Abstract
Renal fibrosis is a failed regenerative process that facilitates chronic kidney disease progression. The current study was designed to study the effect of erlotinib, a receptor tyrosine kinase inhibitor, on the progression of renal fibrosis. The study included four groups of mice: control group; adenine group: received adenine (0.2% w/w) daily with food for 4 weeks; erlotinib group: received 80 mg/kg/day erlotinib orally (6 ml/kg/day, 1.3% w/v suspension in normal saline 0.9%) for 4 weeks; adenine + erlotinib group: received adenine and erlotinib concurrently. Kidney function and antioxidant biomarkers were measured. Renal expression of Bcl2 and p53 and histopathological changes (tubular injury and renal fibrosis) were scored. Renal tissue levels of transforming growth factor-β1, p-ERK1/2 and p-STAT3 were measured. Results obtained showed significant decrease (P < 0.001) in serum creatinine, urea and uric acid in erlotinib + adenine group. Level of malondialdehyde was decreased significantly (P < 0.001) while reduced glutathione and catalase levels were increased (P < 0.01) by erlotinib concurrent administration. Erlotinib markedly reduced fibrosis and tubular injury and decreased TGF-β1, p-ERK1/2 and p-STAT3 (P < 0.5). In addition, expression level of Bcl-2 was elevated (P < 0.001) while that of p53-was reduced compared to adenine alone. Erlotinib can attenuate renal fibrosis development and progression through anti-fibrotic, antioxidant and anti-apoptotic pathways.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a global health burden and is considered as an independent risk factor for cardiovascular diseases. Its prevalence is correlated to many health problems as diabetes, hypertension and obesity1, 2.
Renal fibrosis is a failed regenerative process that facilitates the development of CKD. The progression of renal insufficiency in patients with CKD is mainly due to tubulointerstitial fibrosis, glomerular inflammation and tubular atrophy3. Many cellular events are involved in tubulointerstitial fibrosis as infiltration of inflammatory cells, fibroblast activation, extracellular matrix (ECM) deposition and microvascular rarefaction4.
Various mediators are involved in the development of CKD and may represent possible targets for therapy such as transforming growth factor (TGF)-β, nuclear factor-erythroid 2 related factor, peroxisome proliferator-activated γ receptor, IL-11 and advanced glycation endproducts5, 6. Recently, studies reported involvement of miRNA in the progression of CKD progression7.
Tyrosine kinases have a pivotal role in cell signalling, and their activity deregulation can promote the development and progression of fibrotic diseases. As a result, pathologic activation of tyrosine kinases can drive fibrogenesis8.
Epidermal growth factor receptor (EGFR), a receptor tyrosine kinase, has been identified in renal tissue mainly in the epithelial cells of distal and collecting tubules, peritubular vessels and glomeruli with more abundant expression in diseased than in normal kidneys9, 10. Activation of these receptors has been found to contribute in various experimental models of CKD as diabetic nephropathy and glomerulonephritis11,12,13. Recently, targeting EGFR by blocking and/or interfering with its signalling pathway showed a protective effect against renal fibrosis14, 15.
Erlotinib is an EGFR tyrosine kinase selective reversible inhibitor that competes with the binding of ATP to the receptor intracellular domain. It thereby inhibits receptor autophosphorylation and blocks downstream signals16. Erlotinib is used in treatment of different types of cancer as lung cancer17 and head and neck squamous cell carcinoma18. Many studies showed beneficial effects of erlotinib in different kidney injuries. Matar et al. (2013) demonstrated that erlotinib can prevent salt retention and partially preserve renal function in nephrotic rats19. It can also suppress progression of glomerulonephritis through attenuating ultrastructural alterations of podocytes20. Yamamoto et al. (2018) revealed that erlotinib can attenuate progression of CKD in 5/6Nx rats21.
Collectively, EGFR signaling presents an interesting therapeutic target in the treatment of fibrotic renal disease. The present study was carried out to investigate effect of erlotinib as an EGFR inhibitor in an experimental model of chemically induced nephrotoxicity and fibrosis.
Results
Kidney function biomarkers, tubular injury and fibrosis
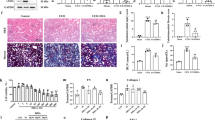
Administration of adenine orally with food daily for 4 weeks resulted in significant elevation in serum creatinine, urea (P < 0.001) and uric acid (P < 0.01) levels compared to control group (Fig. 1a, b, c respectively). Also, there was a significant high albumin level in urine collected from this group (P < 0.001) (Fig. 1d). In line with these results, histopathological examination revealed marked tubular injury and significant fibrosis in adenine received group (Fig. 2a). Kidney sections isolated from adenine received mice were infiltered with remarkable mixed inflammatory cells (macrophages and neutrophils) indicating inflammation (Fig. 2b).
Representative photographs for kidney sections stained with a. Masson trichrome stain (× 400) showing fibrotic areas (arrows) (b). Hematoxylin–eosin stain (× 400) showing tubular injury and infiltration of inflammatory cells (arrows). A: control group B: adenine group C: erlotinib group D: adenine + erlotinib group.
Administration of erlotinib concurrently with adenine resulted in significant (P < 0.01) decrease in levels of kidney function biomarkers compared to their elevated levels in group received adenine alone. The measured biomarkers (albumin in urine, serum creatinine and uric acid) in adenine + erlotinib group were comparable to control group level but urea level remained significantly high compared to control group level (P < 0.01).
Administration of erlotinib with adenine significantly decreased tubular injury score by about 1.5-fold when compared to adenine group score. Besides, it significantly decreased fibrosis score by about 2.4-fold when compared to fibrosis score in adenine group (Table 1).
Lipid peroxidation and antioxidant biomarkers
Adenine administration increased level of malondialdehyde (MDA) significantly (P < 0.001) while the group received erlotinib concurrently with adenine showed a decreased (P < 0.001) MDA level compared to adenine group (Fig. 3a). Reduced glutathione (GSH) level in kidney homogenate (Fig. 3b) was significantly high (P < 0.001) in adenine + erlotinib group compared with its level in adenine group. Notably, GSH level in the former group was still lower than the control group level (P < 0.001).
Effect of erlotinib (80 mg/kg) administration on renal (a) malondialdehyde (MDA) level, (b) reduced glutathione (GSH) level, (c) total antioxidant capacity and (d) catalase activity in adenine induced nephrotoxicity in mice. Significance: *, **, *** P < 0.05, 0.01, 0.001 compared to control group; ‡‡‡ P < 0.001 compared to adenine group.
Erlotinib significantly increased (P < 0.001) total antioxidant capacity when administered with adenine compared to adenine alone (Fig. 3c). Catalase level in renal tissue was significantly low in groups received adenine alone (P < 0.001) and erlotinib alone (P < 0.01). Concurrent administration of both adenine and erlotinib significantly elevated catalase level (P < 0.001) but remained significantly lower than control group level (P < 0.05) (Fig. 3d).
Profibrogenic marker TGF-β1, p-ERK1/2 and phospho-STAT3
Measuring level of TGF-β1 in kidney tissue showed an elevated level of the fibrogenic marker significantly (P < 0.001) in adenine group compared to control group (Fig. 4a). In adenine + erlotinib group, TGF-β1 level was significantly (P < 0.001) less than its level in adenine group. Similar results were obtained when measuring kidney levels of phosphorylated extracellular signal-regulated kinases-1 and 2 (p-ERK1/2) [Fig. 4b].
Effect of erlotinib (80 mg/kg) administration on renal levels of (a) transforming growth factor (TGF)-β1, (b) phospho-signal transducer and activator of transcription (STAT)-3, (c) p-extracellular signal-regulated kinase (ERK)-1/2 in adenine induced nephrotoxicity in mice. Significance: *, **, *** P < 0.05, 0.01, 0.001 compared to control group; ‡‡‡ P < 0.001 compared to adenine group.
Renal phosphorylated signal transducer and activator of transcription-3 (phospho-STAT3) level (Fig. 4c) was significantly (P < 0.001) high in adenine received group compared to its level in control group. The group received erlotinib alone also showed a significant (P < 0.05) high level of phospho-STAT3. Erlotinib administration with adenine significantly lowered (P < 0.001) phospho-STAT3 level compared to adenine group level. Phospho-STAT3 level in adenine + erlotinib group was significantly higher than control group level (P < 0.01).
Expression of Bcl-2 and p-53
Figure 5a shows expression scores of B-cell lymphoma (Bcl)-2 in immuno-stained kidney sections (Fig. 5b). Adenine administration resulted in 1.8-fold decrease in Bcl-2 expression compared to control group. Administration of erlotinib concurrently with adenine significantly increased Bcl-2 expression (P < 0.001) compared to adenine alone. Expression scores in erlotinib and adenine + erlotinib groups (P < 0.01, 0.05 respectively) were significantly higher than control group score.
(a) Effect of erlotinib (80 mg/kg) administration on kidney expression scores of B-cell lymphoma (Bcl)-2 in adenine induced nephrotoxicity in mice. Significance: *, ** P < 0.05, 0.01 compared to control group; ‡‡‡ P < 0.001 compared to adenine group. (b) Representative photographs for immuno-stained kidney sections isolated from A: control group B: adenine group C: erlotinib group D: adenine + erlotinib group showing expression of Bcl-2 (arrows).
On the other hand, expression of the tumor protein p-53 in adenine group was markedly increased by ≈ 3-fold compared to its expression in control group (Fig. 6a). Expression was significantly decreased in erlotinib and adenine + erlotinib groups (P < 0.001) compared to adenine group but still significantly (P < 0.05, 0.001 respectively) higher than control group. Immuno-stained sections representing p-53 expression is shown in Fig. 6b.
(a) Effect of erlotinib (80 mg/kg) administration on kidney expression scores of tumor protein p-53 in adenine induced nephrotoxicity in mice. Significance: *, *** P < 0.05, 0.001 compared to control group; ‡‡‡ P < 0.001 compared to adenine group. (b) Representative photographs for immuno-stained kidney sections isolated from A: control group B: adenine group C: erlotinib group D: adenine + erlotinib group showing expression of p-53 (arrows).
Discussion
Renal fibrosis has been reported to be a final common pathway in progressive renal diseases that subsequently leads to end-stage renal disease20. Currently, no effective treatment can reverse or even halt the progression of CKD and therapies that target fibrosis underlying mechanisms remains a challenge.
The current study was designed to investigate the effect of erlotinib as an EGFR blocker in protection against adenine induced renal fibrosis in mice. Effect of erlotinib on kidney function biomarkers, oxidative stress and antioxidant biomarkers was evaluated. In addition, renal sections were examined for inflammatory cells infiltration, tubular injury and collagen deposition. Effect on fibrogenic pathways was assessed by measuring TGF-β1 level. We also evaluated effect of erlotinib on expression of p53, Bcl2, phosphor-STAT3 and p-ERK1/2 to track possible mechanism underlying its renoprotective effect.
Dietary adenine has been extensively used to induce progressive kidney injury that highly mimic human CKD. Adenine model was established mainly in mice or rats and was used to test therapeutic strategies that may slow progression or even reverse renal injury and fibrosis22. After oral administration, adenine metabolites precipitates as crystals in renal tubules leading to tubulointerstitial nephritis and vascular calcification as well23.
To our knowledge there is no reported data that signify harmful effects of erlotinib on kidney function. In addition, it has been used safely as an anticancer for small cell lung cancer in hemodialysis patients24. Otherwise, concerns about liver complications has been raised with erlotinib use in treating pancreatic cancer and liver transaminases monitoring was recommended25.
Obtained results showed an elevation in serum urea level in erlotinib group suggesting a metabolic impact of erlotinib as a tyrosine kinase inhibitor on liver cells. Previous studies on cancer cell lines showed an effect of tyrosine kinase inhibitors on N-acetylglutamate synthase a key enzyme in the urea cycle26, 27. We also postulate that erlotinib may interfere with glutamate incorporation in ERK1/2 stimulatory signals through halting glutamate receptors activation by Src tyrosine kinases28, 29. This effect may in turn direct glutamate towards urea formation elevating its blood level.
EGF/EGFR system is involved in several biological processes including proliferation, differentiation and survival. It activates proliferation of tubular cells in autocrine/paracrine manner30. The glomerulus and the tubulointerstitial compartment show specific localization of EGFR10 and interestingly, upregulation of the EGFR and their pathway activation was reported in both human and experimental chronic renal diseases31.
Various publications postulate a key role for EGFR pathway in the pathogenesis of rapidly progressive glomerulonephritis and other forms of glomerulosclerosis, diabetic nephropathy and chronic allograft nephropathy32. In the present study, the elevated levels of serum creatinine, uric acid and urine albumin in adenine group were decreased by concurrent erlotinib administration. This is consistent with the effect of targeting EGF/EGFR by erlotinib in protection against glomerular injury and improvement of glomerular filtration in nephrectomy model21.
The kidney is a highly energetic organ and so it is liable to oxidative stress induced damage. In turn, oxidative stress can enhance renal disease progression33. Studies reported a link between EGF-EGFR and reactive oxygen species (ROS) production. For example, a cross-talk between EGF-EGFR axis and NADPH oxidase mediated ROS signaling pathways was reported. The enzyme NADPH oxidase is considered a major source of ROS in many cell types such as endothelial and smooth muscle cells34, 35. Additionally, EGF addition to human epidermoid carcinoma A431 cells, which highly express the EGFR, resulted in increased levels of intracellular ROS36. Furthermore, a key role of EGFR/AKT/ROS stress signaling in the development of nephropathy has been proved37.
Administration of adenine in our study resulted in high levels of MDA and a reduction in endogenous antioxidant biomarkers. Elevation of renal MDA level was observed in different experimental models of renal fibrosis as unilateral ureteral obstruction and lipopolysaccharide induced renal fibrosis38, 39. Malondialdehyde is an indirect marker of lipid peroxidation. High level of MDA indicates ROS production that initiates tissue injury as an early leading step to fibrosis. In line with previous researches, data obtained suggests a renoprotective effect of erlotinib through an antioxidant capability. It reduces MDA level and promotes different antioxidant pathways such as GSH production, catalase activation and increasing total antioxidant capacity.
In renal injuries, the kidney activates various signaling networks as those containing mitogen-activated protein kinases (MAPKs)40, 41 including ERK1/2 that is mainly activated by mitogenic stimuli such as growth factors and hormones42. Robust ERK activation has been reported in many kidney diseases as polycystic kidney disease43 glomerulonephritis44 and many others. Nowak (2002) suggested a role of ERK1/2 inhibition in attenuating cisplatin induced renal apoptosis and tissue injury45. Moreover, targeting EGFR/ERK1/2 pathway using inhibitors showed slow renal fibrosis progression in different models of fibrosis46,47,48.
In various experimental and human nephropathies, STAT3 is activated in many compartments of the injured kidney49, 50. It contributes to fibrosis by inducing collagen type 1 synthesis with subsequent increased ECM deposition. Kidney fibrosis models attribute TGF-β1 increased level and the subsequent collagen I production to STAT351. Interestingly, both STAT3 pharmacologic inhibition and/or haploinsufficiency have been reported to decrease injury progression in different types of nephropathy52, 53. Our study showed ability of erlotinib to inhibit STAT3 and ERK1/2 phosphorylation activated by adenine and as a result renal injury and fibrosis are attenuated.
Being an important fibrogenic cytokine, TGF-β1 induces production of ECM and alters its degradation and involved in the major pathogenic mechanisms of CKD. In models of renal fibrosis, activation of EGFR signaling was critical for increasing production of TGF-β152, 54. Overexpression of TGF-β1 was found to be mediated through ERK1/2 downstream of EGFR in vitro55. Chen et al. (2011) reported that inhibition of EGFR can attenuate TGF-β1 induced cell mitogenesis via the EGFR-ERK1/2/p38 kinase pathway in cultured renal interstitial fibroblasts56. In line with these findings, erlotinib herein decreased the profibrogenic marker TGF-β1 an effect that may be mediated by halting the activation of ERK1/2 pathway.
Apoptosis is a cell-suicide program that occurs through either death receptor-mediated extrinsic pathway and/or the mitochondria-mediated intrinsic pathway57. The mitochondrial pathway involves the key regulators Bcl-2 family proteins. The balance between the anti-apoptotic member Bcl-2 and the pro-apoptotic member Bax can regulate the activation of caspases. Recent studies reported the downregulation of Bcl-2 in renal tissue and activation of caspase pathway in chronic renal failure58.
Likewise, our results showed that the expression of Bcl-2 was markedly decreased in mice received adenine whereas treatment with erlotinib increased its renal expression suggesting anti-apoptotic effect of erlotinib and so protection against renal tissue damage.
Moreover, activation of the tumor suppressor p53 in tubular cells also play a role in acute kidney injury initiation and maladaptive kidney repair. Inhibition of p53 could be a novel and potential strategy for the treatment acute injury and its progression into CKD59. Samarakoon et al. (2013) suggested that p53 is profibrotic effector required for expression of a subset of profibrotic genes in response to TGF-β160. They demonstrated cooperativity between p53, SMAD3 and EGFR signaling in inducing the profibrotic gene expression. Accordingly, erlotinib ability to regress renal fibrosis in the current study may be, at least in part, attributed to the reduction in p-53 expression in kidney tissue.
Conclusion
The EGFR inhibitor erlotinib can attenuate progression of kidney injury and renal fibrosis. This effect may be mediated at least by antioxidant effect, targeting STAT3 activation, ERK1/2 pathway and the profibrogenic marker TGF-β1. Interestingly, erlotinib was found to modulate apoptosis through up-regulating the anti-apoptotic member Bcl-2. Finally, it interferes with p-53 induced fibrotic genes. Since erlotinib was administered concurrently with adenine and showed an ability to slow fibrosis progression, future research about its ability to reverse fibrosis is warranted. Figure 7 summarizes proposed mechanisms underlying erlotinib renoprotective effect.
Graphical abstract. Bcl B-cell lymphoma, EGF epidermal growth factor, EGFR epidermal growth factor receptor, p-STAT3 phosphorylated signal transducer and activator of transcription-3, p-ERK phosphorylated extracellular signal-regulated kinase, ROS reactive oxygen species, TGF transforming growth factor.
Materials
Animals: Albino mice (n = 40, 25–30 g, adult male, Swiss CD-1 strain) were purchased from VACSERA [Agouza, Giza, Egypt] and housed 10 mice/cage randomly. During accommodation period of 2 weeks mice were allowed food and water ad libitum. The experimental protocol approved by the Scientific Research Ethics Committee (Faculty of Pharmacy, Mansoura University) for the care and use of laboratory animals under approval number 2019-151. The study was carried out according to the guidelines and ethical principles adopted by the mentioned committee.
Chemicals: Adenine (99%) as fine white powder (Oxford laboratory Pvt. Ltd., Mumbai, India; PubChem CID: 190); Erlotinib free base as white powder (LC laboratories, MA, USA; PubChem CID: 176870).
Induction of renal fibrosis
Mice average food consumption in each cage was reported during accommodation. Each cage was found to consume about 50 g food/day. Food was restricted to this average in all groups during the study. Renal fibrosis was induced in mice by receiving adenine with food (0.2% w/w) daily for 4 weeks as previously described by Nemmar et al.61.
Experimental design
Four groups of mice (n = 10 each) were used as follows: control group: received erlotinib vehicle (6 ml/kg/day normal saline orally using oral gavage for 4 weeks); adenine group: received adenine (0.2% w/w) daily with food four 4 weeks in addition to erlotinib vehicle as described in control group; erlotinib group: received 80 mg/kg/day erlotinib62 orally (6 ml/kg/day, 1.3% w/v suspension in normal saline 0.9%) for 4 weeks; adenine + erlotinib group: received adenine and erlotinib concurrently as described above. Schematic presentation of experimental design is shown in Table 2.
Methods
Twenty-four hours following the last adenine dose, mice were anesthetized (50 mg/kg thiopental, i.p), blood samples were collected through cardiac puncture and serum was separated by centrifugation (4000 rpm, 4 °C, 15 min). Kidneys were isolated, washed with saline and cut into pieces longitudinally. To prepare kidney homogenate, kidney pieces were minced manually PBS (10% w/v) using a handheld homogenizer on ice, centrifuged (4000 rpm at 4 °C for 20 min) and the supernatant was separated. Other kidney pieces were fixed in neutral buffered formalin (8% v/v) for 24 h for preparation of paraffin blocks. Urine samples were collected 24 h before sacrifice through transferring mice to metabolic cages along 24 h.
Kidney function and antioxidant biomarkers
Levels (mg/dl) of serum creatinine, urea and uric acid were measured spectrophotometrically as indicated by the manufacturer (Biomed Diagnostics, Egy-Chem, Egypt). Albumin level (μg/dl) in urine was measured using commercial kits (ABC Diagnostics, Egypt).
Level of MDA (nmol/g tissue) as a lipid peroxidation indirect marker was measured in kidney homogenate as described by Ohkawa et al.63. Total antioxidant capacity and GSH level were measured spectrophotometrically (Bio-diagnostic, Giza Egypt) at 505 and 405 nm respectively. Activity of catalase was determined as previously described by Aebi64.
Measurement of renal phospho-STAT3, TGF-β1 and p-ERK1/2
Transforming growth factor- β1 (Cloud Clone Corp., USA), phospho-STAT3 and p-ERK1/2 (Assay Biotechnology Co., CA, USA) were measured in kidney homogenate using sandwich ELISA technique according to the manufacturer’s instructions.
Histopathological examination and immunohistochemistry (IHC)
Kidney sections of 4 μm thickness were prepared from paraffin wax blocks, stained with hematoxylin–eosin (H&E) and examined for tubular injury using light microscope (× 400) according to Kinomura et al. (2008) modified scoring system65. Briefly, randomly selected 20 fields were quantified for: swelling, desquamation from the tubular basement membrane, necrosis and vacuolar degeneration using a score from 0 to 5 as follows: 0: normal; 1: < 20% of the tubules are involved; 2: 20–40%; 3: 40–60%; 4: 60–80%; and 5: 80–100%).
Other kidney sections were stained with Masson trichrome stain for interstitial fibrosis quantification as described by Yamate et al.66. Five random fields were selected and examined with the Image J 1.32 as a color image analyzer where fibrotic area was measured avoiding the blood vessels in the OSOM and cortico-medullary junction.
For IHC, kidney sections were deparaffinized with xylene, dehydrated in decreasing concentrations of ethanol. Sections were treated with 3% H2O2/methanol for 10 min to block endogenous peroxidase activity. For antigen retrieval, sections were processed in EDTA then pre-incubated with normal rabbit serum to block nonspecific binding of antibodies. Kidney sections were incubated overnight with rabbit polyclonal primary antibodies (dilution 1:50) against p53 (ab131442, Abcam) Bcl-2 (PA5-20068, Invitrogen, Thermo Fisher Scientific, CA) at 4 °C.
Sections were incubated with secondary antibodies as described by Kane and Greenhalgh67 after washing with phosphate buffer saline. Slides were then stained (with H&E as a counter stain) and visualized for antibody binding using horseradish peroxidase- diaminobenzidine detection system (GBI Labs, WA, USA). Using a light microscope (X100), expression of p53 (cytoplasmic and nuclear) and Bcl-2 (cytoplasmic) was detected as % of positive cells in 100 cells counted per each high definition field and calculated as the mean of 20 fields in each group68.
Statistical testing
Data (n = 10) are expressed as mean ± S.E.M except IHC scores that are expressed as median with interquartile range. Level of α was set to 0.05 and obtained data were compared using one-way analysis of variance (ANOVA), followed by Tukey–Kramer multiple comparison test as the post-Hoc test. Tubular injury and IHC scores were analyzed with Kruskal Wallis test by rank followed by Dunn's multiple comparison test. Statistical analysis and figures construction were performed using GraphPad Prism V5.01 (GraphPad Software Inc, CA, USA).
Data availability
All data generated or analyzed during this study are included in this published article.
References
Taylor, D. M. et al. Health literacy and patient outcomes in chronic kidney disease: a systematic review. Nephrol. Dial. Transplant. 33, 1545–1558 (2018).
Wu, P. Y., Chao, C. T., Chan, D. C., Huang, J. W. & Hung, K. Y. Contributors, risk associates, and complications of frailty in patients with chronic kidney disease: a scoping review. Ther. Adv. Chronic Dis. 10, 1–23 (2019).
Meran, S. & Steadman, R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 92, 158–167 (2011).
Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7, 684 (2011).
Chen, Y. Y. et al. Redox signaling in aging kidney and opportunity for therapeutic intervention through natural products. Free Radic. Biol. Med. 141, 141–149 (2019).
Corden, B., Adami, E., Sweeney, M., Schafer, S. & Cook, S. A. IL-11 in cardiac and renal fibrosis: late to the party but a central player. Br. J. Pharmacol. 177, 1695–1708 (2020).
Zhao, H., Ma, S. X., Shang, Y. Q., Zhang, H. Q. & Su, W. microRNAs in chronic kidney disease. Clin. Chim. Acta. 491, 59–65 (2019).
Beyer, C. & Distler, J. H. Tyrosine kinase signaling in fibrotic disorders: translation of basic research to human disease. Biochim. Biophys. Acta Mol. Basis Dis. 1832, 897–904 (2013).
Nakopoulou, L. et al. Immunohistochemical study of epidermal growth factor receptor (EGFR) in various types of renal injury. Nephrol. Dial. Transplant. 9, 764–769 (1994).
Yoshioka, K. et al. Identification and localization of epidermal growth factor and its receptor in the human glomerulus. Lab. Invest. 63, 189–196 (1990).
Overstreet, J. M. et al. Selective activation of epidermal growth factor receptor in renal proximal tubule induces tubulointerstitial fibrosis. FASEB J. 31, 4407–4421 (2017).
Lim, A. K. H. & Tesch, G. H. Inflammation in diabetic nephropathy. Mediat. Inflamm. 2012, 146154. https://doi.org/10.1155/2012/146154 (2012).
Bollée, G. et al. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat. Med. 17, 1242–1250 (2011).
Skibba, M. et al. New EGFR inhibitor, 453, prevents renal fibrosis in angiotensin II-stimulated mice. Eur. J. Pharmacol. 789, 421–430 (2016).
Yang, L. et al. Epidermal growth factor receptor mimotope alleviates renal fibrosis in murine unilateral ureteral obstruction model. Clin. Immunol. 205, 57–64 (2019).
Dowell, J., Minna, J. D. & Kirkpatrick, P. Erlotinib hydrochloride. Nat. Rev. Drug Discov. 4, 13–14 (2005).
Lis, C. et al. Development of erasin: a chromone-based STAT3 inhibitor which induces apoptosis in erlotinib-resistant lung cancer cells. Sci. Rep. 7, 1–10 (2017).
Jain, A. P. et al. MAP2K1 is a potential therapeutic target in erlotinib resistant head and neck squamous cell carcinoma. Sci. Rep. 9, 18793. https://doi.org/10.1038/s41598-019-55208-5 (2019).
Matar, R. N., Klein, J. D. & Sands, J. M. Erlotinib preserves renal function and prevents salt retention in doxorubicin treated nephrotic rats. PLoS One 8, e54738. https://doi.org/10.1371/journal.pone.0054738 (2013).
Boor, P., Sebekova, K., Ostendorf, T. & Floege, J. Treatment targets in renal fibrosis. Nephrol. Dial. Transplant. 22, 3391–3407 (2007).
Yamamoto, Y. et al. Erlotinib attenuates the progression of chronic kidney disease in rats with remnant kidney. Nephrol. Dial. Transplant. 33, 598–606 (2018).
Diwan, V., Brown, L. & Gobe, G. C. Adenine-induced chronic kidney disease in rats. Nephrology 23, 5–11 (2018).
Claramunt, D., Gil-Peña, H., Fuente, R., Hernández-Frías, O. & Santos, F. Animal models of pediatric chronic kidney disease. Is adenine intake an appropriate model?. Nefrologia 35, 517–722 (2015).
Togashi, Y. et al. Pharmacokinetics of erlotinib and its active metabolite OSI-420 in patients with non-small cell lung cancer and chronic renal failure who are undergoing hemodialysis. J. Thorac. Oncol. 5, 601–605 (2010).
Gunturu, K. S., Abu-Khalaf, M. & Saif, M. W. Hepatic failure and hepatorenal syndrome secondary to erlotinib: a possible etiology of complications in a patient with pancreatic cancer. JOP 11, 484–485. https://doi.org/10.6092/1590-8577/3443 (2010).
Li, J. et al. Computational analysis of receptor tyrosine kinase inhibitors and cancer metabolism: implications for treatment and discovery of potential therapeutic signatures. BMC Cancer 19, 600. https://doi.org/10.1186/s12885-019-5804-0 (2019).
Pham-Danis, C. et al. Urea cycle sustains cellular energetics upon EGFR inhibition in EGFR-mutant NSCLC. Mol. Cancer Res. 17, 1351–1364 (2019).
Mao, L. M. & Wang, J. Q. Tyrosine phosphorylation of glutamate receptors by non-receptor tyrosine kinases: roles in depression-like behavior. Neurotransmitter (Houst) 3, e1118 (2016).
Kacharmina, J. E., Job, C., Crino, P. & Eberwine, J. Stimulation of glutamate receptor protein synthesis and membrane insertion within isolated neuronal dendrites. Proc. Natl. Acad. Sci. USA 97, 11545–11550 (2000).
Isaka, Y. Epidermal growth factor as a prognostic biomarker in chronic kidney diseases. Ann. Transl. Med. 4(Suppl 1), S62. https://doi.org/10.21037/atm.2016.10.64 (2016).
Sis, B. et al. Epidermal growth factor receptor expression in human renal allograft biopsies: an immunohistochemical study. Transpl. Immunol. 13, 229–232 (2004).
Harskamp, L. R., Gansevoort, R. T., van Goor, H. & Meijer, E. The epidermal growth factor receptor pathway in chronic kidney diseases. Nat. Rev. Nephrol. 12, 496–506 (2016).
Daenen, K. et al. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 34, 975–991 (2019).
Bir, S. C., Kolluru, G. K., Fang, K. & Kevil, C. G. Redox balance dynamically regulates vascular growth and remodeling. Semin. Cell Dev. Biol. 23, 745–757 (2012).
Lassegue, B., San Martin, A. & Griendling, K. K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 110, 1364–1390 (2012).
Weng, M. S., Chang, J. H., Hung, W. Y., Yang, Y. C. & Chien, M. H. The interplay of reactive oxygen species and the epidermal growth factor receptor in tumor progression and drug resistance. J. Exp. Clin. Cancer Res. 37, 61 (2018).
Xu, Z. et al. EGFR inhibition attenuates diabetic nephropathy through decreasing ROS and endoplasmic reticulum stress. Oncotarget 8, 32655–32667 (2017).
Ren, Y. et al. The Sirt1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int. J. Mol. Med. 39, 1317–1324 (2017).
Bargi, R. et al. Thymoquinone protects the rat kidneys against renal fibrosis. Int. J. Pharm. Sci. Res. 12, 479 (2017).
Kasinath, B. S. et al. Novel mechanisms of protein synthesis in diabetic nephropathy—role of mRNA translation. Rev. Endocr. Metab. Disord. 9, 255–266 (2008).
Lieberthal, W. & Levine, J. S. The role of the mammalian target of rapamycin (mTOR) in renal disease. J. Am. Soc. Nephrol. 20, 2493–2502 (2009).
Kyriakis, J. M. & Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869 (2001).
Omori, S. et al. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J. Am. Soc. Nephrol. 17, 1604–1614 (2006).
Bokemeyer, D. et al. Differential activation of mitogen-activated protein kinases in experimental mesangioproliferative glomerulonephritis. J. Am. Soc. Nephrol. 11, 232–240 (2000).
Nowak, G. Protein kinase C-and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na transport, and cisplatin-induced apoptosis in renal cells. J. Biol. Chem. 277, 43377–43388 (2002).
Rodríguez-Peña, A. B. et al. Activation of Erk1/2 and Akt following unilateral ureteral obstruction. Kidney Int. 74, 196–209 (2008).
Wada, Y. et al. Epidermal growth factor receptor inhibition with erlotinib partially prevents cisplatin-induced nephrotoxicity in rats. PloS one 9, e111728. https://doi.org/10.1371/journal.pone.0111728 (2014).
Qian, Y. et al. Novel epidermal growth factor receptor inhibitor attenuates angiotensin II-induced kidney fibrosis. J. Pharmacol. Exp. Ther. 356, 32–42 (2016).
Hernandez-Vargas, P. et al. Suppressors of cytokine signaling regulate angiotensin II-activated Janus kinase-signal transducers and activators of transcription pathway in renal cells. J. Am. Soc. Nephrol. 16, 1673–1683 (2005).
Leonhard, W. N. et al. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model. Am. J. Physiol. Renal Physiol. 300, F1193-1202 (2011).
Li, C. et al. Noncanonical STAT3 activation regulates excess TGF-beta1 and collagen I expression in muscle of stricturing Crohn’s disease. J. Immunol. 194, 3422–3431 (2015).
Lu, T. C. et al. Knockdown of Stat3 activity in vivo prevents diabetic glomerulopathy. Kidney Int. 76, 63–71 (2009).
Pang, M. et al. A novel STAT3 inhibitor, S3I–201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 78, 257–268 (2010).
Liu, N. et al. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J. Am. Soc. Nephrol. 23, 854–867 (2012).
Chen, J. et al. EGFR signaling promotes TGF beta-dependent renal fibrosis. J. Am. Soc. Nephrol. 23, 215–224 (2012).
Chen, S. C. et al. Gefitinib attenuates transforming growth factor-beta1-activated mitogen-activated protein kinases and mitogenesis in NRK-49F cells. Transl. Res. 158, 214–224 (2011).
Gajate, C., Gonzalez-Camacho, F. & Mollinedo, F. Lipid raft connection between extrinsic and intrinsic apoptotic pathways. Biochem. Biophys. Res. Commun. 380, 780–784 (2009).
Wu, D. et al. Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-κ B signaling pathways. Sci. Rep. 7, 455 (2017).
Tang, C. et al. P53 in kidney injury and repair: mechanism and therapeutic potentials. Pharmacol Ther. 195, 5–12 (2019).
Samarakoon, R. et al. Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species. Cell Signal. 25, 2198–2209 (2013).
Nemmar, A. et al. Prolonged pulmonary exposure to diesel exhaust particles exacerbates renal oxidative stress, inflammation and DNA damage in mice with adenine-induced chronic renal failure. Cell. Physiol. Biochem. 38, 1703–1713 (2016).
Zhang, M. Z., Wang, Y., Paueksakon, P. & Harris, R. C. Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes 63, 2063–2072 (2014).
Ohkawa, H., Ohishi, N. & Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358 (1979).
Aebi, H. E. Catalase. In Methods of enzymatic analysis (eds Bergmeyer, H. U. et al.) 273–286 (Verlag Chimie, Weinheim, 1985).
Kinomura, M. et al. Amelioration of cisplatin-induced acute renal injury by renal progenitor-like cells derived from the adult rat kidney. Cell Transplant. 17, 143–158 (2008).
Yamate, J. et al. Immunohistochemical observations on the kinetics of macrophages and myofibroblasts in rat renal interstitial fibrosis induced by cis-diamminedichloroplatinum. J. Comp. Pathol. 112, 27–39 (1995).
Kane, C. D. & Greenhalgh, D. G. Expression and localization of p53 and bcl-2 in healing wounds in diabetic and nondiabetic mice. Wound Repair Regen. 8, 45–58 (2000).
Percicote, A. P. et al. Immunohistochemical expression of p53, BCL-2, BAX and VEGFR1 proteins in nephroblastomas. J. Bras. Patol. Med. Lab. 49, 50–56 (2013).
Acknowledgements
Authors represent their grateful thanks to Prof. Amr A. El-Karef, professor of pathology-Faculty of Medicine-Mansoura University, for carrying out the histopathological examination and scoring.
Author information
Authors and Affiliations
Contributions
A.M.A.: carried out the experimental work; samples collection and parameters analysis; shared in writing the manuscript draft. M.A.S.: shared in writing the manuscript draft and publication. N.M.A.: defined the experimental design and the study protocol; carried out data statistics and interpretation, shared in writing the manuscript and publication. T.M.I.: defined the experimental design, shared in manuscript writing and revision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Awad, A.M., Saleh, M.A., Abu-Elsaad, N.M. et al. Erlotinib can halt adenine induced nephrotoxicity in mice through modulating ERK1/2, STAT3, p53 and apoptotic pathways. Sci Rep 10, 11524 (2020). https://doi.org/10.1038/s41598-020-68480-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68480-7
This article is cited by
-
Ameliorative effect of montelukast against STZ induced diabetic nephropathy: targeting HMGB1, TLR4, NF-κB, NLRP3 inflammasome, and autophagy pathways
Inflammopharmacology (2024)
-
Genistin modulates high-mobility group box protein 1 (HMGB1) and nuclear factor kappa-B (NF-κB) in Ehrlich-ascites-carcinoma-bearing mice
Environmental Science and Pollution Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.