Abstract
Carrot (Daucus carota L.), which is one of the 10 most important vegetable crops worldwide, is an edible root vegetable desired for its taste as well as its medicinal uses. However, a fungus isolated from carrot seeds was observed to substantially decrease the germination rate. The isolate was identified as Alternaria alternata based on morphological and molecular characteristics as well as a phylogenetic tree. The maximum seed infection rate of selected carrot cultivars was approximately 60%, with the main infection site just underneath the seed shell. Additionally, the germination rate of infected seeds decreased by 28.7%. However, the seed infection rate varied among the examined carrot cultivars. Regarding the effects of chemical fungicides, the optimal treatment involved immersing seeds in amistar top suspension concentrate (SC) (effective concentration of 0.65 g/L) for 6 h, which effectively killed the fungi inside the carrot seeds. The results of this study provide a theoretical basis for the development of efficient methods for preventing the infection of carrot seeds by specific fungi and increasing the germination rate and vigour index.
Similar content being viewed by others
Introduction
Carrot (Daucus carota L.), which is cultivated worldwide, is one of the most important root vegetables in the family Apiaceae1. The importance of carrots in fulfilling the nutritional needs of communities has long been known2. In addition to being an edible root vegetable with a desirable taste, carrots have a medicinal use. The largest carrot producer worldwide is China, wherein the vegetable is cultivated mainly in the northern, northeastern, central, and southwestern parts of the country to generate 43% of the global carrot yield3.
Current studies on carrots mainly focus on cultivation, breeding, tissue culture, nutrient content, increasing yield, and regulating carotenoid synthesis1,4,5. There has been comparatively less research regarding fungal infections of seeds. We recently revealed that some carrot varieties have a low germination rate and a weak seedling growth potential. Preliminary analyses have suggested that these characteristics may be related to the infection of seeds by specific fungi. Seeds form the basis of crop production and are vital for plant associations with microorganisms, which may be damaging to the seeds or the seedlings that germinate from infected seeds6. Of the 16% annual crop loss due to plant diseases, at least 10% is caused by seed-borne diseases7.
During seed production, storage, and transport, the seeds are exposed to many kinds of microorganisms, ultimately resulting in fungal infections that may adversely affect seeds by decreasing germination and vigour, shortening the storage period, and inducing physiological changes7,8. Seeds infected by fungi may survive for 5 years if they are air-dried and stored at 4 °C9. However, seed quality directly determines the quality of agricultural products10. Additionally, seed-borne pathogens can be the primary source of infection and disease transmission11.
Fungi infecting seeds can attach to the seed surface or penetrate the seeds. Consequently, the effectiveness of chemical seed treatments may vary because deep-seated infections may be unaffected12. The application of chemical pesticides is a fundamental agronomic practice regarding crop protection13. However, their excessive use may decrease the sensitivity of the target pathogens to these chemicals. Therefore, developing novel fungicides with low toxicity and establishing appropriate application protocols for managing fungal infections of seeds are crucial. For example, Sudisha et al. (2006) revealed that a carbendazim wettable powder, captan 50 WP, and dithane M-45 can inhibit melon stem blight due to infected seeds14. Wang et al. observed that treatments with 75% chlorothalonil, 50% thiram, or 80% carbendazim are significantly inhibitory to the pathogens infecting seeds, and that 75% chlorothalonil and 80% carbendazim can promote seed germination15. Thus, research involving the screening and use of chemical fungicides may result in effective methods for controlling fungal infections of seeds.
The aim of the current study was to isolate the fungi associated with carrot seeds, evaluate the efficacy of chemical fungicides against the detected fungi, and optimize the application of fungicides to maintain a relatively high germination rate and vigour index.
Materials and methods
Isolation and identification of fungi infecting carrot seeds
The tested carrot seeds (cultivars Kaixinmintu and Berlin) were purchased from a commercial market in Shuangyashan, Heilongjiang province, China. The seeds (30 seeds/cultivar) were surface-sterilized with 0.5% NaOCl for 5 min, rinsed three times with sterilized distilled water, placed on potato dextrose agar (PDA), and incubated at 26 °C. Single spores were obtained from the fungal cultures for morphological and molecular analyses as previously described16. The fungi were identified based on morphological characteristics as described in published methods17,18,19. To further identify the fungi, genomic DNA was extracted from the isolates with the Fungal Genomic DNA Kit (Tiangen, Beijing, China), after which it was amplified by PCR with the universal fungal primers ITS1 and ITS420 and EF1-728F/EF1-986R21. The PCR amplification was conducted in a 50 µl solution consisting of 25 μl Taq mixture (Promega, Madison, WI, USA), 2 µl ITS1 primer (10 μM), 2 µl ITS4 primer (10 μM), 2.0 µl DNA template, and 19 µl ddH2O. The PCR was completed with the T100™ thermocycler (Bio-Rad Laboratories Inc, CA) and the following program: 94 °C for 5 min; 36 cycles of 94 °C for 1 min, 58 °C for 1 min, and 72°C for 1.5 min; 72 °C for 10 min. The amplicons were purified and sequenced by Shanghai Biological Engineering Co., Ltd. (Shanghai, China).
Effects of fungal infections on carrot seed germination
Carrot seeds (cv. Hanhong) were collected to analyse the impact of fungal infections on germination. amistar top SC (325 g/L) was diluted to 0.65 g/L (effective concentration), after which carrot seeds were immersed in the diluted fungicide solution for 8 h to kill all of the fungi within the seeds. Control seeds were treated with sterile water. All seeds were then air-dried and analysed. The seed infection and germination rates were determined by culturing on PDA. Specifically, the seeds were sterilized, after which 30 seeds per treatment were placed on PDA medium and incubated at 25 °C for 5 days. The appearance of the resulting fungal colonies was recorded. This analysis was completed with three replicates for a total of 90 seeds, which were used to calculate the seed infection and germination rates with the following equations:
Carrot seed parts infected by fungi
Carrot seeds (cultivar Hanhong) were surface-sterilized with three replicates for a total of 90 seeds. The 90 seeds were cut in the middle with sterilized scissors and the halved seeds were placed on PDA medium. Additionally, 90 intact seeds were placed on PDA medium. After a 5-day incubation at 25 °C, the appearance of the fungal colonies was recorded. The seed infection rate was calculated to evaluate the seed parts infected by fungi.
Determination of the seed infection rate of 10 carrot cultivars
The following 10 carrot cultivars were analysed: Hanhong (Beijing Baikutian Seedling Co., Ltd., China); Zhimeihong, Qiuhongyun, Hongyu 6, Kaixinmingtu, Kaixin666, Kaixin668, Kaixin838, and Kaixin1 (Qingdao Yuanshengtai Seed Industry Co., Ltd., China); and Berlin (Bejo Zaden B.V., Harenkarspel BEJO, Harenkarspel, The Netherlands).
The tested carrot seeds were surface-sterilized. For each carrot cultivar, 30 seeds per cultivar were placed on PDA medium, with three replicates for a total of 90 seeds per cultivar. The seeds were incubated at 26 °C for 5 days, after which the seed infection rate was calculated with the formula provided above and compared among the 10 cultivars.
Screening of chemical fungicides
Carrot seeds (cultivar Hanhong) were treated with the following fungicides: azoxystrobin (250 g/L SC) (Shanghai Future Industry Co., Ltd., Shanghai, China); boscalid (50% WG) and sercadis plus (12% SC) [BASF Plant Protection (Jiangsu) Co., Ltd., Rudong, China]; difenoconazole (10% WG), fipronil (25 g/L DS), amistar top SC (325 g/L SC), and procymidone (18.7% SC) [Syngenta (Nantong) Crop Protection Co., Ltd., Nantong, China]; carbendazim (80% WP) (Shandong Haier Sanli Biochemical Co., Ltd., Zhucheng, China); and captan (80% WG) (Adama Makhteshim Ltd., Beersheba, Israel).
The chemical fungicides were diluted for an effective concentration of 0.65 g/L. Carrot seeds (180 seeds/fungicide) were immersed in the diluted solutions or sterile water (control) for 6 h. After air-drying the seeds, 90 of them were added to PDA medium to assess the effects of the fungicides on the seed infection rate. This analysis was completed with three replicates for a total of 90 seeds. The remaining 90 seeds were added to pots containing sterilized soil, with 10 seeds per pot and nine pots per treatment. The seeds were covered with 1 cm of soil. After a 15-day incubation in the greenhouse, the germination rate, seedling height, and fresh weight were determined. The seed infection rate was calculated with the formula provided above. The following formula was used to calculate the control effect:
Optimizing fungicide applications on carrot seeds
Fungicide applications were optimized, including the amistar top SC concentration and treatment time. Specifically, carrot seeds (90 seeds/treatment) were treated with amistar top SC (effective concentrations of 0.33, 0.41, or 0.65 g/L) or sterile water (control) for 6 h, after which 30 seeds per treatment were surface-sterilized and added to PDA medium (10 seeds/plate) and incubated at 26 °C. The remaining 90 seeds were added to a culture bowl (10 seeds/bowl). The seeds were incubated for 20 days in a greenhouse with a 28 °C (day): 22 °C (night) cycle. The germination rate, plant height, and fresh weight of the carrot seedlings were determined.
Sterilized carrot seeds (90 seeds/treatment) were immersed in amistar top SC solution (effective concentration of 0.65 g/L) or sterile water (control) for 2, 4, 6, or 8 h. The seed infection rate was calculated with the formula provided above. Additionally, the effect of the treatment on seedling growth was assessed.
Statistical analysis
All experiments were conducted twice under similar conditions. Data underwent an analysis of variance with the SPSS Statistics 19.0 program (IBM/SPSS, Chicago, IL, USA). The significance of any differences in the mean values for treatments was determined with Duncan’s multiple range test (P < 0.05).
Results
Isolation and identification of fungi infecting carrot seeds
Ten fungi were isolated from cultivars Kaixin666 and Berlin and then subcultured by transferring hyphal tips. Single-microconidium isolates were generated from the fungal cultures as previously described16 and the morphological characteristics of the isolates were analysed. Colonies on PDA medium consisted of dark grey mycelium (Fig. 1). Conidia produced after a 4-day incubation at 26 °C in darkness were pale brown to dark brown, straight or flexuous, obclavate to obpyriform or ellipsoid, and had a short conical beak at the tip or were beakless. The spores had a smooth or verrucose surface and were 3.6–11.5 μm × 6.3–35.2 μm, with 0–4 transverse septa and 0–1 longitudinal septa. Ten fungal isolates had identical morphological characteristics and were identified as Alternaria alternata19.
Genomic DNA was extracted from the single conidial cultures of two representative isolates, Cbailin and Akaixin, and the internal transcribed spacer (ITS) regions and translation elongation factor 1-alpha (TEF-1ɑ) genes and RNA polymerase II second largest subunit gene (RPB2) were amplified by PCR with primers ITS1 and ITS420, EF1-728F/EF1-986R21 and RPB2–5F2/fRPB2–7cR22. A BLAST analysis revealed the amplified sequences of Cbailin and Akaixin were 100% identical to sequences from A. alternata isolates Alt-C81 (MN044802.1) and 13A (MK248606.1) for ITS and isolates Aa1 (MK733276.1) and PaF-3 (MN692926.1) for TEF-1ɑ, isolates 15–239 (LC132700.1) and 14–358 (LC132699.1) for RPB2, respectively.
A combined tree based on the ITS, TEF-1ɑ and RPB2 sequences indicated that Cbailin and Akaixin were A. alternata (Fig. 2). The sequences of the Cbailin and Akaixin amplicons were deposited in the GenBank database (accession numbers MN337233 and MK332248 for ITS, and MT178330 and MT178329 for TEF-1ɑ, and MT593329 and MT593330 for RPB2, respectively). To the best of our knowledge, this is the first report of the isolation of A. alternata from carrot seeds.
Effects of fungal infections on carrot seed germination
The carrot seeds treated to kill all fungi germinated better than the control carrot seeds (Table 1). Specifically, the mean germination rate of seeds lacking A. alternata was 28.7% higher than that of control seeds infected by fungi.
Detection of carrot seed parts infected by fungi
An analysis of the carrot seed parts infected by fungi revealed the seed infection rates of the whole seed and the cut seed were both 53.3% (Fig. 3). The lack of significant difference (P < 0.05) between the seed infection rates suggested that the fungi were just underneath the seed shell (i.e., internally seed-borne and extra-embryonic).
Calculation of the seed infection rate of 10 carrot cultivars
Significant differences in the seed infection rates were revealed among the 10 analysed carrot cultivars (Table 2). Cultivars Hanhong and Kaixinmingtu were the most heavily infected, with seed infection rates of 60.0% and 23.3%, respectively. Second, cultivars Kaixin 1 and Bailin were very few with seed infection rates of 6.7% and 3.3%. Finally, other varieties were free of fungi.
Seed treatments with various fungicides
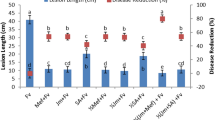
The treatments with nine chemical fungicides resulted in considerable variability in the seed infection rate, germination, and seedling growth (Table 3). For example, amistar top SC killed all of the fungi within carrot seeds (100% control of fungal infection), whereas captan, procymidone, and azoxystrobin were less effective (40% control of fungal infection). Moreover, boscalid, difenoconazole, carbendazim, benzofuramide, and fipronil were relatively ineffective for controlling fungal infections of seeds. On the basis of the treatment effects on seed germination and seedling growth, amistar top SC was considered to be the most appropriate chemical fungicide.
Seed treatments with varying amistar top SC concentrations
The three tested amistar top SC concentrations were inhibitory to the fungal infection of carrot seeds (Table 4). The 0.65 g/L treatment was the most effective (99.2% control of fungal infection). Additionally, seed germination and seedling growth were not inhibited.
Effects of varying amistar top SC seed treatment times
The four tested amistar top SC treatment times inhibited the infection of seeds by fungi, with obvious differences among the analysed times (Table 5). Specifically, the 6-h and 8-h treatment times were the most effective, with no significant difference between these two time-points. However, plant height was significantly lower after the 8-h treatment. The 6-h seed treatment with amistar top SC was the most appropriate based on the overall performance.
Discussion
Carrots are an important vegetable grown worldwide and represent a source of carotenoids in the human diet23. However, the emergence rate of some carrot cultivars was relatively low. The decreased germination rate was due to an infection of the seeds by A. alternata. The genus Alternaria Nees includes imperfect fungal species that are cosmopolitan and economically important. In the current study, the germination rate of seeds not infected with A. alternata was 28.7% higher than that of the control seeds infected with fungi. Thus, screening for suitable fungicides and optimizing their applications are important.
Seeds are critical for viable crop production. Pathogen-free seeds are essential for generating healthy plant populations and a good harvest7. In many crops, fungal infections are responsible for low-quality seeds24. Additionally, the presence of seed-borne pathogenic fungi in beans results in decreased germination, emergence, growth, and yield25. This is consistent with our finding that the highest carrot seed infection rate was approximately 60%. Moreover, seed infection rates differed among the tested carrot cultivars. Selecting carrot cultivars uninfected by fungi represents a good agronomic practice for minimizing the chances of fungal infections.
Microorganisms associated with seeds may be pathogens, weak parasites, or saprophytes. They may be present within or on the surface of seeds and may infect seeds via exposures to contaminated sclerotia, galls, fungal bodies, infected plant parts, and soil particles6. Fungal pathogens may be externally or internally seed-borne, extra- or intra-embryonic, or associated with seeds as contaminants26. To clarify which carrot seed parts are infected by fungi, the seed infection rates were calculated for surface-sterilized whole and cut seeds. Our results suggest the primary fungal infection site of carrot seeds is just underneath the seed shell (internally seed-borne and extra-embryonic). However, a more precise localization of the fungi is necessary.
Seeds infected by fungi influence the germination, overall health, and final crop stand under field conditions7. Seed-borne as well as seed-associated fungal infections can be effectively inhibited if the seeds are treated with fungicides before sowing6. The application of chemical fungicides can completely control fungal infections, but it can be costly and harmful to human health and the environmentyyyyy6. To prevent the undesirable effects of fungicides on carrots, we systematically screened for effective fungicides and examined the effects of their application. The optimal seed treatment involved a 6-h immersion in amistar top SC (effective concentration of 0.65 g/L), which killed all of the fungi infecting the seeds, with no deleterious effects on the seeds or seedlings.
The results of this study provide a theoretical basis for the development of effective methods for controlling the fungal infections of carrot seeds, thereby increasing the germination rate and vigour index.
References
Que, F. et al. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. Engl. 6, 69–82 (2019).
Sumekar, Y. Weed diversity in carrot (Daucus carota L.) crop in Majalengka Regency, West Java Province. Indonesia. Res. Crops 20, 109–115 (2019).
Fu, Y. L., Liu, T. Z., Fan, J. Y., Zhao, X. & Niu, R. S. Present Situation and development trend of carrot breeding in China. Hebei Agri. Sci. 14, 100–101 (2010).
Michael, T. P. & VanBuren, R. Progress, challenges and the future of crop genomes. Curr. Opin. Plant Biol. 24, 71–81 (2015).
Luby, C. H., Maeda, H. A. & Goldman, I. L. Genetic and phenological variation of tocochromanol (vitamin E) content in wild (Daucus carota L. var. carota) and domesticated carrot (D. carota L. var. sativa). Hortic. Res. Engl. 1, 14015 (2014).
Biswas, S. et al. Isolation of seed-borne and seed associated fungi of lablab purpureus (l) Sweet and their biological control. J. Microbiol. Biotech. Food Sci. 5, 136–141 (2015).
Baka, Z. A. M. Biological control of the predominant seed-borne fungi of tomato by using plant extracts. J. Phytopath. Pest Manage. 1, 10–22 (2014).
Duan, C. X., Wang, X. M., Zhu, Z. D. & Wu, X. F. Testing of seedborne fungi in wheat germplasm conserved in the National Crop Genebank of China. Agric. Sci. China 6, 682–687 (2007).
Maude, R. B. Seed-borne diseases and their control: principles and practice (CAB International, Wallingford, UK, 1997).
Ilieva, V., Mitrev, S., Karov, I., Markova, N., & Todorovska, E. Seed quality and its importance in agricultural production and safety of agricultural products. In International Conference “Quality and Competence 2013”, pp. 1–11 (2013).
Agarval, V. K. & Sinclair, J. B. Principles of seed pathology (CRC Press Inc, Boca Raton FL, 1987).
Dube, E., Sibiya, J. & Fanadzo, M. Early planting and hand sorting effectively controls seed-borne fungi in farm-retained bean seed: research article. S. Afr. J. Sci. 110, 75–80 (2014).
Miyake, T., Tateishi, H., Sakuma, Y. & Saishoji, T. A novel soil-type biopesticide KNB422-soil against rice seedling diseases. J. Pestic. Sci. 37, 129–134 (2012).
Sudisha, C. I. J., Niranjana, S. R., Umesha, S., Prakash, H. S. & Shetty, H. S. Transmission of seed-borne infection of muskmelon by Didymella bryoniae and effect of seed treatments on disease incidence and fruit yield. Biol. Control 37, 196–205 (2006).
Wang, H., Li, K. M., Wang, L. L., Fan, J. X. & Qiereyazidan, J. Y. Inhibitory effect of 6 kinds of fungicides on alfalfa seed carrying pathogens and its seed development. Xinjiang Agr. Sci. 54, 931–937 (2017).
Leslie, J. F. & Summerell, B. A. The Fusarium Laboratory Manual (Blackwell Publishing, Victoria, Australia, 2006).
Ellis, M. Dematiaceous hyphomycetes 601–608 (Commonwealth Mycol. Institute. Kew, Surrey, England, 1971).
Aleoxopolous, J. & Mims, W. Introductory Mycology (Third Edi. John Wiley and Sons. Inc, USA, 1979).
Li, Y. G. et al. Occurrence of leaf spot of early lilac caused by Alternaria alternata in Heilongjiang Province in China. Plant Dis. 101, 1048 (2017).
Yin, J. et al. Aggressiveness and diversity of Phytophthora capsici on vegetable crops in Georgia. Ann. Appl. Biol. 160, 191–200 (2012).
Carbone, I. & Kohn, I. M. A method for designing primer sets forspeciation studies in filamentous ascomycetes. Mycologia 91, 553–556 (1999).
Woudenberg, J. H. C., Groenewald, J. Z., Binder, M. & Crous, P. W. Alternaria redefined. Stud. Mycol. 75, 171–212 (2013).
Macura, R., Michalczyk, M., Fiutak, G. & Maciejaszek, I. Effect of freeze-drying and air-drying on the content of carotenoids and anthocyanins in stored purple carrot. Acta Sci. Pol. Technol. Aliment. 18, 135–142 (2019).
Neergaard, P. Seed pathology Vol. 1, 839 (The Macmillan Press, New York, 1979).
Marcenaro, D. & Valkonen, J. P. T. Seedborne pathogenic fungi in common bean (Phaseolus vulgaris cv. INTA Rojo) in Nicaragua. PLoS ONE 11, e0168662 (2016).
Singh, D. & Mathur S. B. Location of fungal hyphae in seeds. in Histopathology of seed borne infections (Singh D, & Mathur SB, Eds.). Boca Raton, FL, USA: CRC Press, pp. 101–168 (2004).
Acknowledgements
This study was financially supported by Shuangyashan Dong Hao Agricultural Science and Technology Development Co., Ltd; the National Natural Science Foundation of China (31971760) and Heilongjiang Collaborative Innovation and Extension System of Modern Agricultural Industry Technology of Forage and Feed.
Author information
Authors and Affiliations
Contributions
X.Z., R.W., H.N. and Y.L. designed the experiment. X.Z., R.W., W.L. and Y.B. conducted the experiment and wrote the article. X.Z., R.W., and Y.L. helped in statistical analysis. H.N. and Y.G.L. revised the article. All authors approved the final article after reading.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Wang, R., Ning, H. et al. Evaluation and management of fungal-infected carrot seeds. Sci Rep 10, 10808 (2020). https://doi.org/10.1038/s41598-020-67907-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67907-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.