Abstract
The periodic changes of atmospheric CO2 and temperature over the last 5 Myr reveal three features that challenge current climate research, namely: (i) the mid-Pleistocene transition of dominant 41-kyr cycles to dominant 100-kyr cycles, (ii) the absence of a strong precession signal of approximately 20 kyr, and (iii) the cooling through the middle and late Holocene. These features are not directly addressable by Earth’s orbital changes described by Milankovitch. Here we show that a closed photochemical system exposed to a constant illumination source can sustain oscillations. In this simple conceptual model, the oscillations are intrinsic to the system and occur even in the absence of periodic radiative forcing. With proper adaptations to the Earth system, this oscillator explains the main features of past climate dynamics. Our model places photosynthesis and the carbon cycle as key drivers of climate change. We use this model to predict the relaxation of a 1,000 PgC pulse of CO2. The removal of 50% of this CO2 will require one century, and will lead to a warmer and wetter future. However, more pronounced glaciation cycles emerge on the millennial timescale.
Similar content being viewed by others
Introduction
Current atmospheric CO2 levels are the highest of the last 2 million years (Myr)1. The identification of the causes and prediction of the consequences of such high CO2 levels triggered intense scrutiny of drivers of past climate changes2,3,4,5,6. The same drivers should be present today and, together with new anthropogenic drivers, need to be considered in models aiming at the prediction of climate changes. The most prominent features of climate change over the last 5 Myr are the global cooling by 2–3 °C between 5.3 and 0.8 Myr ago, the change from a dominant periodicity of 40 kyr between 5 and 3.5 Myr ago to a dominant periodicity of 100 kyr in the last 800 kyr, and the occurrence of glaciations cycles at both poles in the last 800 kyr1,7,8,9,10. The power spectra of temperature changes captured by Antarctic ice records over the last 420 to 720 kyr confirms the dominance of 100-kyr period followed by minor 40-kyr and ca. 23-kyr periods11,12. The change in the dominant periodicity from approximately 40 kyr to approximately 100 kyr is known as the middle Pleistocene transition (MPT).
Explanations of past climate changes, namely of glaciation cycles, continue to favor orbital forcing13,14 due to Milankovitch cycles15, possibly amplified first by greenhouse gases and then by deglaciation and ice-albedo feedback. Milankovitch cycles relate cyclic variations in insolation to three main orbital parameters: eccentricity (Earth’s orbit changes with periods of 96 and 125 kyr), obliquity (changes in the tilt of the Earth’s axis of rotation with period of 41 kyr) and precession (precession of the equinoxes and movement of the perihelion, with periodicities of 23 and 19 kyr). Precession dominates insolation mainly in the equatorial regions, with the contribution of obliquity reinforced at the solstices and at high latitudes16, while eccentricity has only a very weak effect on insolation. Figure 1 shows changes in summer energy at 65°N17 or in March at 25°N18,19 over the last 5 Myr with respect to their average. Their frequency distributions reveal the irrelevance of eccentricity. Climate changes driven by orbital forcing in the last 800 kyr would require nonlinear effects to amplify insolation changes due to eccentricity that are not present in obliquity or in precession. This anomaly of the astronomical forcing hypothesis is widely recognized17,20,21,22.
(A) Changes in summer energies at 65°N above the insolation threshold of 275 W/m2 relative to average (ca. 5 GJ/cm2 17, centered in zero, red line), and corresponding Fourier series (blue dashed line). (B) Changes in solar energy received at 25°N in the month of March relative to average (ca. 1 GJ/cm2, centered in zero)18,19. Corresponding frequency distributions calculated for the time interval from – 5 Myr to present (C,E), or for the last 500 kyr (D,F).
Figure 1 also shows that the oscillations of summer energy did not systematically depart from the 5 GJ/cm2 average in the last 5 Myr, but eight glacial-interglacial cycles occurred over the last 800 kyr. Attempts to explain these intriguing observations have followed two strategies23. One approach argues that the 100 kyr periods are in fact cycles of approximately 80 kyr or approximately 120 kyr quantized in multiples of the basic obliquity cycle24, and relies on frequency-locking of obliquity and/or precession to an external forcing to allow for skipping some of the obliquity cycles6. The other approach3 associates MPT with an oscillator with a limit-cycle of 100 kyr which, for example, with a linear decrease in atmospheric CO2 reaches an abrupt bifurcation between the linear response to obliquity forcing and the oscillator regime25. However, three major issues remain unresolved: why did ice sheets start to develop over the Northern Hemisphere approximately 3.3–2.7 Myr ago? Why did climate cycles evolve from 40- to 100-kyr periodicities ca. 1 Myr ago? What is the origin of the dominant 100-kyr frequency of the last 5 glacial cycles? Abandoning the hypothesis of orbital forcing raises an even more fundamental question: how can a closed system such as Earth trigger and sustain oscillations on the millennia time scale? The answer to this question should point to a physical mechanism capable of sustained oscillations in a closed system such as Earth.
According to the second law of thermodynamics, in an isolated system, exchanging neither energy nor matter with the outside world, entropy increases monotonically to its maximum at equilibrium. An open system can exhibit persistent oscillations of some of its components because there is a constant influx of another component. The first efforts to find open systems with undamped oscillations appeared in chemical sciences and were authored by Lotka26, but it was the explanation of Volterra for predator–prey population relations27 that popularized undamped oscillations. Prigogine demonstrated the possibility of having chemical oscillations of intermediates of an overall reaction A + B → C + D28. The mechanism proposed, named Brusselator29, is the prototypical model of an autocatalytic chemical reaction providing oscillations and even chaos when two Brusselators are coupled30. The first experimental observation of oscillations was the Belousov-Zhabotinsky (BZ) reaction31, that undergoes approximately 100 oscillations before achieving the final equilibrium state. Field and Noyes proposed a general kinetic scheme, named Oregonator, to explain such oscillations and showed that sustained oscillations required some chemical components to remain constant32, i.e., sustained oscillations required influx of material. This led to the believe that a closed system at constant temperature and pressure should attain equilibrium33. Sustained oscillations were only anticipated for open systems described by a set of non-linear chemical reactions with autocatalysis and feedback34.
Earth is a (nearly) closed system receiving an influx of energy from the Sun. The oscillators mentioned above cannot represent an internal Earth system mechanism with glaciation cycles. However, a closed chemical reaction system where a net product of other steps is photochemically transformed back in the reactant may have sustained oscillations35. Photosynthesis can maintain the state of disequilibrium on Earth, but storage and release of free energy alone are insufficient to drive oscillations. Persistent oscillations also require autocatalysis, where one component X is a catalyst of its own production, D + X → Z + 2X.
We present a simple conceptual model for intrinsic oscillations in a closed system under constant illumination, such as the Earth, that accounts for the major trends of temperature, CO2 and biomass cycles over the last 5 million years. We design the simplest oscillator for a closed system that can sustain periodic changes of its composition under constant illumination, and then add the minimum complexity required to represent the Earth system. The model only employs two adjustable parameters, which are fitted to historic CO2 cycles, but incorporates a mechanism that triggers oscillations. This trigger is associated with a change in the global efficiency of photosynthesis in the Pliocene. The oscillations are enabled by an autocatalytic step. The dominant periodicity of 100 kyr arises naturally from the timescales of CO2 land uptake, ocean invasion, reaction with calcium carbonate and silicate weathering, once the efficiency of photosynthesis increased. We use this model to predict long-term climate consequences of a large CO2 pulse.
Results
Oscillations in a closed system under illumination
Dynamical systems are often described by a set of ordinary differential equations (ODEs) that contain parameters in addition to variables. In some systems, similar sets of parameter values lead to dynamic behaviors of qualitatively different nature. For example, one set of parameters may lead to a stable equilibrium point—an attractor—whereas the other set leads to an asymptotically stable periodic solution—a limit cycle. Such models are said to contain bifurcation points. The case given above, where an attractor becomes a limit cycle as a parameter is varied, is named a supercritical Hopf bifurcation. When such a bifurcation is present, the system may exhibit exponentially damped oscillations leading to equilibrium or to limit cycle oscillations36. These limit cycles are periodic orbits when represented as trajectories in the phase space of the variables. Chemical oscillations arise from Hopf bifurcations in the Oregonator32, in the oscillations of glycolysis37 and in the Brusselator29.
Following the tradition of naming oscillators based on their geographical origin, we name Coimbrator the set of ODEs represented in Fig. 2. They enable “self-oscillations” of composition variables as a parameter crosses a critical value. Figure 2 emphasizes that the Coimbrator is a closed system with two photochemical steps. The equations are at most bimolecular, contain three composition variables (X, Y, Z) and include an autocatalytic reaction, which are the minimal sufficient conditions for realistic oscillatory behavior38.
The Coimbrator. Representation of the reactions taking place in the closed system, where C is obtained from A by electronic excitation with hν1, and D is obtained from B by electronic excitation with hν2. The photochemical steps are shown in red and raise the free energy of the system. Under conditions where A, B, C and D are approximately constant, the changes in the concentrations of the composition variables X, Y, and Z are determined by the reactions \({\text{C}} + {\text{Y}}\mathop{\longrightarrow}\limits^{{k_{1} }}{\text{B}},{\text{X}} + {\text{Y}}\mathop{\longrightarrow}\limits^{{k_{2} }}A,{\text{D}} + {\text{X}}\mathop{\longrightarrow}\limits^{{k_{3} }}{\text{Z}} + 2{\text{X}}\,{\text{and}}\,{\text{Z}}\mathop{\longrightarrow}\limits^{{k_{4} }}2{\text{Y}}\).
This closed system can be simplified when the concentrations of dyes A and B are high compared with the photon flux, and can be considered constant. Moreover, if the photon fluxes with energies hν1 and hν2 are also approximately constant, the concentrations of C and D will remain approximately constant (steady-state). Hence, for large A and B and constant illumination, A, B, C and D can be treated as constants and the system can be decoupled from the variations of X, Y and Z. “Methods” section shows how to express these species in dimensionless variables (x, y, z) to obtain the following ODEs
where the dot notation denotes the derivative with respect to dimensionless time; α = (k3D)/(k1C) and β = k4/(k1C) are parameters. We define 1/(k1C) as the characteristic time.
The equilibrium (fixed) points of this three-dimensional system are obtained solving \(\dot{x}\) = 0, \(\dot{y}\) = 0 and \(\dot{z}\) = 0 simultaneously. They are O = (0,0,0) and P = (1, α, α/β). When β < [α–2 + √(α + 4)]/2 the point P becomes a repelling equilibrium point and a periodic orbit appears. “Methods” section presents the treatment of the bifurcation. The Hopf bifurcation is supercritical in the whole region depicted in Fig. 3. Attracting limit cycles emerge when crossing the bifurcation curve with \(\beta\) decreasing or α increasing. Figure 3 illustrates the bifurcation selecting a segment in the parameter space β = 0.6180 and α ∈ [0.80, 1.25].
Bifurcation diagram of the Coimbrator. Left panel: the Hopf bifurcation is supercritical and the oscillations arise for parameter values to the bottom-right side of the bifurcation curve. Right panel: the onset of the cycle as α increases along the green segment (β = 0.6180) depicted in the left panel (z = blue, y = green, x = red).
Figure 4 presents phase space trajectories to illustrate the crossing of the supercritical Hopf bifurcation. The numerical simulations in the upper panel illustrate a trajectory for certain values of the parameters (e.g., α = 1 and β = 0.7) that is attracted to an equilibrium point. This is true independently of the initial values of the variables (x, y, z). The trajectories in the lower panel calculated with another set of parameters (e.g., α = 1 and β = 0.6) go to an asymptotically stable periodic orbit. A Hopf bifurcation occurs for β = 0.618 when α = 1. Figure 4 also represents the values of the variables as a function of time. For α = 1 and β = 0.7 we see exponentially damped oscillations of the variables leading to a final stable value. This closed system sustains oscillations of the variables for α = 1 and β < 0.618. The Coimbrator leads to oscillations when β crosses a critical value.
Phase space plots obtained by solving numerically the differential equations of the Coimbrator. (A) Dynamics with α = 1 and β = 0.7 make the system evolve to equilibrium, and (C) corresponding damped oscillations of the xyz variables. (B) Dynamics with α = 1 and β = 0.6 lead to a periodic orbit and (D) corresponding sustained oscillations of the xyz variables.
The Earth system as an oscillator
The Coimbrator shows that the internal Earth system mechanism that drives climate changes can be an oscillator with parameters that in the Pleistocene crossed those of a supercritical Hopf bifurcation and triggered glacial cycles. We explore this hypothesis incorporating the most essential elements of the Earth system in the oscillator.
Photosynthesis in plants involves two photons of different energies to produce a high-energy intermediate,
This overall reaction of oxygenic photosynthesis represents the buildup of biomass. Hence, we identify CH2O with Z. It represents both live biomass and dead organic carbon. Its consumption is the oxidation reaction
The amount of oxygen in the atmosphere decreased by less than 1% in the last 5 million years and remained at least one order of magnitude higher than that of H2O or CO2. Assuming that O2 is approximately constant and can be assimilated in k4, the autocatalysis reaction becomes
where D comprises elements from the photosynthetic systems and carbon fixation cycle. A, B and C are also part of this carbon fixation cycle. This leads to the assignment of X as water vapor and, consequently, Y = CO2. This approach emphasizes the analogy with the Coimbrator and helps to identify both the photochemical reaction that drives the system and the autocatalysis required for oscillations. These aspects and the presence of a quadratic term, make the representation of the oscillator as a compartmental model that exchanges substances between physical spaces (e.g., atmosphere, sea, biosphere) less insightful than the representation of Fig. 2.
The main control of water vapor in the atmosphere is the vapor–liquid (X ↔ X0) equilibrium. We add the (X ↔ X0) equilibrium to the oscillator to account for the saturation of water vapor pressure in moist air. Similarly, the CO2 solubility (Y ↔ Y0) equilibrium is included to the oscillator, together with the reactions of CO2 dissolved in water, CO2(aq). “Methods” section show how choices of physically motivated parameters constrain changes in X and Y. Adding these equilibria to the oscillator changes its nature. We name the new oscillator Glaciator.
Figure 5 illustrates how the Glaciator incorporates the basic features of the Earth system. “Methods” section shows how to formulate the Glaciator in terms of dimensionless variables. Assuming that Xo and Y0 are constant, the new set of ODEs involves four parameters (ε, σ, ω, ρ)
The Glaciator. Representation of the reactions taking place in the closed system emphasizing the analogy between photochemical reactions and photosynthesis, and their generation of a high-energy intermediate (CH2O)2. The green shade covers the photosynthetic steps and the orange shade covers the steps in the biomass. Under conditions where A, B, C and D are approximately constant, the changes in the concentrations of the composition variable X = H2O, Y = CO2 and Z = CH2O are determined by the reactions of the Coimbrator and, additionally, by \({\text{X}}\mathop{\longrightarrow}\limits^{{k_{5} }}{\text{X}}_{0} ,{\text{X}}_{0} \mathop{\longrightarrow}\limits^{{k_{6} }}{\text{X}},{\text{Y}}\mathop{\longrightarrow}\limits^{{k_{7} }}{\text{Y}}_{0} \,{\text{and}}\,{\text{Y}}_{0} \mathop{\longrightarrow}\limits^{{k_{8} }}{\text{Y}}\).
where ε = (k6x0)/(k1C), σ = k5/(k1C), ω = (k8y0)/(k1C) and ρ = k7/(k1C), in addition to α = (k3D)/(k1C) and β = k4/(k1C). The rates of the processes represented by k3D and k4 depend on the biomass, which is not constant, and the corresponding parameters are better treated as time dependent, α(t) and β(t). We recall that in the Coimbrator we assumed a constant irradiation source, and consequently constant α and β, but this is no longer valid when we account for the insolation changes of Fig. 1 in the Glaciator. The rate constants k5 and k6 for the (X ↔ X0) equilibrium depend on the temperature and, consequently, ε and σ should also be time dependent. For simplicity, that is not considered in this study.
Figure 5 represents the dissolution of carbonates explicitly for CaCO3 and MgCO3. Identically, CO2 reactions with calcium- or magnesium-containing silicate minerals (CaSiO3 or MgSiO3) are equally present. The overall reactions
represent from left-to-right Ca-Mg silicate weathering plus sedimentation of marine carbonates. From right-to-left these reactions represent thermal decomposition of carbonates at depth resulting in degassing of CO2 to the surface39.
Timescales of climate changes
We show in “Methods” section that meaningful values of the parameters in Eq. (5) require ε ≈ 33ω and σ ≈ 3.3ρ. The independent parameters α(t), β(t), ω and σ are defined as ratios of rate constants (or time constants). This dependence on the rates places the timescales of the processes in Fig. 5 under careful scrutiny.
The major processes for CO2 removal from the atmosphere are those of land uptake (timescale 1–102 yr), ocean invasion (10–103 yr), reaction with calcium carbonate (103–104 yr) and silicate weathering (104–106 yr)40. In the Glaciator, CO2 removal is represented by
We associate the rate of land uptake with
Considering that globally water vapor pressure is much larger than CO2 pressure, pH2O ≫ pCO2, it is reasonable to incorporate the water vapor pressure in the rate constant and relate 1/(k2pH2O) to the timescale of CO2 removal by land uptake, 130 yr41. This, together with present day pH2O ≈ 11 matm, discussed in “Methods” section, allow us to calculate k2 ≈ 0.7 atm–1 yr–1. We associate the timescale of silicate weathering with 1/k7 and make 1/k7 ≈ 100 kyr. This is also the residence time of inorganic carbon in the ocean39. The value of 1/(k1C), defined as the characteristic time, must be related to the timescales of ocean invasion and reaction with calcium carbonate. Admittedly, this step of the Glaciator could be divided in two steps, because at least these two physical processes are present. This would increase the complexity and the number of parameters in the calculations. We favored simplicity and use 1/(k1C) to describe the two processes and represent the mean atmospheric lifetime of CO2. The mean atmospheric lifetime of CO2 after a large emission pulse is 12–14 kyr42. Hence, we make 1/(k1C) ≈ 12 kyr, which is in the upper limit of the geometric mean of ocean invasion and calcium carbonate reaction timescales.
These timescales determine the characteristic concentration (k1C)/k2 = 0.12 matm, and allow for the conversion of the dimensionless variables (x, y, z) to absolute values of H2O, CO2 and biomass. The value of the parameter ρ is given by its definition, ρ = k7/(k1C), and using the timescales discussed above we obtain ρ = 0.11. This restricts the number of adjustable parameters to three: α(t), β(t) and ω. The parameter ω relates the timescale of CO2 release from the surface ocean to the atmosphere with its mean atmospheric lifetime. We employed ω = 5 but the calculations are not very sensitive to this value. The values of the timescales employed in this work are presented in Table 1. They lead to a peak in atmospheric CO2 in the Holocene before the onset of anthropogenic perturbations43.
“Methods” section present a study of the Hopf bifurcation in the Glaciator. For the fixed values ω = 5, ρ = 0.11, ε = 33ω and σ = 3.3ρ, the Hopf bifurcation is supercritical for the values of α and β shown in Fig. 6. This figure shows the crossing of the bifurcation curve when β = 0.46 and α increases in the segment α \(\in\) [0.8,0.9]. The Glaciator may trigger sustained oscillations when β decreases or α increases from a set of parameters corresponding to a stable equilibrium.
Bifurcation diagram of the Glaciator. Left panel: the Hopf bifurcation is supercritical and the oscillations arise for parameter values to the bottom-right side of the bifurcation curve. Right panel: the onset of the cycle as α increases along the green segment (β = 0.46) depicted in the left panel (z = blue, y = green, x = red).
Photosynthetic forcing
Given the definition of α and the processes in Fig. 5, α can be regarded as the ratio between the rate of formation of the biomass and the rate of incorporation of CO2. Hence, α is a measure of the efficiency of photosynthesis and should not exceed unity.
There are two major types of photosynthesis, C3 and C4 photosynthesis, named after the number of carbon atoms of the first compound in which CO2 is incorporated. C3 plants (e.g., trees, wheat, rice, soybean) were spread in ancestral atmospheres, characterized by elevated CO2 and low O2 levels, whereas C4 plants (e.g., corn, sugarcane, many grasses) became dominant at mid-to-low latitudes in the last 5 Myr44, and now represent ¼ of the primary productivity on the planet45. C4 plants are able to concentrate CO2 and minimize photorespiration. This contributes to a specific activity (i.e., mol CO2 fixed per mass of enzyme per unit time) of C4 plants that can be twice as large as that of C3 plants46, and yields 50% greater efficiencies in radiation use47. Given the limit of α, we make αC3 ≈ 0.65 for C3 plants and, because the efficiency of C4 plants is 50% higher than that of C3 plants, we make αC4 ≈ 1. “Methods” section describe how to obtain α(t) from the changes in populations C3 and C4 plants. Using β = 0.46 and an increase in α from 0.84 to 0.925 over the last 10 Myr ago, we calculate that the Hopf bifurcation was crossed ca. 5 Myr ago and triggered a trajectory towards the limit cycle oscillation.
The value of β depends on production of (CH2O). Higher numbers of photons, larger areas covered by biomass and hydrological cycles driven by latitudinal insolation gradients should lead to temporary increases in β. Inter- or intra-hemispheric insolation gradients show the same features as the 65°N insolation curve, namely the dominant 41 kyr period that is the signature of obliquity48,49,50. We incorporate photosynthetic-orbital forcing in the Glaciator making β(t) = β0[1 + 0.09F(t)], where the zero of F(t) is the average summer energy of the Northern Hemisphere over the last 5 Myr (ca. 5 GJ/m2)17, and F(t) is normalized. The scaling factor 0.09 accounts for relative energy changes. A larger scaling factor could be used to account for the other mechanisms contributing to changes in β. Reasonably higher values did not change the nature of our results. This also means that choice of the insolation function is not critical for the results. The parameter β0 = 0.46 was selected to reproduce climate changes over the last 5 Myr. Its value implies that respiration, burning or other processes associated with the consumption of the biomass are in the same timescale as the incorporation of CO2 in the biomass.
The Glaciator can be regarded as a case of slow passage through a Hopf bifurcation51,52. Parameters α and β change slowly with respect to time and, as exhibited in the simulations, solutions stay near the unstable stationary state after the point (α, β) has crossed the curve of instantaneous Hopf bifurcation; oscillations emerge only after a delay. As already mentioned, Hopf bifurcation is crossed ca. 5 Myr ago, but large oscillations appear ca. 2 Myr later (Fig. 7). These slow passages through bifurcations have been observed in other climate models6,53.
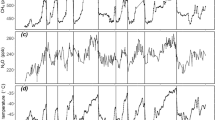
(A) Benthic δ18O records of the LR04 stack (red)7 matched to temperatures derived from Mg/Ca ratios of benthic foraminifera (black)9 and temperatures calculated from changes in CO2 radiative forcing with S = 1.35 °C/(W/m2) added to the pre-industrial global surface land temperature Ts = 8 °C (blue), and (B,C,D) their frequency distributions. (E) Historical (red)11 and Glaciator (blue) changes in atmospheric concentration of CO2, and (F,G) their frequency distributions.
Periodicities of past climate changes
The Glaciator has two adjustable parameters (β0 and ω) and one parameter with constrained values (0.84 < α(t) < 0.925) over the last 5 Myr. The values of three parameters are imposed by their assigned physical meanings: ε ≈ 33ω, σ ≈ 3.3ρ and ρ = k7/(k1C) = 0.11. The characteristic concentration allows for the conversion between the adimensional variables x and y to H2O and CO2 pressures. Using the parameters in Table 1, the Glaciator gives x = 95.5 at t = 0 in the absence of a CO2 pulse (i.e. pH2O = 11.6 matm), and y = 2.19 (i.e., pCO2 = 262 ppm) at t = 0. These absolute values are in reasonable agreement with the pre-industrial CO2 pressure of 280 ppm and with the water vapor pressure expected for the temperature of pre-industrial times discussed in “Methods” section. The calculated atmospheric CO2 in the Last Glacial Maximum (LGM), pCO2 = 171 ppm, is in good agreement with the Antarctic ice-core record of 185 ppm54. These results come from the assignment of the timescales and not from an arbitrary scaling.
Figure 7 compares reconstructions of geological records with simulations of CO2 concentration and temperature over the last 5 Myr using the parameters in Table 1. The absolute values of CO2 pressure were obtained using the characteristic concentration. The temperature was estimated adding changes in CO2 radiative forcing to the pre-industrial global surface land temperature Ts = 8°C55.
The average global surface temperature change (∆T) is often related to radiative forcing changes (∆R) externally imposed at the top of the atmosphere by the long-wavelength absorption of greenhouse gases. The most important of them is CO2, and its concentration changes have been associated with global temperature changes over the last 420 million years56. Temperature and radiative forcing are related by the climate sensitivity parameter S
The change in radiative forcing when the concentration of CO2 changes from that of a reference state (C0) to the state studied (C) is given by40
where the constant is in units of W/m2. Studies of past temperature changes showed that changing CO2 concentrations by a factor of 2 is consistent with ∆T = 2.8 °C, which is within the range 2.3–3.0 °C suggested by various climate models56. Taking ∆T = 2.8 °C for ∆R = 3.7 W/m2, gives S = 0.76 °C/(W/m2). An alternative approach for the long timescales considered here is to include slow feedbacks in the CO2 sensitivity parameter and employ a higher value, S = 1.35 °C/(W/m2)1. We adopted this approach for temperature calculations of the past.
The Mg/Ca ratios of foraminifera are one of the most reliable proxies of the paleo-temperatures of seawater9,57. A simple relation to obtain deep-sea temperature (in °C) was proposed57
Figure 7 uses this relation and the Mg/Ca ratios available from a 1.5-million-year record9 to obtain the corresponding Antarctic deep-sea temperatures. The benthic δ18O records of the LR stack7 were aligned with Tw from the Mg/Ca ratios to extend the estimated paleo-temperatures to 5 Myr ago.
Calculated global surface temperatures (Ts) are 3 to 5 °C higher than historical Antarctic deep-sea temperatures (Tw), as expected. Remarkably, 5 Myr ago both exhibited a dominant period of 42 kyr, which changed to a dominant 100 kyr period ca. 1 Myr ago. This reflects the change in the main driver of climate from orbital forcing due to obliquity to a limit-cycle oscillation. Figure 8 presents a detail of the MPT. Ca. 2 Myr ago a stronger 100 kyr period started imposing on the older 42 kyr period to become clearly dominant in the last 1 Myr. According to the Glaciator, this change results from the increase of α(t) in the Pleistocene with the expansion of C4 plants, which increased the efficiency of photosynthesis and led to the crossing of a Hopf bifurcation.
Mid-Pleistocene Transition. The benthic δ18O records of the LR04 stack (red)7 are compared with the changes in CO2 calculated with the Glaciator (blue). The vertical lines are separated by 42 kyr on the left and by 100 kyr on the right.
The relative changes in terrestrial carbon stock can also be compared with observations. The extreme values of z in the last cycle of the Glaciator are 216 and 278, i.e., CH2O changes by ca. 24%. A recent estimate of the total carbon stocks (soils and vegetation) is 2,807 PgC58. Data-based estimates of the difference between the LGM and pre-industrial land carbon storage range from 330 PgC59 to 821 PgC60 less carbon in the LGM, which correspond to deficits between 10 and 30%. Hence, the change in terrestrial carbon stock given by the oscillator is consistent with the current estimates.
Climate response to a CO2 pulse
Industrialization released approximately 300 PgC and business-as-usual predictions indicate a total release of 1,000 PgC (471 ppm of CO2) by the end of the century42. Climate models are often asked to predict the relaxation time of such a pulse on the current pCO2 level. In the Glaciator, the CO2 pulse comes with a H2O pulse. The scenario in Fig. 9 is a simulation of an instantaneous pulse of 1,000 PgC at present time, with the corresponding increase in water vapor, followed by relaxation assuming that all other parameters remain constant. Figure 8 shows that the relaxation time is approximately 100 years but the decay is non-exponential. Half of the CO2 pulse to the atmosphere is removed in 85 years. The IPCC estimate is that, for an emission pulse of about 1,000 PgC, about half is removed within a few decades40. Using the climate sensitivity parameter more appropriate for short timescales, S = 0.76 °C/(W/m2), this CO2 pulse corresponds to ∆T = 4.4 °C.
Figure 9 also illustrates a major difference between the Glaciator and simulations that do not take into account the dominant 100-kyr period of climate change. The CO2 pulse will eventually be absorbed and a new glacial cycle will begin. The next glacial maximum is predicted to occur in 50 kyr and be cooler than the preceding ones. This is due to the approach to the limit cycle shown in Fig. 4. The amplitude of the cycles depends on the final value of α, α(∞). Historical records show that the mid Pleistocene transition from 40-kyr to 100-kyr dominant cycles took more than half a million years. We estimate that the approach to the limit cycle started 5 Myr ago when α(t) became larger than 0.84 for β0 = 0.46, and the Hopf bifurcation was first crossed. However, as discussed in “Methods” section, the attraction to the limit cycle just after the bifurcation is very weak.
Discussion
Our work addresses the internal Earth system mechanism that drives climate changes. We propose that this mechanism is an oscillator with the following steps: (i) photosynthesis uses CO2 and H2O to generate high-energy intermediates that store free energy for an extended period of time; (ii) the stored energy is released at the same time as CO2 and H2O that re-initialize the cycle; (iii) the minimal sufficient conditions for oscillatory behavior are met due to the presence of an autocatalytic step. According to this mechanism, the expansion of C4 plants, with the concomitant increase in radiation use efficiency of photosynthesis, triggered glaciation cycles.
The Glaciator with meaningful timescales leads to intrinsic oscillations consistent with the periods observed in records of historical climate changes, and to a good agreement with the absolute values of CO2 and H2O in the atmosphere over the last 5 Myr. This simple conceptual model explains major puzzles of past climate dynamics: the absence of a strong 20 kyr precession signal8 (Fig. 7F), the MPT of 41 kyr cycles to 100 kyr cycles61 (Figs. 7C, F), the onset of glaciation cycles one million years ago with a dominant 100-kyr frequency22 (Fig. 8), and the Holocene temperature conundrum62 (Fig. 7D).
An unexpected prediction of the Glaciator is that further expansion of C4 plants will drive more pronounced glaciations in the future. Climate is a complex dynamic system and the simple conceptual model presented here only captures the most fundamental mechanisms underlying its dynamics. Nevertheless, it seems that extreme climate changes may happen in the next 50 kyr. Feedback mechanisms not included in the Glaciator may change this view, but our present understanding suggests that dampening the amplitudes of climate changes may be achieved recovering land from C4 plants to C3 plants.
The Glaciator is open to improvements as our knowledge of the various processes responsible for atmospheric CO2 release and removal evolves. Regardless of possible refinements, in their present forms our oscillators reveal that closed photochemical systems are capable of producing sustained oscillations of their chemical compositions when absorbing light from a constant irradiation source.
Methods
Numerical simulations
Simulations were carried with Matlab R2018a. We used the ode45 solver to integrate each of the systems of differential equations: the Coimbrator and the Glaciator. This solver is based in a Runge–Kutta–Fehlberg method. In both cases we have fixed a relative error tolerance of 10–12.
The Coimbrator is a closed system of autonomous differential equations with a Hopf bifurcation. The Hopf bifurcation is supercritical and oscillations arise for parameter values below the bifurcation curve. The Glaciator is described by non-autonomous differential equations because the parameters α and β are time-dependent: α(t) = PC3(t)αPC3 + PC4(t)αPC4 and β(t) = β0[1 + 0.09F(t)]. Note that the expression for β(t) involves an unknown function F(t) related with the summer insolation in the Northern Hemisphere available in the literature17. Values of F(t) are available in steps of 1 kyr along the last 5 Myr. However, a sample of values of F is not enough for numerical simulations. We approach the values of F(t) at arbitrary t with two different methods. On one hand, when running the Glaciator to get simulations of the last 5 Myr we use a linear interpolation in each time interval corresponding to consecutive sample points. Alternatively, we have used the same sample points to get a Fourier series approaching F(t) in the whole period of 5 Myr. The good fitting obtained is illustrated in Fig. 1A. Both methods are used to obtain the simulations for the past, but no remarkable sensitivity is observed. To get extrapolations of the insolation values in the future, we have used the approach of the Fourier series. In the case of the Glaciator, when running the simulation in the past, initial time is fixed in the value − 5 Myr and the numerical integration extends up to present time. Initial conditions for the dependent variables are chosen close to the equilibrium point of the system when α(t) = PC3(t)αPC3 + PC4(t)αPC4 and β = β0, namely, x = 133.8721, y = 1.710171, z = 245.4826.
Reduction of variables in the Coimbrator
The Coimbrator is defined by the reactions
The time dependence of all the chemical species involved in the model is described by
where the photon fluxes were represented by h1 and h2. If A, B, C and D represent species in the solid state, by definition they have unitary concentrations. Hence, [A], [B], [C] and [D] are constant, and the system above can be simplified to the following set of differential equations
It is convenient to express these equations in terms of dimensionless variables to obtain a general solution. We follow the procedure of Field and Noyes32 to cast the concentrations of X, Y and Z in the dimensionless variables x, y, z. We define g = k1C as a pseudo-first order rate under continuous irradiation if A is not depleted from the system. Thus, the quotient g/k2 has the dimensions of a concentration and is defined as the characteristic concentration. This is used to make the following dimensionless measures of the species:
The characteristic concentration g/k2 can later be used to obtain the values of X, Y and Z in units of concentration. The same procedure is applied to the conversion of time t in dimensionless variable τ. The derivatives become
Making the appropriate replacements we obtain a simplified system of differential equations, in dimensionless units
where α = (k3D)/(k1C) and β = k4/(k1C).
Hopf bifurcation in the Coimbrator
In a physically meaningful system all the concentrations [C], [D], [X], [Y] and [Z] and all the constants k1, k2, k3 and k4 are positive. This three-dimensional system has two equilibrium points
The Jacobian matrix of the vector field is
The characteristic polynomial at O is
and gives the characteristic equation at O
The eigenvalues of J(0,0,0) are λ1 = α, λ2 = – 1, and λ3 = – β. Hence O is an equilibrium point of saddle type. Moreover, one can check that x = 0 is an invariant plane, namely, the stable invariant manifold at O. The unstable invariant manifold is tangent to y = z = 0.
The characteristic polynomial at P is
and gives the characteristic equation at P
There are pure imaginary eigenvalues if and only if
and
Condition (25) is satisfied along the curve defined by (24) for all β > 0. These eigenvalues are \(\lambda_{ \pm } = \pm i\sqrt {2\beta - \alpha }\). The third eigenvalue λ3 = –(β + 2) is negative for all β > 0. The distinction between subcritical and supercritical Hopf bifurcation depends on the value of the first Lyapunov coefficient36. Although such coefficient can be obtained for the given system, calculations are quite laborious and we prefer to use MATCONT63, a MATLAB numerical continuation package for bifurcation analysis, to check that the Hopf bifurcation is supercritical (see Fig. 3).
Reduction of variables in the Glaciator
The Glaciator is defined by the reactions
The time dependence of all the chemical species involved in the Glaciator is described by
For constant [A], [B], [C] and [D], this reduces to following set of differential equations
The following meanings can be assigned to terms in the equations above: k2[X][Y]—land uptake of H2O and CO2, k3[D][X]—a step in the biosynthesis of CH2O (e.g., condensation reactions in the formation of a glycosidic bond to make cellulose or in the formation of a peptide bond to yield a protein), k5[X]—condensation of water, k6[X0]—evaporation of water, k4[Z]—combustion/respiration, k7[Y]—loss of CO2 into the ocean leading to silicate weathering, k7[Y]—release of CO2 from the ocean in all its forms. As mentioned above and further discussed below, 1/(k1C) is the characteristic time and is associated with more than one physical process in this simplified description of the Earth system.
Making, as for the Coimbrator, g = k1C, the quotient g/k2 is defined as the characteristic concentration. Additionally, 1/g is defined as the characteristic time. The characteristic time and concentration are used to obtain the dimensionless variables
In terms of the adimensional variables, we obtain
where
Considering that k6x0 and k8y0 are proportional to the water evaporation and to the CO2 desorption fluxes, respectively, the following relation must be obeyed
The desorption flux of CO2 from water at 313 K is jCO2 ≈ 7 × 10–4 mol/(m2 s)64. At this temperature, the water evaporation flux from seawater ranges from 1.5 × 10–2 to 4.6 mol/(m2 s), depending on the air velocity65. Using jH2O ≈ 2.3 × 10–2 mol/(m2 s) we estimate ε ≈ 33ω.
Constrains of the parameters in the Glaciator
The saturation vapor pressure can be calculated from an approximation to the Clausius–Clapeyron equation
where the constants where chosen to yield the vapor pressure in mbar, T is in K and a = 0.064 K–1 66. Using the average Earth surface land temperature Ts = 8 °C to represent pre-industrial global temperature (ca. 1900)55, we obtain e0* ≈ 10.9 mbar, which corresponds to pH2O ≈ 11 matm.
Water vapor equilibrates relatively rapidly with liquid water (k5x ≈ k6x0). Assuming CO2 in the atmosphere also equilibrates relatively rapidly with CO2 dissolved in the sea (k7y ≈ k8y0), the following relations can be established
Given that
we reduced the number of variables needed to apply the Glaciator making x ≈ 10y and using ε ≈ 33ω, to set σ ≈ 3.3ρ.
Hopf bifurcation in the Glaciator
The Glaciator, as the Coimbrator, has two equilibrium points. We have to study the following system of nonlinear equations
From the third equation we obtain
After substituting z in the second equation we get
and finally, substituting y in the first equation, we obtain the following equation for x
Since [(α – ρ)(ρ + 1) – ω + ε)2 + 4ε (ρ + 1) (α + σ)] > 0, we always have two equilibria. In this case the analytical characterization of the Hopf bifurcation is plausible but more involved. Using again, as we did for the Coimbrator, the package MATCONT, we obtain the results depicted in Fig. 6, for which we have fixed the values
As shown in Fig. 10, the first Lyapunov coefficient is negative in the whole range of parameters considered in Fig. 6 and hence the Hopf bifurcation is supercritical. A periodic orbit in a three-dimensional phase space has Floquet multipliers 1, m1 and m2 (m1 and m2 are the eigenvalues of the linearization of the first return map defined around the periodic orbit). If |mi|< 1, for i = 1,2, then the periodic orbit is attracting. The right panel in Fig. 10 shows the value of Floquet multipliers for the periodic orbits born at α = 0.84 when β = 0.46. Because the first Lyapunov coefficient is very small, the attraction of the limit cycle just after the bifurcation is very slow. For the computation of the first Lyapunov coefficient, instead of using the approximations obtained with MATCONT, we wrote our own code, designed for this specific model, to obtain more accurate numerical results.
Populations of C3 and C4 plants
The value of α in Eq. (5) must take in consideration the population of C3 and C4 plants
We simulate C4 plants expansion with the logistic equation often used to describe population dynamics
where Nmax is the population maximum at t = ∞, N0 is the population at – t0, which is selected to be the origin of the expansion, and r is the rate of population increase. The sudden C4 plants expansion occurred ca. 10 Myr ago44, which leads to t0 = 10 Myr. Nmax = 0.25 was selected in view of the fact that C4 plants account for 25% of terrestrial photosynthesis. With these values of t0 and Nmax, the known profile of C4 plants expansion44 can be reproduced with the population N0 = 0.15 and the rate r = 0.3 Myr–1. The expansion of C4 plants was achieved, in part, at the expense of C3 plants. We modelled the reduction of C3 plants population as
where the factor 0.15 was selected to reflect evolution from nearly exclusive C3 vegetation 20 Myr ago to the composition of biomass today: 389.3 PgC of C3 vegetation and 18.9 PgC of C4 vegetation67. This corresponds to a fraction of C3 biomass of 0.954, whereas Eq. (43) gives PC3(0) = 0.96. Equation (43) assumes that only 15% of the increase in C4 plants is made at the cost of C3 plants. Figure 11 presents the changes in the populations of C3 and C4 plants. It also shows the changes in α(t) calculated with Eq. (41).
Left axis: changes in the relative populations of C3 and C4 plants, where the logistic equation was employed to express the explosive spread of C4 plants ca. 10 Myr ago at some cost of the C3 plants population. Right axis: value of α given the contributions of C3 and C4 plants weighted by their relative populations.
Data availability
Data described in this study will be available from the corresponding authors upon request.
References
Hönisch, B., Hemming, N. G., Archer, D., Siddall, M. & McManus, J. F. Atmospheric carbon dioxide concentration across the mid-Pleistocene transition. Science 324, 1551–1554 (2009).
Huybers, P. Combined obliquity and precession pacing of late Pleistocene deglaciations. Nature 480, 229–232. https://doi.org/10.1038/nature10626 (2011).
Crucifix, M. Oscillators and relaxation phenomena in Pleistocene climate theory. Philos. Trans. R. Soc. A 370, 1140–1165 (2012).
Martinez-Botí, M. A. et al. Plio-Pleistocene climate sensitivity evaluated using high-resolution CO2 records. Nature 518, 49–54. https://doi.org/10.1038/nature14145 (2015).
Zeebe, R. E., Westerhold, T., Littler, K. & Zachos, J. C. Orbital forcing of the Paleocene and Eocene carbon cycle. Paleoceanography 32, 440–465 (2017).
Nyman, K. H. M. & Ditlevsen, P. D. The middle Pleistocene transition by frequency locking and slow ramping of internal period. Clim. Dyn. 53, 3023–3038 (2019).
Lisiecki, L. E. & Raymo, M. E. A Pliocene-Pleistocene stack of 57 globally distributed benthic d18O records. Paleoceanography 20, PA1003 (2005).
Patterson, M. O. et al. Orbital forcing of the East Antarctic ice sheet during the Pliocene and Early Pleistocene. Nature Geosci. 7, 841–847. https://doi.org/10.1038/ngeo2273 (2014).
Elderfield, H. et al. Evolution of ocean temperature and ice volume through the mid-Pleistocene climate transition. Science 337, 704–709 (2012).
Erb, M. P., Broccoli, A. J. & Clement, A. C. The contribution of radiative feedbacks to orbitally driven climate change. J. Climate 26, 5897–5914 (2013).
Petit, J. R. et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Anarctica. Nature 399, 429–436 (1999).
Uemura, R. et al. Asynchrony between Antarctic temperature and CO2 associated with obliquity over the past 720,000 years. Nature Comm. 9, 961. https://doi.org/10.1038/s41467-018-03328-3 (2018).
Hays, J. D., Imbrie, J. & Shackleton, N. J. Variations in the Earth’s Orbit: Pacemakes of the Ice Ages. Science 194, 1121–1132 (1976).
Jochum, M. et al. True to Milankovitch: Glacial inception in the new community climate system model. J. Climate 25, 2226–2239 (2012).
Milankovitch, M. Canon of Insolation and the Ice-Age Problem. 848 (Israel Program for Scientific Translations, 1941).
Berger, A. & Loutre, M. F. Insolation values for the climate of the last 10 million years. Quat. Sci. Rev. 10, 297–317 (1991).
Huybers, P. Early Pleistocene glacial cycles and the integrated summer insolation forcing. Science 313, 508–511 (2006).
Laskar, J., Robutel, P., Joutel, F., Gastineau, M. & Correia, A. C. M. A long-term numerical solution for the insolation quantities of the Earth. Astron. Astrophys. 428, 261–285 (2004).
Berger, A., Loutre, M.-F. & Yin, Q. Total irradiation during any time interval of the year using elliptic integrals. Quat. Sci. Rev. 29, 1968–1982 (2010).
Raymo, M. E. & Huybers, P. Unlocking the mysteries of the ice ages. Nature 451, 284–285 (2008).
Maslin, M. A. & Brierley, C. M. The role of orbital forcing in the Early Middle Pleistocene Transition. Quatern. Int. 389, 47–55. https://doi.org/10.1016/j.quaint.2015.01.047 (2015).
van Nes, E. H. et al. Causal feedbacks in climate changes. Nature Clim. Change 2015, 445–448 (2015).
Daruka, I. & Ditlevsen, P. D. A conceptual model for glacial cycles and the middle Pleistocene transition. Clim. Dyn. 46, 29–40 (2016).
Huybers, P. Glacial variability over the last two million years: an extended depth-derived agemodel, continuous obliquity pacing, and the Pleistocene progression. Quat. Sci. Rev. 26, 37–55 (2007).
Saltzman, B. & Maasch, K. A. A first-order global model of late Cenozoic climate change. II. Further analysis based on a simplification of CO2 dynamics. Clim. Dyn. 5, 201–210 (1991).
Lotka, A. J. Undamped oscillations derived from the law of mass action. J. Am. Chem. Soc. 42, 1595–1599 (1920).
Volterra, V. Variazioni e fluttuazioni del numero d’individui in specie animali conviventi. Mem. Acad. Lincei Roma 2, 31–113 (1926).
Prigogine, I. & Lefever, R. Symmetry breaking instabilities in dissipative systems. II.. J. Chem. Phys. 48, 1695–1700 (1968).
Prigogine, I. Time, structure, and fluctuations. Science 201, 777–785 (1978).
Drubi, F., Ibáñez, S. & Rodriguez, J. A. Coupling leads to chaos. J. Differ. Equ. 230, 371–385 (2007).
Zaikin, A. N. & Zhabotinsky, A. M. Concentration wave propagation in two-dimensional liquid-phase self-oscillating system. Nature 225, 535–537 (1970).
Field, R. J. & Noyes, R. M. Oscillations in chemical systems. IV. Limit cycle behavior in a model of a real chemical reaction. J. Chem. Phys. 60, 1877–1884 (1974).
Noyes, R. M. Effects of global constraints on permissible local behavior. Ber. Bunsenges. Phys. Chem. 89, 620–629 (1985).
Epstein, I. R. & Showalter, K. Nonlinear chemical dynamics: Oscillations, patterns, and chaos. J. Phys. Chem. 100, 13132–13147 (1996).
Li, R.-S. & Ross, J. Chemical instabilities in closed systems with illumination. J. Phys. Chem. 95, 2426–2430 (1991).
Strogatz, S. H. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering 2nd edn. (Westview Press, Boulder, 2015).
Sel’kov, E. E. Self-oscillations in glycolysis 1. A simple kinetic model. Eur. J. Biochem. 4, 79–86 (1968).
Wilhelm, T. & Heinrich, R. Smallest chemical reaction system with Hopf bifurcation. J. Math. Chem. 17, 1–14 (1995).
Berner, R. A. The Phanerozoic Carbon Cycle: CO 2 and O 2 (Oxford University Press, Oxford, 2005).
IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. (Cambridge University Press, 2013).
Eby, M. et al. Lifetime of antropogenic climate change: Millennial time scales of potential CO2 and surface temperature perturbations. J. Climate 22, 2501–2511 (2009).
Archer, D. et al. Atmospheric lifetime of fossil fuel carbon dioxide. Annu. Rev. Earth Planet. Sci. 37, 117–1134 (2009).
Marcott, S. A. et al. Centennial-scale changes in the global carbon cycle during the last deglaciation. Nature 514, 616–619 (2014).
Edwards, E. J., Osborne, C. P., Strömberg, C. A. E., Smith, S. A. & Consortium, C. G. The origins of C4 grasslands: Integrating evolutionary and ecosystem science. Science 328, 587–591 (2010).
Sage, R. F. The evolution of C4 photosynthesis. New Phytol. 161, 341–370 (2004).
Seemann, J. R., Badger, M. R. & Berry, J. A. Variations in the specific activity of ribulose-1,5-bisphophate carboxylase between species utilizing different photosynthetic pathways. Plant Physiol. 74, 791–794 (1984).
Sage, R. F. & Zhu, X.-G. Exploiting the engine of C4 photosynthesis. J. Exp. Botany 62, 2989–3000 (2011).
Raymo, M. E. & Nisancioglu, K. The 41 kyr world: Milankovitch’s other unsolved mystery. Paleoceanography 18, 1011 (2003).
Bosmans, J. H. C., Hilgen, F. J. & Lourens, L. J. Obliquity forcing of low-latitude climate. Clim. Past 11, 1335–1346 (2015).
Kuechler, R. R., Dupont, L. M. & Shefuß, E. Hybrid insolation forcing of Pliocene monsoon dynamics in West Africa. Clim. Past 14, 73–84 (2018).
Baer, S. M., Erneux, T. & Rinzel, J. The slow passage through a Hopf bifurcation: delay, memory effects, and resonance. SIAM J. Appl. Math. 49, 55–71 (1989).
Hayes, M. G., Kaper, T. J., Szmolyan, P. & Wechselberger, M. Geometric desingularization of degenerate singularities in the presence of fast rotation: A new proof of known results for slow passage through Hopf bifurcations. Indag. Math. 27, 1184–1203 (2016).
Ashwin, P. & Ditlevsen, P. The middle Pleistocene transition as a generic bifurcation on a slow manifold. Clim. Dyn. 45, 2683–2695 (2015).
Monnin, E. et al. Atmospheric CO2 concentrations of the Last Glacial Termination. Science 291, 112–114 (2001).
Rohde, R. et al. A new estimate of the average Earth surface land temperature spanning 1753 to 2011. Geoinform. Geostat. Overv. 1, 1. https://doi.org/10.4172/2327-4581.1000101 (2013).
Royer, D. L., Berner, R. A. & Park, J. Climate sensitivity constrained by CO2 concentrations over the past 420 million years. Nature 446, 530–532 (2007).
Elderfield, H. et al. A record of bottom water temperature and seawater ∂18O for the Southern Ocean over the apst 440 kyr based on Mg/Ca of benthic foraminiferal Uvegina spp. Quat. Sci. Rev. 29, 160–169 (2010).
Carvalhais, N. et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 514, 213–217 (2014).
Ciais, P. et al. Large inert carbon pool in the terrestrial biosphere during the Last Glacial Maximum. Nat. Geosci. 5, 74–79 (2012).
Kaplan, J. O., Prentice, I. C., Knorr, W. & Valdes, P. J. Modeling the dynamics of terrestrial carbon storage since the Last Glacial Maximum. Geophys. Res. Lett. 29, 31-1-31–4 (2002).
Snyder, C. W. Evolution of global temperature over the past two million years. Nature 538, 226–228 (2016).
Liu, Z. et al. The Holocene temperature conundrum. Proc. Natl. Acad. Sc. USA 111, E3501–E3505. https://doi.org/10.1073/pnas.1407229111 (2014).
Dhooge, A., Govaerts, W., Kuznetsov, Y. A., Meijer, H. G. E. & Sautois, B. New features of the software MatCont for bifurcation analysis of dynamical systems. MCMDS 14, 147–175. https://doi.org/10.1080/13873950701742754 (2008).
Tunnat, A., Behr, P. & Görner, K. Desorption kinetics of CO2 from water and aqueous amine solutions. Energy Procedia 51, 197–206 (2014).
El-Dessouky, H. T., Ettouney, H. M., Alatiqi, I. M. & Al-Shamari, M. A. Evaporatoin rates from fresh and saline water in moving air. Ind. Eng. Chem. Res. 41, 642–650 (2002).
Stephens, G. L. On the relationship between water vapor over the oceans and sea surface temperature. J. Clim. 3, 634–645 (1990).
Still, C. J., Berry, J. A., Collatz, G. J. & Defries, R. S. Global distribution of C3 and C4 vegetation: Carbon cycle implications. Global Geobiochem. Cycles 17, 1006 (2003).
Acknowledgements
This work was financially supported by the Portuguese Science Foundation (UIDB/QUI/00313/2020) and by the Spanish Research projects MTM2014-56593-P and MTM2017-87697-P. We thank C. Fernández and J. A. Rodríguez for their valuable suggestions and help.
Author information
Authors and Affiliations
Contributions
L.G.A. did the conceptualization and wrote the manuscript; S.I. implemented the model and run the calculations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arnaut, L.G., Ibáñez, S. Self-sustained oscillations and global climate changes. Sci Rep 10, 11200 (2020). https://doi.org/10.1038/s41598-020-68052-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68052-9
This article is cited by
-
The Mid-Pleistocene Transition: a delayed response to an increasing positive feedback?
Climate Dynamics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.