemic stroke but is also associated with worse prognosis after events1,2,3,4. Ischemic stroke patients with coexisting AF (e.g., AF-related stroke) have higher initial NIH Stroke Scale (NIHSS) scores and poor short- and long-term outcomes2,3,5. Thus, efforts have been made to identify high-risk groups among AF-related stroke patients, but mostly relying on clinical markers6. It would be helpful if we had an objective and reliable biomarker.
Cardiac troponin I (cTnI) is an intracellular protein that controls calcium-mediated myocardial contraction7,8. With high sensitivity and specificity, elevated cTnI levels have recently been included in the universal definition of acute myocardial infarction (MI)9. Interestingly, elevated cTnI is also commonly found in up to 34% of the acute ischemic stroke patients10,11,12,13. The exact reasons why cTnI increases during the acute stage of ischemic stroke are still unclear. However, previous studies reported that elevated serum cTnI was associated with initial severe stroke and poor prognosis7,11,13,14,15,16,17,18. In these backgrounds, the American Heart Association (AHA)/American Stroke Association (ASA) guidelines recently recommended the evaluation of cTnI levels in all patients with acute ischemic stroke in consideration of the pathological overlap between cardiovascular and cerebrovascular diseases and their clinical impact on the prognosis of ischemic stroke19.
Patients with AF have vulnerable cardiac environments with increased oxygen demand due to tachycardia and decreased myocardial oxygen supply due to the shortening of diastole8,13. In these patients, cTnI reflects AF-related cardiac structural changes [e.g., left atrial (LA) enlargement, endothelial dysfunction, and fibrosis] and secondary thrombus formation (e.g., LA thrombus, and spontaneous echocardiographic contrast)20,21. Furthermore, elevated cTnI is associated with poor prognosis in patients with AF20,22,23. Thus, serum cTnI may be closely related to the prognosis of AF-related stroke patients with both AF and ischemic stroke. In this study, we aimed to evaluate the association between serum cTnI and early neurological deterioration (END) in patients with AF-related stroke.
Results
A total of 1,133 patients with AF-related stroke were evaluated. The mean age of the cohort was 74 years, and 51.1% were male. The median initial NIHSS score was 10 [3–16]. END occurred in 164 (14.5%) patients and the median time to admission was 0 [0–1] day. The median cTnI level was 0.02 [0.02–0.04] ng/mL. Other baseline characteristics are presented in Supplementary Table 1.
In univariate analysis, END was significantly associated with older age, higher frequencies of hypertension and previous stroke history, lesser use of anticoagulants, and higher initial NIHSS score and serum cTnI levels (Table 1). In multivariable logistic regression analysis, cTnI [adjusted odds ratio (aOR) = 1.16, 95% confidence interval (CI) 1.00–1.34, P = 0.047] remained significant after adjusting for confounders. Furthermore, initial NIHSS score (aOR = 1.03, 95% CI 1.00–1.06; P = 0.043) was positively associated with END, independent of cTnI (Table 2). The use of anticoagulants was negatively associated with END in both vitamin K antagonists (VKA) (aOR = 0.35, 95% CI 0.23–0.54; P < 0.001) and new oral anticoagulants (NOAC) (aOR = 0.41, 95% CI 0.19–0.89; P = 0.024) (Table 3). These positive and negative associations continued when the variable “cTnI > 0.03 ng/mL” was used to conduct additional sensitivity analyses (Table 3).
When the relationship between serum cTnI and vascular risk factors/echocardiographic parameters was analyzed, cTnI showed a positive association with the initial NIHSS scores, regional wall motion abnormalities, and LA thrombus, and negative associations with body mass index and left ventricular ejection fraction (LVEF) (Table 4). In addition, the END group showed more frequent 7d-poor outcome (80.0% versus 44.9%, P < 0.001) and 3m-poor outcome (78.3% versus 41.6%, P < 0.001), showing an effect on subsequent prognosis (Table 5).
Discussion
In this study, we found that high serum cTnI levels were associated with END in patients with AF-related stroke. Furthermore, cTnI was not associated with any avascular risk factors, but was associated with LVEF or regional wall motion abnormality (Table 4). Thus, the close relationship between the two seems to be linked by more cardio-specific mechanisms.
The exact mechanisms explaining the close relationship between cTnI and END are unclear. Serum cTnI is a simple indicator of damaged myocytes, and it is difficult to say that it is a substance that can cause END in itself. Therefore, it is reasonable to say that the heart was damaged by any causes, and that the decreased cardiac function caused END. We found the cause of the cardiac dysfunction in (1) neurogenic-heart syndrome after stroke and (2) hidden cardiac disease before stroke.
First, we can think about how cTnI is associated with END in neurogenic-heart syndrome condition after a stroke. In this situation, increased cTnI may reflect a severe stroke that is vulnerable to early progression. When an ischemic stroke occurs, circulating catecholamine increases with activation of the hypothalamus-pituitary gland-adrenal gland axis7,10,24,25. This increased catecholamine injures myocytes via calcium channel activation, called “myocytolysis”, and elevates serum cTnI levels7,10,12,24,25,26. At this time, the degree of cTnI level increase is proportional to the severity of index stroke, which can be confirmed in our data (Table 4)11,17,22,25,26. The severity of index stroke, which is represented by initial NIHSS score, is the most well-known risk factor of END27. Eventually, it can be interpreted that elevated cTnI serves as a marker for patients with severe stroke who are originally well progressed. However, the authors also thought about the possibility of elevated cTnI linking to END through a mechanism other than simply reflecting severe stroke. To prove this, an additional subgroup analysis was performed on the patients with mild (initial NIHSS score < 5) AF-related stroke. Even in the situation where the effect of the initial stroke severity was limited to a minimum, cTnI was closely related to END (Supplementary Table 2). Therefore, the relationship between two seems to be also explained by a mechanism other than the initial stroke severity. On the one hand, catecholamine-induced myocardial damage can also provoke transient left ventricular dysfunction, called Takotsubo cardiomyopathy20,24,28,29,30. In previous studies, ischemic stroke patients with Takotsubo cardiomyopathy had worse initial clinical outcomes, including END. Also, like those with Takotsubo cardiomyopathy, our data showed that patients with elevated cTnI were closely related to systolic dysfunction (e.g., LVEF) or regional wall motion abnormality and not to diastolic dysfunction (e.g., DT, E/e′) or global wall motion abnormality (Table 4)28,29,30. Thus, it may not be involved in all patients with END, but we suggest that Takotsubo cardiomyopathy may be a sufficient mechanism to explain this phenomenon.
Next, the possibility of hidden cardiac disease before stroke can be considered. Patients with ischemic stroke are often unaware of accompanying heart disease due to symptom masking by the stroke itself7,11,24,31. These hidden cardiovascular diseases could lead to END, at the same time increasing serum cTnI levels. Additionally, as seen in the Troponin Elevation in Acute Ischemic Stroke (TRELAS) study, unknown coronary culprit lesions are found in up to 25% of the ischemic stroke patients32. These lesions are chronic but unstable, able to cause problems at any time32. It is difficult to know whether these heart diseases were exactly accompanied by the data we present in our cohort. However, given the high prevalence of accompanying heart disease in previous studies, this is quite possible.
Interestingly, in this study, the use of early anticoagulant showed a negative correlation with the occurrence of END. This negative correlation was found in both VKA and NOAC regardless of the type of anticoagulant. Simply, it can be thought that the use of anticoagulant suppressed further embolism in the acute period, which resulted in less END. However, on the contrary, it may be a result of the tendency not to use anticoagulants in severe strokes that are good at early progression. Actually, our data showed that the patients without anticoagulant showed higher initial NIHSS score and more frequent hemorrhagic transformation than the rest of the patients (Supplementary Table 3). This study is a cross-sectional study, so it is difficult to obtain further information on causal relationships or related information. Further prospective studies addressing the relationship between the use or type of early anticoagulant and the development of END would provide an impression that is clinically interesting.
The sensitivity, specificity, positive predictive value, and negative predictive value based on cTnI > 0.03 ng/mL, which we used as the reference value of sensitivity analysis, can be seen relatively high specificity and negative predictive values (Supplementary Fig. 2). This seems to mean that the clinical probability of END is very low if serum cTnI is lower than 0.03 ng/mL. However, relatively low sensitivity and positive predictive value also mean that cut-off point 0.03 ng/mL is somewhat high to be used as a screening tool for END. It may be necessary to consider the new appropriate reference value.
Our study had several limitations. First, this study was designed as a retrospective study. Although we included large numbers of participants at multiple centers, selection bias may have occurred. Second, due to the limitations of cross-sectional analysis, we could only indicate association, not causality. Further large, prospective studies are needed to confirm the causality. Third, we defined END by a relatively sensitive definition33. Thus, the END events could have been overestimated. However, this sensitive definition of END has been validated in previous studies34, and it also has a significant effect on further prognosis (e.g., 7d- and 3m-poor outcome) in our data. Therefore, it is believed that our END definition will have no problems using it. Fourth, this study did not include information related to imaging parameters. If there was information on lesions and vascular occlusion on the initial MRI and follow-up MRI images within 72 h, it would be helpful to infer the mechanism of END occurrence. Fifth, we analyzed the relationship with END using only baseline cTnI values at the time of admission. If information such as the change in cTnI within 72 h and its highest value can be known, it is likely to be able to analyze the more certain relationship between cTnI and END and infer its mechanism. Lastly, considering the definition of END, we included ischemic stroke patients within 72 h from symptom onset. Therefore, if the END event occurs before the patient visits, it may be underestimated than the actual prevalence of END. However, since most of the participants (91.5%) in our dataset visited within 24 h, there will be very few missing cases that actually happen, and not enough to affect our main results.
We demonstrated that high serum cTnI levels at admission were associated with END events in patients with AF-related stroke using data from 11 large centers in Korea. These positive associations were observed within the range of cTnI levels recognized as “subclinical”. Thus, even slight increases in cTnI should be cautiously interpreted and the patients need close observation. However, our insights must be validated with further large, prospective studies.
Methods
Patients and population
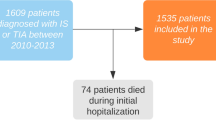
The current study is a sub-study of K-ATTENTION (Korean ATrial fibrillaTion EvaluatioN regisTry in Ischemic strOke patieNts) that was a real-world cohort composed of prospective stroke registries from 11 large centers in South Korea between January 2013 and December 2015 (n = 3,213)35. The K-ATTENTION study aimed to investigate the relationships between the use of oral anticoagulants and outcomes of AF-related stroke patients35. From this large dataset, we included consecutive AF-related stroke patients who were admitted within 72 h after symptom onset (n = 2,960). The exclusion criteria were patients without (1) END data (n = 771) or (2) serum cTnI levels (n = 821) or (3) patients who suffered severe heart diseases (e.g., congestive heart failure, MI, severe valvular heart disease, etc.) (n = 235). A total of 1,133 patients were included in the final analyses (Supplementary Fig. 1). All patients were evaluated and treated according to the individual center’s protocol, including brain magnetic resonance imaging, echocardiography, and laboratory evaluations.
This retrospective study was approved by the Institutional Review Board (IRB) at Samsung Medical Center (SMC-2016-07-011). The requirement to obtain written informed consent from the participants was exempted by the IRB due to the retrospective design using anonymous and de-identified information. All experiments were performed in accordance with the Declaration of Helsinki and relevant guidelines and regulations.
Clinical assessment
We evaluated demographic factors, clinical factors, and vascular risk factors, including age, sex, body mass index, hypertension, diabetes, hyperlipidemia, previous stroke history, initial NIHSS score, types of AF, thrombolysis therapy, the use of anticoagulants, and systolic/diastolic blood pressure34. The initial NIHSS score was rated daily from admission to discharge by well-trained neurologists who were not involved in the study34 AF was documented by electrocardiogram, 24-h Holter monitoring, and/or continuous electrocardiogram monitoring during hospitalization. The types of AF were classified as paroxysmal or sustained. The use of anticoagulants was defined as using any type of these drugs during the acute period within 72 h from admission. Considering the difference in acute period pharmacological action according to the type of anticoagulant, we divided the entire participants into 3 groups: no use, VKA, and NOAC. Patients who used intravenous anticoagulants were excluded from the initial process because K-ATTENTION study, which corresponds to the original study of this study, was intended to examine the clinical prognosis of oral anticoagulants in AF-related stroke patients35. Laboratory examinations, including glucose profiles, lipid profiles, complete blood cell counts, creatinine kinase-MB, and cTnI, were obtained within the first 24 h of admission. As an outcome, END was defined as an increase ≥ 2 in the total NIHSS score or ≥ 1 in the motor NIHSS score within the first 72 h of admission34. We also measured the participants’ 7d-mRS and 3m-mRS scores. Based on this score, mRS score > 3 was defined as “poor outcome”36.
Echocardiographic evaluation
Comprehensive 2-dimensional and Doppler echocardiography (TTE) examinations were performed by skilled cardiac sonographers and the findings were interpreted by cardiologists at each center. Considering the differences between the TTE protocols among the centers, we extracted common echocardiographic parameters, including cardiac structural parameters [e.g., LA diameter, left ventricular (LV) end-systolic diameter, LV end-diastolic diameter, interventricular septal dimension, and LV posterior wall thickness], LVEF, deceleration time (DT), the peak trans-mitral filling velocity (E)/mean mitral annular velocity at early diastole (e′) ratio, and the presence of LA thrombus37. LVEF was obtained using Simpson’s method with estimations of the end-systolic and end-diastolic LV volume37. E and DT were measured by the pulse wave Doppler method at the tip of the mitral leaflets from an apical 4-chamber view37,38. Tissue Doppler imaging was applied to the apical 4-chamber view at the septal mitral annulus to determine e′. To calculate the E/e′ ratio, the maximum velocity of the E-wave of mitral valve inflow was divided by the maximum velocity of e′37,38.
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 (IBM, SPSS, Chicago, IL, USA). Univariate analyses for assessing the possible predictors of END were performed using Student’s t-test or the Mann–Whitney U-test for continuous variables. Variables with severely skewed data were transformed into a log scale. The Chi-squared test or Fisher’s exact test was used for categorical variables. Based on the results of the univariate analyses, variables with P < 0.10 were introduced into the multivariable logistic regression analysis. To confirm our results, we conducted additional sensitivity analysis using the variable of “cTnI > 0.03 ng/mL” that has been used in previous studies10,13,18.
To understand the underlying pathological mechanisms between cTnI and END, the relationship between serum cTnI and vascular risk factors/echocardiographic parameters was evaluated using simple linear regression analysis. In addition, in order to examine the effect of END on the subsequent clinical prognosis, we compared the frequencies of 7d- and 3m-poor outcome between END group and non-END group. All variables with P < 0.05 were considered significant in this study.
References
Wolf, P. A., Abbott, R. D. & Kannel, W. B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22, 983–988 (1991).
Kamel, H. & Healey, J. S. Cardioembolic stroke. Circ. Res. 120, 514–526 (2017).
Ferro, J. M. Cardioembolic stroke: an update. Lancet Neurol. 2, 177–188 (2003).
Steger, C. et al. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur. Heart J. 25, 1734–1740 (2004).
Dulli, D. A., Stanko, H. & Levine, R. L. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 22, 118–123 (2003).
Tanaka, K. et al. Pre-admission CHADS2, CHA2DS2-VASc, and R2CHADS2 scores on severity and functional outcome in acute ischemic stroke with atrial fibrillation. J. Stroke Cerebrovasc. Dis. 24, 1629–1635 (2015).
Kerr, G., Ray, G., Wu, O., Stott, D. J. & Langhorne, P. Elevated troponin after stroke: a systematic review. Cerebrovasc. Dis. 28, 220–226 (2009).
Jeremias, A. & Gibson, C. M. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann. Intern. Med. 142, 786–791 (2005).
Thygesen, K. et al. Fourth universal definition of myocardial infarction (2018). J. Am. Coll. Cardiol. 72, 2231–2264 (2018).
Etgen, T., Baum, H., Sander, K. & Sander, D. Cardiac troponins and N-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke 36, 270–275 (2005).
Di Angelantonio, E. et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 76, 76–81 (2005).
Barber, M. et al. Elevated troponin levels are associated with sympathoadrenal activation in acute ischaemic stroke. Cerebrovasc. Dis. 23, 260–266 (2007).
Bugnicourt, J.-M. et al. Troponin levels help predict new-onset atrial fibrillation in ischaemic stroke patients: a retrospective study. Eur. Neurol. 63, 24–28 (2010).
Sandhu, R. et al. Relation of cardiac troponin I levels with in-hospital mortality in patients with ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. Am. J. Cardiol. 102, 632–634 (2008).
Hijazi, Z. et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 125, 1605–1616 (2012).
Raza, F., Alkhouli, M., Sandhu, P., Bhatt, R. & Bove, A. A. Elevated cardiac troponin in acute stroke without acute coronary syndrome predicts long-term adverse cardiovascular outcomes. Stroke Res. Treat. https://doi.org/10.1155/2014/621650 (2014).
Hasırcı, B., Okay, M., Ağırcan, D. & Koçer, A. Elevated troponin level with negative outcome was found in ischemic stroke. Cardiovasc. Psychiatry Neurol. 2013, 953672 (2013).
Beaulieu-Boire, I., Leblanc, N., Berger, L. & Boulanger, J.-M. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J. Stroke Cerebrovasc. Dis. 22, 978–983 (2013).
Jauch, E. C. et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44, 870–947 (2013).
Providência, R., Barra, S. & Paiva, L. Atrial fibrillation, elevated troponin, ischemic stroke and adverse outcomes: understanding the connection. Clin. Res. Cardiol. 102, 701–711 (2013).
Providência, R. et al. Cardiac troponin I: prothrombotic risk marker in non-valvular atrial fibrillation. Int. J. Cardiol. 167, 877–882 (2013).
Ahn, S. H. et al. Cardiac vulnerability to cerebrogenic stress as a possible cause of troponin elevation in stroke. J. Am. Heart Assoc. 5, e004135 (2016).
Hijazi, Z. et al. High-sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation 129, 625–634 (2014).
Scheitz, J. F., Nolte, C. H., Laufs, U. & Endres, M. Application and interpretation of high-sensitivity cardiac troponin assays in patients with acute ischemic stroke. Stroke 46, 1132–1140 (2015).
Chen, Z. et al. Brain–heart interaction: cardiac complications after stroke. Circ. Res. 121, 451–468 (2017).
Chalela, J. A., Ezzeddine, M. A., Davis, L. & Warach, S. Myocardial injury in acute stroke. Neurocrit. Care 1, 343–346 (2004).
Miyamoto, N. et al. Demographic, clinical, and radiologic predictors of neurologic deterioration in patients with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 22, 205–210 (2013).
Jung, J.-M. et al. Takotsubo-like myocardial dysfunction in ischemic stroke: a hospital-based registry and systematic literature review. Stroke 47, 2729–2736 (2016).
Templin, C. et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 373, 929–938 (2015).
Citro, R. et al. Standard and advanced echocardiography in Takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J. Am. Soc. Echocardiogr. 28, 57–74 (2015).
Jensen, J. K., Atar, D. & Mickley, H. Mechanism of troponin elevations in patients with acute ischemic stroke. Am. J. Cardiol. 99, 867–870 (2007).
Mochmann, H.-C. et al. Coronary angiographic findings in acute ischemic stroke patients with elevated cardiac troponin: the troponin elevation in acute ischemic stroke (TRELAS) study. Circulation 133, 1264–1271 (2016).
Siegler, J. E. & Martin-Schild, S. Early Neurological Deterioration (END) after stroke: the END depends on the definition. Int. J. Stroke 6, 211–212 (2011).
Nam, K. W. et al. D-dimer as a predictor of early neurologic deterioration in cryptogenic stroke with active cancer. Eur. J. Neurol. 24, 205–211 (2017).
Baek, I.-Y. et al. Characteristics and factors for short-term functional outcome in stroke patients with atrial fibrillation, nationwide retrospective cohort study. Front. Neurol. 10, 1101 (2019).
Bath, P. M. & Optimising Analysis of Stroke Trials (OAST) Collaboration. Can we improve the statistical analysis of stroke trials? Statistical re-analysis of functional outcomes in stroke trials. Stroke 38, 1911–1915 (2007).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 16, 233–271 (2015).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. J. Echocardiogr. 17, 1321–1360 (2016).
Acknowledgements
The study was partially supported by the Korean Neurological Association (KNA-17-MI-10) and the National Research Foundation of Korea (NRF-2019R1A2C2008788).
Author information
Authors and Affiliations
Contributions
Study concept and design: K.-W.N., C.K.K. and W.-K.S.; Acquisition, analysis, or interpretation of data: S.W.Y., J.-W.C., T.-J.S., Y.-J.K., B.J.K., S.H.H., K.-Y.P., J.-M.K., J.-H.P., J.C.C., M.-S.P., J.-T.K., K.-H.C., and Y.H.H.; Drafting of the manuscript: K.-W.N. and C.K.K.; Critical revision of the manuscript for important intellectual content: O.Y.B., G.-M.K., and J.-M.J.; Statistical analysis: K.-W.N. and C.K.K.; Obtained funding: C.K.K. and W.-K.S.; Supervision: W.-K.S. and K.M.O.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nam, KW., Kim, C.K., Yu, S. et al. Elevated troponin levels are associated with early neurological worsening in ischemic stroke with atrial fibrillation. Sci Rep 10, 12626 (2020). https://doi.org/10.1038/s41598-020-69303-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69303-5
This article is cited by
-
Threshold adjusted vagus nerve stimulation after asphyxial cardiac arrest results in neuroprotection and improved survival
Bioelectronic Medicine (2022)
-
SARS-COV-ATE risk assessment model for arterial thromboembolism in COVID-19
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.