Abstract
It has been shown that contrast-induced nephropathy (CIN) can be attenuated by the administration of PGE1. As an enzyme responsible for the production of PGE1, PTGS1 was confirmed in this study as a miR-512 target. Meanwhile, HULC has been identified as a competing endogenous RNA of miR-512. Therefore, in this study, we tested the diagnostic value of HULC and miR-512 in subjects with or without CIN. In addition, we evaluated the regulatory relationship among HULC, miR-512, PTGS1 and PGE1 in vitro. We enrolled 320 patients with coronary heart disease and divided them into a CIN group and a non-CIN group. Subsequently, we detected the differential expression of miR-512, HULC and PGE1 in the two groups. We also used a dual luciferase reporter assay to evaluate the regulatory relationship among HULC, miR-512, PTGS1 and PGE1 in THP-1 cells. In patients with CIN, the expression levels of HULC and PGE1 were lower, but the expression level of miR-512 was higher. MiR-512 could directly bind to and negatively regulate the expression of PTGS1 and HULC. The expression of HULC was positively correlated with the expression of PTGS1 and PGE1, while negatively correlated with the expression of miR-512. The findings of this study demonstrated that deregulation of lncRNA-HULC/miR-512/PTGS1/PGE1 might be involved in the pathogenesis of CIN.
Similar content being viewed by others
Introduction
As a form of kidney damage induced by recent contrast medical imaging exposure without other significant reasons for acute kidney injury, contrast-induced nephropathy (CIN) represents a disorder featured by a rise in serum creatinine (Scr) > 25% from baseline and/or an absolute increased Scr from baseline of > 0.5 mg/dL, which usually occurs after the use of a contrast media1. CIN can cause high mortality and morbidity, especially in patients undergoing percutaneous coronary intervention (PCI) or coronary angiography. There is no effective way to prevent CIN, and hence many researchers tried to develop new pharmacological agents with the potential to reduce the risk of CIN2.
Miao et al. found that prostaglandin E1 (PGE1) inhibits the development of CIN in patients receiving contrast computed tomography3. Another study also showed that the oral administration of pentoxifylline can prevent CIN to a certain degree4. In addition, as a widely used prophylactic agent in patients receiving computed tomography, N-acetylcysteine reduces CIN risk5. In many cancers such as bladder cancer, the level of cyclooxygenase-2 (COX-2), an enzyme participating in the biosynthesis of prostaglandin E2 (PGE2), is elevated6. In a rat model of bladder cancer triggered by N-butyl-4-N-(4-hydroxybutyl) nitrosamine, the level of COX-2 is also shown to be increased along with higher expression of PGE27,8. In humans, the expression of COX-2 can be easily detected in glomerular podocytes, while the patients suffering from acute rejection of renal allograft show up-regulated expression of PGE29,10. Similarly, it was shown that the number of COX-2-expressing podocytes, macula densa cells and mesangial cells was increased in adult rats of diabetes, whereas the suppression of COX-2 reduced the severity of proteinuria and delayed the progression of diabetic nephropathy11.
Small RNA molecules do not code for proteins, and microRNAs (miRNAs), one of the small RNA molecules, were reported to mediate gene expression post-transcriptionally by targeting the 3′ UTRs of target mRNAs12. Moreover, long-noncoding RNAs (lncRNAs) have a length of greater than 200 nt and can also regulate gene expression. Recently, many studies demonstrate that lncRNAs and miRNAs are involved in cell growth and differentiation, whereas the interactions between lncRNAs and microRNAs may be implicated in many diseases13,14.
It has been shown that CIN could be attenuated by the administration of PGE115,16. As an enzyme responsible for the production of PGE1, PTGS1 was confirmed in this study as a target of miR-512. Meanwhile, we also found that HULC has been reported to be a competing endogenous RNA (ceRNA) to sponge miR-51217,18,19. Therefore, in this study, we tested the diagnostic value of HULC and miR-512 in subjects with or without CIN. In addition, we evaluated the regulatory relationship among HULC, miR-512, PTGS1 and PGE1 in vitro.
Materials and methods
Patient grouping
In this study, we enrolled 320 subjects of coronary heart diseases. All patients were treated by coronary intervention, including 78 CIN subjects (the CIN group) and 242 patients free of CIN (non-CIN group). All subjected enrolled in this study were diagnosed with coronary heart disease and have received angiogram. Those who were diagnosed with CIN (CIN are defined as a rise in SCr > 25% from baseline, or by an absolute increase from baseline of > 0.5 mg/dL) were divided into CIN group and those who are free with CIN were divided into non-CIN group. In addition, those who were diagnosed with any renal disease other than CIN or have been currently or recently (within past 3 months) diagnosed with any infection, inflammation or immune diseases were excluded from this study.
Peripheral blood samples were collected and HULC and miR-512 expression were detected in these two groups. This study was approved by the Ethics Committee of the First Affiliated Hospital of the Second Military Medical University and was performed in accordance with the latest vision of the Declaration of Helsinki. All patients signed the form of informed consent before the study was initialized.
RNA isolation and real-time PCR
Total RNA from cultured cells and peripheral blood samples was extracted using Trizol. The concentration of extracted RNA was then measured by spectrometer. Subsequently, a TaqMan Reverse Transcription Kit (Applied Biosystems, Foster City, CA) was adopted to convert total RNA into cDNA, followed by real-time PCR on an ABI Prism 7,500 system (ABI, Foster City, CA) using a SYBR green mix following the kit instruction. The relative expression of HULC, miR-512, and PTGS1 mRNA was calculated using the 2−ΔΔCT method with GAPDH (for lncRNA and mRNA) and U6 (for miRNAs) being utilized as the internal control.
Cell culture and transfection
THP-1 cells were maintained in DMEM containing 10% FBS, 4.5 g/L glucose, and 100 units/mL of streptomycin-penicillin. The cells were incubated at 37 °C in an atmosphere of 5% CO2. Subsequently, the cells were transfected with the control or HULC siRNA at the concentration of 50 nM, and an empty vector or pcDNA-HULC using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) at the concentration of 200 ng/L, followed by the measurement of target gene expression at 48 h post transfection.
Vector construction, mutagenesis and luciferase assay
PCR was used to amplify the full length of HULC and 3ʹ-UTR PTGS1 mRNA containing the binding site for miR-512, and the PCR products were inserted into pcDNA vectors (Promega, Madison, WI) to obtain the plasmids for the wild-type HULC and PTGS1 3ʹ-UTR. Subsequently, a QuickChange Mutagenesis kit (Stratagene, La Jolla, CA) was adopted to carry out site-directed mutagenesis in the miR-512 binding site of HULC and PTGS1 mRNA, and the mutagenesis products were also inserted into pcDNA vectors to obtain the plasmids for the mutant HULC and PTGS1 3ʹ-UTR. Subsequently, THP-1 cells were co-transfected with the mutant-type or wild-type HULC/PTGS1 3′-UTR, in conjunction with miR-512 mimics or a negative control (NC), using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). During transfection, a Renilla luciferase reporter construct (pRL-TK) was used as the internal standard. Finally, the transfected THP-1 cells were collected 48 h later and the luciferase activity in the cells was measured on a Berthold AutoLumat LB9507 rack luminometer (Berthold Technologies, Shanghai, China) using a Dual Luciferase Assay (Promega, Madison, WI).
Western blot analysis
Peripheral blood samples and transfected THP-1 cells were lysed and the proteins in the supernatant were collected by centrifugation. Then, the isolated proteins were resolved by 10% SDS-PAGE and blotted onto a PVDF membrane. Subsequently, the membrane was blocked by 5% non-fat milk for 1 h at 37 °C and then incubated at 4 °C overnight with anti-PTGS1, anti‑ATG7, anti‑LC3‑II and anti-β-actin (Internal control) primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), followed by 1 h of room temperature incubation with HPR-conjugated secondary antibodies (Abcam, Cambridge, MA). Then, an ECL kit (GE Healthcare, Little Chalfont, UK) was used to develop the membrane and the target bands were quantified using Image J (NIH Image, Bethesda, MD) .
Cell apoptosis analysis
The apoptosis of THP-1 was detected by flow cytometry using an Annexin V FITC‑conjugated/propidium iodide (PI) apoptosis kit (Nanjing Keygen Biotech Co., Ltd., Nanjing, China). Different groups of THP-1 cells were seeded into a 24-well plate at a density of 1 × 105 cells/well and were incubated with serum-free medium for 12 h after the cell confluence reached 70–80%. The incubated cells were subsequently collected and incubated with 5 μl Annexin V‑FITC and 5 μl PI for 30 min at room temperature before being analyzed with a flow cytometer (BD Biosciences, San Jose, CA, USA).
Statistical analysis
All results were presented as mean ± standard deviations. ANOVA was employed to compare the results among different groups. ROC curves were plotted and analyzed using Chi-square test and Pearson correlation analysis. All analyses were conducted in SPSS 21.0 (IBM, Chicago, IL). P < 0.05 was considered significant.
Results
Diagnostic value of HULC and miR-512 in CIN
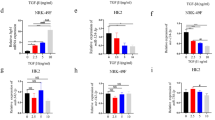
In this study, we enrolled 320 patients with coronary heart disease. All patients were treated by coronary intervention, including 78 patients with CIN (CIN group) and 242 patients without CIN (non-CIN group). The clinicopathological data of all patients were listed in Table 1. A further analysis on the curves of receiver operating characteristics (ROC) showed that the AUC of HULC and miR-512 was 0.78 and 0.62, respectively (Fig. 1).
MiR-512 is negatively correlated with HULC and PGE1
We then detected the expression of HULC, miR-512 and PGE1 in these two groups. As shown in Fig. 2, the patients with CIN showed higher expression of miR-512 (Fig. 2A) and lower expression of HULC (Fig. 2B) and PGE1 (Fig. 2C). We further analyzed the correlation between the expression of miR-512 and HULC (Fig. 3A) as well as the correlation between the expression of miR-512 and PGE1 (Fig. 3B). The results revealed a negative correlation between miR-512 and HULC/PGE1.
Regulatory relationships between miR-512 and HULC/PTGS1
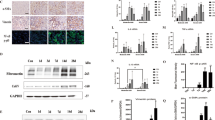
TargetScan, Pictar-Vert, and Microrna.org were employed in this study to search for potential targets of miR-512 involved in CIN. HULC and PTGS1 were identified as potential targets of miR-512. Both the 3′-UTRs of HULC and PTGS1 carried a binding site for miR-512 (Figs. 4A, 5A), suggesting that HULC and PTGS1 mRNAs may act as direct targets of miR-512. To verify the role of HULC and PTGS1 mRNAs as targets of miR-512, we constructed vectors containing wild-type or mutant 3′-UTRs of HULC/PTGS1 (Figs. 4B, 5B). The wild-type and mutant vectors were then co-transfected into THP-1 cells with miR-512 or miR-512 NC. The transfection efficiency was normalized by the transfection with a Renilla reporter vector. As shown in Figs. 4B and 5B, miR-512 significantly decreased the relative luciferase activity of wild-type HULC and PTGS1 3′-UTRs (by more than 60%), whereas the reduction in the luciferase activity of mutant HULC and PTGS1 3′-UTRs was not as significant, suggesting that miR-512 could directly bind to the 3′-UTRs of HULC and PTGS1. Also, THP-1 cells were respectively transfected with miR-512 precursors or scramble control miRNAs. Accordingly, the expression of PTGS1 mRNA (Fig. 5C) and protein (Fig. 5D) were both down-regulated by the transfection of miR-512 precursors. Taken together, these findings indicated that HULC and PTGS1 are direct targets of miR-512.
MiR-512 negatively regulated HULC expression in THP-1 cells. (A) Putative binding sites of miR-512 on the 3′-UTR of HULC (white sequences) were predicted by TargetScan. (B) MiR-512 down-regulated the luciferase activity of wild-type HULC 3′-UTR, but did not affect the luciferase activity of mutant HULC 3′-UTR (the white sequences were mutated).
MiR-512 negatively regulated PTGS1 expression in THP-1 cells. (A) Putative binding sites of miR-512 on the 3′-UTR of PTGS1 (white sequences) were predicted by TargetScan. (B) MiR-512 down-regulated the luciferase activity of wild-type PTGS1 3′-UTR, but did not affect the luciferase activity of mutant PTGS1 3′-UTR (the white sequences were mutated). (C) MiR-512 precursors down-regulated the expression of PTGS1 mRNA. (D) MiR-512 precursors down-regulated the expression of PTGS1 protein.
Regulatory relationships among HULC, miR-512, PTGS1 and PGE1
To further evaluate the regulatory relationships among HULC, miR-512, PTGS1 and PGE1, we transfected THP-1 cells with si-HULC or pcDNA-HULC. As shown in Fig. 6, upon the successful transfection of si-HULC (Fig. 6A), the level of miR-512 (Fig. 6B) was increased in the THP-1 cells, along with decreased levels of PTGS1 mRNA (Fig. 6C) and PGE1 protein (Fig. 6D). Moreover, by observing the effect of si-HULC on autophagy indicative marker proteins (Fig. 6E), a significant increase of ATG7, LC3-II and caspase-3 upon the transfection of si-HULC in THP-1 cells was presented by the Western blot results, indicating the promoted level of autophagy. And the according apoptosis of THP-1 cells was also aggravated by the knockdown of HULC (Fig. 6F). On the contrary, in the THP-1 cells transfected with pcDNA-HULC or co-transfection of pcDNA-HULC and scramble controls (Fig. 7A), the level of miR-512 (Fig. 7B), as well as autophagy indicative marker proteins (Fig. 7E), was decreased along with increased levels of PTGS1 mRNA (Fig. 7C) and PGE1 protein (Fig. 7D), while the co-transfection of pcDNA-HULC and miR-512 precursors in THP-1 cells (Fig. 7A) partly reverted the increased PTGS1 mRNA (Fig. 7C) and PGE1 mRNA (Fig. 7D) levels. And the cell apoptosis was also suppressed by the presence of pcDNA-HULC, which was partly restored by the co-transfection of miR-512 precursors (Fig. 7F). These results indicated the presence of a negative relationship between HULC and miR-512, whereas a positive relationship was present between HULC and PTGS1/PGE1.
Regulatory relationships among HULC, miR-512, PTGS1 and PGE1 in THP-1 cells transfected with si-HULC. (A) Expression level of HULC in THP-1 cells transfected with si-HULC or control siRNA. (B) Expression level of miR-512 in THP-1 cells transfected with si-HULC or control siRNA. (C) Expression levels of PTGS1 mRNA in THP-1 cells transfected with si-HULC or control siRNA. (D) Expression level of PGE1 mRNA in THP-1 cells transfected with si-HULC or control siRNA. (E) Expression level of autophagy indicative marker proteins including ATG7, LC3-II and caspase-3 in THP-1 cells transfected with si-HULC or control siRNA. (F) Cell apoptosis of THP-1 cells transfected with si-HULC or control siRNA.
Regulatory relationships among HULC, miR-512, PTGS1 and PGE1 in THP-1 cells transfected with an empty vector, pcDNA-HULC, pcDNA-HULC + scramble control, or pcDNA-HULC + miR-512 precursors. (A) Expression level of HULC in THP-1 cells transfected with an empty vector, pcDNA-HULC, pcDNA-HULC + scramble control, or pcDNA-HULC + miR-512 precursors. (B) Expression level of miR-512 in THP-1 cells transfected with an empty vector, pcDNA-HULC, pcDNA-HULC + scramble control, or pcDNA-HULC + miR-512 precursors. (C) Expression levels of PTGS1 mRNA in THP-1 cells transfected with an empty vector, pcDNA-HULC, pcDNA-HULC + scramble control, or pcDNA-HULC + miR-512 precursors. (D) Expression level of PGE1 mRNA in THP-1 cells transfected with an empty vector, pcDNA-HULC, pcDNA-HULC + scramble control, or pcDNA-HULC + miR-512 precursors. (E) Expression level of autophagy indicative marker proteins including ATG7, LC3-II and caspase-3 in THP-1 cells transfected with an empty vector, pcDNA-HULC, pcDNA-HULC + scramble control, or pcDNA-HULC + miR-512 precursors. (F) Cell apoptosis of THP-1 cells transfected with an empty vector, pcDNA-HULC, pcDNA-HULC + scramble control, or pcDNA-HULC + miR-512 precursors.
Discussion
CIN is a frequently diagnosed complication in diabetes patients undergoing contrast-enhanced CT20. Due to its serious consequences, such as extended hospitalization and increased mortality/mortality associated with the increased risks of renal dysfunction, CIN has become a research hotspot in recent years21. In this study, we enrolled 320 patients with coronary heart disease. All patients have undergone coronary intervention, including 78 patients with CIN and 242 patients without CIN. The patients with CIN showed lower levels of HULC/PGE1 expression and a higher level of miR-512 expression. In addition, a negative correlation between miR-512 and HULC/PGE1 was found.
PGE1 is a naturally occurring prostaglandin that can exert multiple effects, such as inhibiting the aggregation and activation of thrombocytes and neutrophils, protecting endothelial cells, and triggering vasodilation22. Moreover, PGE1 has been widely used to treat ischemic heart diseases. Clinical studies have shown the potential of using PGE1 to alleviate ischemia–reperfusion injury and to improve myocardial microcirculations23. Cyclooxygenases, also termed as prostaglandin H synthases, have two isoforms, i.e., COX-1/COX-2. Recently, COX-1 has emerged as an important player in the neuroinflammation of the CNS24. In aging rats, the brain expression of COX-1 and TXB2 is increased to render brain more susceptible to neurodegeneration and inflammation. Indeed, COX-1 is implicated in many inflammatory processes by increasing the level of PG25. In this study, HULC and PTGS1 were identified as potential targets of miR-512. The validity of such regulatory relationships was confirmed by co-transfecting THP-1 cells with wild-type/mutant 3′-UTRs of HULC/PTGS1 and miR-512/NC. The results revealed that miR-512 could directly bind to the 3′-UTRs of HULC and PTGS1. Meanwhile, after THP-1 cells were transfected with si-HULC or pcDNA-HULC, the results indicated the presence of a negative relationship between HULC and miR-512, as well as the presence of a positive relationship between HULC and PTGS1/PGE1.
COX-2, an inducible type of COX, acts as an important mediator of cancer metastasis. In fact, the overexpression of COX-2 is frequently seen in advanced lung cancer with poor prognosis26. The elevation of COX-2 expression in tumor tissues also promotes the expression of prostaglandin E2 (PGE2), a metabolite of COX-2. PGE2 can act as a ligand for G protein-coupled receptors to stimulate the activation of ERK1/2 and PI3K/Akt pathways, which in turn increase the invasiveness of cancer cells and induce tumorigenesis27. Recently, it was shown that, by reducing the expression of protein phosphatase 2A (PP2A), COX-2/PGE2 can induce the expression of migration inducing gene-7 (MIG-7) to activate the PI3K/Akt/GSK-3β pathway and subsequently promote lung cancer metastasis28. Many strategies, including the use of statins, antioxidants, and hydration agents, have been adopted to prevent CIN5. As an exogenous form of PGE1, Alprostadil can reduce radiocontrast-associated nephrotoxicity29. Sketch also showed that PGE1 apparently decreased the level of serum creatinine in patients of renal insufficiency, suggesting that vasodilators may reduce the risk of CIN30. COX-2 can be found in different parts of the kidney. By promoting the synthesis of prostaglandins, COX-2 is also implicated in the continuous synthesis of prostanoids by the kidney to control renal functions. The most important function of COX-2 is to convert arachidonic acids to PGD2, TXA2, PGI2, and PGE2 via specific synthases31. As a key product derived from the metabolism of arachidonic acids, PGE2 plays important roles in regulating inflammatory responses and pain32. An isoform of PGES, mPGES-1, is highly expressed in the lungs, spleen and macrophages upon proinflammatory stimulation33,34. Furthermore, COX-2 also exerts an important effect on cisplatin-induced renal injury35. For example, three days of treatment with 20 mg/kg of cisplatin significantly upregulated the levels of COX-2, mPGES-1 and PGE2 in the kidney, although the treatment with cisplatin showed no effect on the expression of COX-2, cytosolic PGES, and mPGES-2. Nevertheless, mPGES-1 knockout mice are resistant to cisplatin-induced renal injury by suppressing the activity of inflammatory cytokines. The above results demonstrated that the renal activation of the COX-2/mPGES-1 pathway may regulate cisplatin-induced nephrotoxicity. Similarly, another study showed that a five-day administration of 13 mg/kg of cisplatin in mice increased the level of COX-2 in renal interstitial cells, while the concomitant administration of a COX-2 selective inhibitor and cisplatin alleviated cisplatin-induced renal injury by reducing the levels of oxidative stress and inflammatory responses36.
There are limitations in this study: firstly, the THP-1 cells used in this study is a cell line of peripheral blood monocytes rather than a renal cell line and more functional analysis using cell lines of different origins especially renal origins are needed to confirm the conclusion of the present study. Secondly, the sample size of this study is relatively small and the sampled were not renal tissuses. Further study with large scale samples especially with kidney samples are warranted. Lastly, more sophisticated and comprehensive functional analysis to further explore the underlying molecular mechanism is also needed to identify the complexed regulatory signaling pathways and networks involved in the pathogenesis of CIN.
Conclusion
In summary, the findings of this study demonstrated that the deregulation of lncRNA-HULC and miR-512 might be involved in the pathogenesis of CIN. Furthermore, we also found that PTGS1, a key enzyme of PGE1, acted as a direct target of miR-512, and the dysregulation of PTGS1/PGE1 could be the downstream effectors of HULC and miR-512 involved in the pathogenesis of CIN.
References
McCullough, P. A. & Sandberg, K. R. Epidemiology of contrast-induced nephropathy. Rev. Cardiovasc. Med. 4(Suppl 5), S3-9 (2003).
Hogan, S. & Gurm, H. S. Preventing contrast-induced nephropathy in patients undergoing primary PCI. J. Invasive Cardiol. 23, 451–452 (2011).
Miao, Y. et al. Alprostadil plays a protective role in contrast-induced nephropathy in the elderly. Int. Urol. Nephrol. 45, 1179–1185. https://doi.org/10.1007/s11255-013-0484-1 (2013).
Firouzi, A. et al. Efficacy of pentoxifylline in prevention of contrast-induced nephropathy in angioplasty patients. Int. Urol. Nephrol. 44, 1145–1149. https://doi.org/10.1007/s11255-011-0053-4 (2012).
Wu, M. Y. et al. The effectiveness of N-Acetylcysteine in preventing contrast-induced nephropathy in patients undergoing contrast-enhanced computed tomography: a meta-analysis of randomized controlled trials. Int. Urol. Nephrol. 45, 1309–1318. https://doi.org/10.1007/s11255-012-0363-1 (2013).
Ristimaki, A. et al. Expression of cyclooxygenase-2 in human transitional cell carcinoma of the urinary bladder. Am. J. Pathol. 158, 849–853. https://doi.org/10.1016/S0002-9440(10)64033-3 (2001).
Kitayama, W., Denda, A., Okajima, E., Tsujiuchi, T. & Konishi, Y. Increased expression of cyclooxygenase-2 protein in rat urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Carcinogenesis 20, 2305–2310 (1999).
Hammam, O. A., Aziz, A. A., Roshdy, M. S. & Abdel Hadi, A. M. Possible role of cyclooxygenase-2 in schistosomal and non-schistosomal-associated bladder cancer. Medscape J. Med. 10, 60 (2008).
Komhoff, M., Grone, H. J., Klein, T., Seyberth, H. W. & Nusing, R. M. Localization of cyclooxygenase-1 and -2 in adult and fetal human kidney: implication for renal function. Am. J. Physiol. 272, F460–F468. https://doi.org/10.1152/ajprenal.1997.272.4.F460 (1997).
Rangel, E. B., Moura, L. A., Franco, M. F. & Pacheco-Silva, A. Up-regulation of cyclooxygenase-2 in different grades of acute human renal allograft rejection. Prostaglandins Leukot Essent. Fatty Acids 76, 235–243. https://doi.org/10.1016/j.plefa.2007.01.005 (2007).
Cheng, H. F. et al. Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney Int. 62, 929–939. https://doi.org/10.1046/j.1523-1755.2002.00520.x (2002).
Berezikov, E., Cuppen, E. & Plasterk, R. H. Approaches to microRNA discovery. Nat. Genet. 38(Suppl), S2-7. https://doi.org/10.1038/ng1794 (2006).
Batista, P. J. & Chang, H. Y. Long noncoding RNAs: cellular address codes in development and disease. Cell 152, 1298–1307. https://doi.org/10.1016/j.cell.2013.02.012 (2013).
Salmena, L., Poliseno, L., Tay, Y., Kats, L. & Pandolfi, P. P. A ceRNA hypothesis: the Rosetta stone of a hidden RNA language?. Cell 146, 353–358. https://doi.org/10.1016/j.cell.2011.07.014 (2011).
Geng, N. et al. Prostaglandin E1 administration for prevention of contrast-induced acute kidney injury: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 97, e11416. https://doi.org/10.1097/MD.0000000000011416 (2018).
Wang, J. et al. Alprostadil protects type 2 diabetes mellitus patients treated with metformin from contrast-induced nephropathy. Int Urol Nephrol 49, 2019–2026. https://doi.org/10.1007/s11255-017-1639-2 (2017).
Tang, S. H. et al. Expression of cyclooxygenase-2 is correlated with lncRNA-COX-2 in cirrhotic mice induced by carbon tetrachloride. Mol. Med. Rep. 15, 1507–1512. https://doi.org/10.3892/mmr.2017.6161 (2017).
Tong, Q. et al. LincRNA-Cox2 modulates TNF-alpha-induced transcription of Il12b gene in intestinal epithelial cells through regulation of Mi-2/NuRD-mediated epigenetic histone modifications. FASEB J. 30, 1187–1197. https://doi.org/10.1096/fj.15-279166 (2016).
Xiong, H. et al. lncRNA HULC promotes the growth of hepatocellular carcinoma cells via stabilizing COX-2 protein. Biochem. Biophys. Res. Commun. 490, 693–699. https://doi.org/10.1016/j.bbrc.2017.06.103 (2017).
Solomon, R. & Dauerman, H. L. Contrast-induced acute kidney injury. Circulation 122, 2451–2455. https://doi.org/10.1161/CIRCULATIONAHA.110.953851 (2010).
Berg, K. J. Nephrotoxicity related to contrast media. Scand. J. Urol. Nephrol. 34, 317–322. https://doi.org/10.1080/003655900750048341 (2000).
Zhao, X. S., Pan, W., Bekeredjian, R. & Shohet, R. V. Endogenous endothelin-1 is required for cardiomyocyte survival in vivo. Circulation 114, 830–837. https://doi.org/10.1161/CIRCULATIONAHA.105.577288 (2006).
Fang, W. T., Li, H. J. & Zhou, L. S. Protective effects of prostaglandin E1 on human umbilical vein endothelial cell injury induced by hydrogen peroxide. Acta Pharmacol. Sin. 31, 485–492. https://doi.org/10.1038/aps.2010.23 (2010).
Garcia-Bueno, B., Serrats, J. & Sawchenko, P. E. Cerebrovascular cyclooxygenase-1 expression, regulation, and role in hypothalamic-pituitary-adrenal axis activation by inflammatory stimuli. J. Neurosci. 29, 12970–12981. https://doi.org/10.1523/JNEUROSCI.2373-09.2009 (2009).
Choi, S. H., Aid, S., Choi, U. & Bosetti, F. Cyclooxygenases-1 and -2 differentially modulate leukocyte recruitment into the inflamed brain. Pharmacogenomics J. 10, 448–457. https://doi.org/10.1038/tpj.2009.68 (2010).
Brown, J. R. & DuBois, R. N. Cyclooxygenase as a target in lung cancer. Clin. Cancer Res. 10, 4266s–4269s. https://doi.org/10.1158/1078-0432.CCR-040014 (2004).
Chell, S., Kaidi, A., Williams, A. C. & Paraskeva, C. Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim. Biophys. Acta 1766, 104–119. https://doi.org/10.1016/j.bbcan.2006.05.002 (2006).
Ho, M. Y., Liang, S. M., Hung, S. W. & Liang, C. M. MIG-7 controls COX-2/PGE2-mediated lung cancer metastasis. Cancer Res. 73, 439–449. https://doi.org/10.1158/0008-5472.CAN-12-2220 (2013).
Koch, J. A., Plum, J., Grabensee, B. & Modder, U. Prostaglandin E1: a new agent for the prevention of renal dysfunction in high risk patients caused by radiocontrast media? PGE1 Study Group. Nephrol. Dial Transplant. 15, 43–49 (2000).
Sketch, M. H. Jr. et al. Prevention of contrast media-induced renal dysfunction with prostaglandin E1: a randomized, double-blind, placebo-controlled study. Am. J Ther. 8, 155–162 (2001).
Khan, K. N. et al. Interspecies differences in renal localization of cyclooxygenase isoforms: implications in nonsteroidal antiinflammatory drug-related nephrotoxicity. Toxicol. Pathol. 26, 612–620. https://doi.org/10.1177/019262339802600504 (1998).
Breyer, M. D. & Breyer, R. M. G protein-coupled prostanoid receptors and the kidney. Annu. Rev. Physiol. 63, 579–605. https://doi.org/10.1146/annurev.physiol.63.1.579 (2001).
Trebino, C. E. et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc. Natl. Acad. Sci. U.S.A. 100, 9044–9049. https://doi.org/10.1073/pnas.1332766100 (2003).
Boulet, L. et al. Deletion of microsomal prostaglandin E2 (PGE2) synthase-1 reduces inducible and basal PGE2 production and alters the gastric prostanoid profile. J. Biol. Chem. 279, 23229–23237. https://doi.org/10.1074/jbc.M400443200 (2004).
Jia, Z. et al. Amelioration of cisplatin nephrotoxicity by genetic or pharmacologic blockade of prostaglandin synthesis. Kidney Int. 79, 77–88. https://doi.org/10.1038/ki.2010.331 (2011).
Honma, S. et al. Amelioration of cisplatin-induced mouse renal lesions by a cyclooxygenase (COX)-2 selective inhibitor. Eur. J. Pharmacol. 715, 181–188. https://doi.org/10.1016/j.ejphar.2013.05.023 (2013).
Author information
Authors and Affiliations
Contributions
L.Z. and P.L. planned the study, B.Z. and M.Y. reviewed the literature, B.Z., M.Y., R.X., H.W. and S.C. collected and analyzed data, L.Z. and B.Z. drafted the manuscript, and all authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, L., Li, P., Zhang, Bl. et al. Dysregulation of HULC promotes contrast-induced nephropathy (CIN) via regulating signaling pathway of miRNA-512 and prostaglandin E1 (PGE1). Sci Rep 10, 11691 (2020). https://doi.org/10.1038/s41598-020-68634-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68634-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.