Abstract
Severe immunodeficient mice are widely used to examine human and animal cells behaviour in vivo. However, mice are short-lived and small in size; while large animals require specific large-scale equipment. Rabbits are also commonly employed as experimental models and are larger than mice or rats, easy to handle, and suitable for long-term observational and pre-clinical studies. Herein, we sought to develop and maintain stable strains of rabbits with X-linked severe combined immunodeficiency (X-SCID) via the CRISPR/Cas9 system targeting Il2rg. Consequently, X-SCID rabbits presented immunodeficient phenotypes including the loss of T and B cells and hypoplasia of the thymus. Further, these rabbits exhibited a higher success rate with engraftments upon allogeneic transplantation of skin tissue than did wild type controls. X-SCID rabbits could be stably maintained for a minimum of four generations. These results indicate that X-SCID rabbits are effective animals for use in a non-rodent model of severe immunodeficiency.
Similar content being viewed by others
Introduction
Immunodeficient animals are important for in vivo transplantation studies using human or animal cells. In particular, immunodeficient mice are most often used because of their high reproductive ability and ease of handling. Nude mice with a Foxn1 mutation have been widely used in studies on cancer as athymic mice1,2. Thereafter, SCID mice with Prkdc mutations were discovered3, which succeeded in differentiating human haematopoietic stem cells in the mouse body upon transplantation of human foetal liver and thymus tissues under the renal capsule; these mice are referred to as SCID-hu and are widely used in studies on HIV4. Il2rg-mutant mice have recently been developed through gene targeting technology5; this gene is essential for signal transduction among interleukins such as IL-2, IL-4, IL-7, IL-9, and IL-15.
Il2rg is located on the X chromosome, and a mutation of the γ chain in humans causes X-SCID6. Examples of X-SCID-animals include rats7, marmosets8, pigs9, and dogs with the gene mutation10. Furthermore, functional defects such as T, B, and/or NK loss lead to severe immunodeficiency.
Although numerous mouse models of immunodeficiency have been reported and handling of animals is relatively easy, they are small in size and short-lived, thus complicating surgical procedures in certain regions including the eye and blood vessels and resulting in inadequate long-term in vivo evaluation. In contrast, large animals such as pigs and monkeys can have a longer lifespan and larger organ size; however, they require advanced handling expertise and a large-scale facility, resulting in a higher cost. Moreover, large animals have a long period of sexual maturity and gestation period.
Rabbits can be operated on relatively easily owing to their size. Furthermore, rabbits are the second most widely used animal after experimental rodent models owing to their long life, ease of handling, and the ability to be reared in a small space. Further, they have a short period of sexual maturity and gestation and are more genetically related to humans than other rodents11. Hence, rabbits are regularly employed in studies of infectious disease12, liver cancer13, adult T cell leukaemia-lymphoma14, joint or bone disease15,16, dentistry17, eye disease (cornea18, retina19), cardiovascular disease20,21, atherosclerosis22,23,24, and lipid metabolism25 and for the production of antibodies26. The detailed rabbit genome has recently been published in the Broad Institute server (https://www.broadinstitute.org/rabbit/rabbit-genome-project) and RGB-Net: Collaborative European Network on Rabbit Genome Biology (http://www.biocomp.unibo.it/rabbit/index.php). Numerous studies have reported rabbits as models of human disease through genetic modification27,28,29.
Although several rabbit models of immunodeficiency30,31 have been developed, no study has functionally analysed the lineage stability of Il2rg gene-deficient rabbits. This study reports the development of Il2rg-KO rabbits and maintenance of a stable strain, using the CRISPR/Cas9 system.
Results
Injection of Il2rg encoding CRISPR/Cas9 mRNA into rabbit embryos
Cas9 and gRNA concentrations were divided into two conditions: low and high concentrations (Table 1). At low concentration conditions, 293 embryos were harvested from ten donor rabbits, of which 241 survived (82.3%). Twenty-two (9.1%) offspring from founder animals were obtained, of which eight (36.3%) were mutated. At high concentration conditions, 259 embryos were harvested from ten donor rabbits, of which 194 survived (74.9%). Eight (4.1%) offspring were obtained from founder animals, of which 6 (75.0%) were mutated. Sequencing analysis of the CRISPR/Cas9 target site in these 14 founder animals revealed that the offspring harboured various mutations including 1–265-bp deletions and a 1–about 100-bp insertion in the region overlapping the CRISPR/Cas9 target site (Fig. 1c,d).
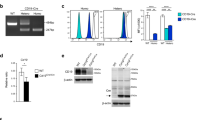
Verification of founder Il2rg-mutant rabbits using the CRISPR/Cas9 system. (a) Schematic representation of rabbit Il2rg. Magnified views illustrate the binding sites for CRISPR. (b) Co-injection of gRNA targeting rabbit Il2rg with Cas9 mRNA into rabbit male pronuclei embryos resulted mutations at the target sites. (c) PCR analysis of 30 offspring obtained through CRISPR/Cas9 injection of JW oocytes. Fourteen mutants (#2, 3, 4, 6, 8, 12, 16, 20, D1, D2, D3, D5, D8, and D9) were identified (asterisk). The product size of the wild-type JW Rabbit allele (arrow) is of 249 bp. (d) Sequencing analysis of CRISPR/Cas9-induced mutations in the target region in JW rabbits. Multiple deletions and insertions are depicted by red dashes and letters, respectively, and are aligned along the WT sequences displayed on the top line.
Three of the five male offspring harboured mosaicism biallelic mutations at the Il2rg locus despite having only one X chromosome, suggesting that mosaicism was induced upon CRISPR/Cas9 treatment, a situation frequently observed in the DNA of transgenic founders. Two of the three female offspring harboured biallelic mutations. We maintained the strain with the 265-bp deletion as it was easy to identify gene disruption and numerous amino acid deletions were expected.
Germline transmission of Il2rg-deficient X-SCID rabbits
Hemizygous male (Il2rg−/Y) rabbits display the severe immunodeficiency phenotype (X-SCID); however, heterozygous female (Il2rg−/+) rabbits display a normal phenotype9,32. Therefore, hemizygous male (F0-8, Il2rg−/ Y) and WT female rabbits were mated to produce heterozygous female (Il2rg−/+) offspring with a 265-bp deletion in the F1 generation (Fig. S1). Furthermore, in the F2 generation, 21 of 80 (26.3%) hemizygous male rabbits were obtained and analysed (Table S1). Moreover, 6 of 22 (27.3%) hemizygous male rabbits among the F3 offspring and 8 of 42 (19.0%) hemizygous male rabbits among the F4 offspring were obtained (Table S1).
Characterisation of Il2rg-deficient X-SCID rabbits
Appearances did not markedly differ between X-SCID and WT rabbits (Fig. 2a). The body weight of male WT rabbits tended to increase until approximately 14 weeks of age, followed by a gradual increase. Furthermore, the body weight of X-SCID rabbits tended to increase until approximately 14 weeks; however, thereafter, individuals with a decreased body weight were noted (Fig. 2b). Survival rates were confirmed among five X-SCID rabbits and five WT in F2 offspring. The mean lifespan of X-SCID rabbits was 29.6 ± 6.8 weeks. Four of the five X-SCID rabbits were euthanised owing to lung abnormalities (black discoloration of lung tissue (Fig. S2)), etc., and the remaining rabbits were experimentally euthanised. Two of five WT animals were experimentally euthanised, while the remaining three were alive during the assessment period (Fig. 2c).
Postnatal growth and survival curve of X-SCID and WT rabbits. (a) Photograph of four-week-old male X-SCID (Il2rg−/Y) and WT (Il2rg + /Y) rabbits. (b) Postnatal growth of rabbits. X-SCID (0–19w; n = 5, 20–22w; n = 4, 23–32w; n = 3, 33w; n = 2), WT (0–32w; n = 5, 33–38w; n = 4, 39–42w; n = 3). (c) Survival curve of rabbits. X-SCID n = 5, WT n = 5. * Two of the five animals were euthanized at 38 and 40 weeks for another experiment.
Haematological parameters determined from peripheral blood (PB) profiles, including the white blood cell (WBC) count, were lower than those in WT rabbits (Table 2), and differential leukocyte counts revealed a significant reduction in lymphocytes in the X-SCID rabbits.
Flow cytometry analysis of cell populations isolated from peripheral blood mononuclear cells revealed a marked reduction in the number of lymphocytes in X-SCID rabbits (Fig. 3a,b). The number of CD3 + T cells (X-SCID, 0.2% ± 0.1%; WT 9.6% ± 1.4%; n = 5 each, p < 0.01) and IgM+ B cells (X-SCID, −0.05% ± 0.06%; WT 11.8% ± 5.2%; n = 5 each, p < 0.05) were significantly decreased in X-SCID rabbits. Heterozygous female rabbits exhibited normal lymphoid development and were indistinguishable from WT rabbits.
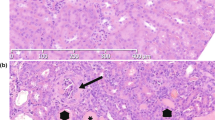
Phenotype of peripheral lymphocytes and thymic and spleen organogenesis in X-SCID rabbits. (a) Flow cytometric analysis of cell populations isolated from peripheral blood lymphocytes. Dot plots for CD3 and IgM for the differentiation of T- and B-cell sub-populations. The numbers shown are mean percentages. *P < 0.05, **P < 0.001. (b) Flow cytometric analysis of cell populations isolated from among peripheral blood lymphocytes. (c) RT-PCR analysis of Il2rg in the spleen in control X-SCID and WT rabbits. Gapdh was considered the internal control. (d) Histological analysis of H&E-stained thymus specimens (yellow circled area) of X-SCID (20× magnification) and WT (20× magnification) rabbits (36 weeks old, male). The thymus of X-SCID rabbits was markedly hypoplastic. Scale bar, 100 μm. (e) Histological analysis of H&E-stained spleen specimens of X-SCID (10× magnification) and WT (10× magnification) rabbits (36 weeks old, male). The yellow circled area indicates white pulp and the white circled area indicates the central artery. In the X-SCID spleen, the white pulp was devoid of lymphocytes. Scale bar, 100 μm.
RT-PCR analysis revealed a reduction in mRNA size in the spleen of X-SCID rabbits in comparison with WT rabbits (Fig. 3c).
Gross and microscopic analyses revealed that X-SCID rabbits underwent abnormal lymphoid development. In the thymus, it was difficult to isolate the exact thymus tissue from X-SCID rabbits for measuring the weights because of the remarkable thymus hypoplasia. Instead, we examined thymus tissue by HE-staining, indicating that thymus from X-SCID rabbits contained almost no lymphocytes (Fig. 3d). Although spleen weight and appearance did not markedly differ, lymphocytes in the white pulp area were reduced (Fig. 3e, Table S2). The mesenteric lymph node and Peyer’s patches in the ileum were not macroscopically identified. The appendix wall in X-SCID rabbits was thinner than that in WT rabbits (Fig. S3a–c).
Allogenic skin transplantation
For skin transplantation, Dutch rabbits (21–22 weeks old, n = 2) were used as donors and X-SCID rabbits (21–22 weeks old, n = 4, F2 offspring) and WT rabbits (21–22 weeks old, n = 4, F2 offspring) were used as recipients (Fig. 4a). No marked difference was observed until three days after transplantation. On day 7, although the exudate was observed in WT rabbits, no clear difference was observed. After 12 days, the skin graft hardened and darkened in all WT rabbits, yet remained soft state in normal skin colour in X-SCID rabbits. On day 21, the skin graft remained dark colour and was not found to aggulutinate in WT, however, remained normal skin colour and aggulutinated in X-SCID rabbits. After 56 days, black hair was observed in X-SCID rabbits, which is characteristic of Dutch rabbit (Figs. 4b, S4a,b).
Allogeneic transplantation of skin. (a) Skin graft method. (b) Morphological observation of the skin graft of time-dependent change. Yellow circled area is black hair of Dutch rabbit. (c) Histological analysis of the day 22 skin graft section of X-SCID and WT rabbits stained with H&E (X4), CD45 (X200), CD3 (X200). Scale bar 500μm. White dot lines are the boundary of the skin graft and original skin. Lymphocytes are infiltrating at the border of WT, but lymphocyte infiltration is scarcely shown in X-SCID.
Histological examination via haematoxylin and eosin staining in X-SCID rabbits revealed marked fibroblast proliferation at the interface between the transplanted and original skin, and no inflammatory cell infiltration, including lymphocytes, was observed. However, in WT rabbits, numerous lymphocytes were observed at the interface (Fig. 4c). During immunostaining, while few positive cells were observed in X-SCID rabbits (Fig. 4c). However, in WT rabbits, CD45-positive cells were confirmed at the border between the site of transplantation and the autologous skin, and CD3-positive cells were confirmed in the dermis (Fig. 4c).
Discussion
This study reports the development of established X-SCID rabbits via the CRISPR/Cas9 system and their stable maintenance, demonstrating that Il2rg-KO rabbits can be generated with a high genome editing efficiency of 46.7%. The efficiency was relatively high compared to that of Il2rg-KO model mice5 (31%; ES cells), and rats7, (24%; ZFN), rabbits31, (16.7%; CRISPR/Cas9), pigs9, (1.25%; somatic cell clone), and marmosets8, i.e., (42.6%; TALEN and ZFN). In a previously generated rabbit model of immunodeficiency30, the genome-editing efficiency was 100%; however, all founder rabbits died in 45 days. Our X-SCID rabbit model and the previously generated rabbit model of immunodeficiency30,31 were subjected to the same Il2rg gene KO; however, the target sites were different, thus also being associated with genome editing efficiency and lineage stability. Non-rodent experimental animals including rabbits are often used for long-term follow-up evaluation studies such as transplantation experiments. Rabbits yield offspring over a short period, with 4–5 months of sexual maturity and a gestational age of approximately 30 days in comparison with other large animals (e.g., cynomolgus monkeys with a 3.5–4-year period of sexual maturity and a gestational period of 150–225 days)33. The ability to obtain highly efficient progeny (F1/F2) that can rectify mosaic mutations and epigenetic defects in the 6th month in the shortest period leads to shortening of the study period, which is advantageous for research and development34.
The Il2rg−/Y (X-SCID) rabbits herein presented marked hypoplasia of the thymus, a decreased lymphocyte count in the white pulp area in the spleen, loss of peripheral lymph nodes, and a significant reduction in the lymphocyte count in peripheral blood, similar to other X-SCID animals. These observations indicate that in rabbits as well, the Il2rg plays contributes to the development and differentiation of lymphocytes and its functional deficiency induces immunodeficiency symptoms. X-SCID rabbits displayed a significant reduction in the number of CD3+ T cells among peripheral blood mononuclear cells during sexual maturation. In X-SCID mice and marmosets, T cells increase in infancy 8,35. In human X-SCID, CD3+ T cells are almost completely obliterated6; hence, rabbit phenotypes can be considered similar to those of humans. Furthermore, the number of B cells remains constant in WT pigs and human X-SCID6,9; however, the number of B cells is significantly reduced in mice and rats5,7. Herein, the number of IgM+ B cells also significantly reduced in the rabbits, displaying the same tendency as that of mice and rats. NK cells were not detected as no specific antibody to rabbit NK cells was identified, as previously reported31. T/B-cell fractions differ among animal species with age. Herein, T and B cells were significantly reduced during sexual maturation of rabbits; however, in X-SCID rabbits, further detailed subset analysis of T and B cells in each immune organ and further analyses are required to elucidate the development and differentiation of T and B cells.
Furthermore, we performed allogeneic transplantation and confirmed skin engraftment among individuals with genetically different characteristics. Skin transplantation is a well-established method often used in studies on the immune response and rejection36,37, and skin transplantation is a non-invasive method. In our skin transplantation experiments, we used Dutch rabbits as donors and Japanese White rabbits (X-SCID rabbits) as recipients. Dutch rabbits have black hair, while X-SCID rabbits have white hair; hence, it is easy to ascertain graft acceptance. The Dutch rabbits belong to the Dutch germline Oryctolagus cuniculus; while the Japanese White species belong to Japanese germline O. cuniculus. The genomes of Dutch and Japanese White rabbits belong to different groups38. Although the innate immune system was functional in the X-SCID rabbits, we were able to confirm the engraftment of allogeneic skin in these rabbits. T and B cells are largely involved in the rejection of transplanted immunity and it has been shown that both the number and function of T and B cells are decreased in X-SCID rabbits. The degree of rejection varies depending on the type of organ (tissue), and among them, skin tissue is susceptible to rejection39. Therefore, survival can potentially be achieved in the transplantation of organs (tissues) other than the skin. Furthermore, in X-SCID pigs9, lymphoid cell reconstruction was confirmed via allogeneic bone marrow transplantation, suggesting that X-SCID rabbits with the same phenotype can be expected to be used as a model for immune system reconstruction through bone marrow transplantation.
We also investigated whether X-SCID rabbits can stably maintain their lineages. Consequently, we obtained a stable line of rabbits up to the F4 generation. The average lifespan of the F2 generation X-SCID rabbits was approximately 200 days, and the X-SCID pigs survive for a longer period than the average period of approximately 70 days9, probably owing to antibiotic administration and housing conditions. Antibiotics were used from 6 weeks after weaning, and the previous studies on X-SCID rats41 have reported that the infection was suppressed through antibiotic administration. Furthermore, in a previous study, rabbits were maintained under conventional housing conditions30, in contrast, our rabbits were maintained in a clean booth until they were approximately 9–15 weeks old. Furthermore, inbreeding degeneration is rapid among rabbits40; however, the issue was resolved through mating between a heterozygous individual with the same phenotype as that of WT and a WT fertile individuals raised in another colony. From the F2 generation, pneumonia symptoms and diarrhoea, which are considered to be caused by Pneumocystis oryctollagi, were observed. This condition has also been reported in X-SCID rats and other immunodeficient rabbits41,42. A previous study reported42 that before weaning, X-SCID rabbits were resistant to Pneumocystis owing to immunity acquired from the mother; however, severe infection was observed sometimes after weaning. These results suggest that the lung abnormality did not result from gene knockout itself but rather from Pneumocystis pneumonia due to a suppressed immune response after weaning. Furthermore, WT rabbits harbouring an antibiotic specific for Pneumocystis pneumonia have reduced P. oryctollagi and the individuals are used as parent WT rabbits, and the offspring are obtained through caesarean sections and reared under aseptic conditions, resulting in the suppression of Pneumocystis infections in the same manner as X-SCID rats43,44,45. Thus, we speculate that X-SCID rabbits survive longer and efficient strain maintenance is possible.
We thus generated highly immunodeficient animals. X-SCID rats were confirmed with regards to human ovarian cancer cells and survival of human embryonic cells or human induced pluripotent stem cell (iPSC)-derived DA neurons46. X-SCID rabbits with the same phenotype can be expected to be used as humanised animal models transplanted with human-type organs (tissues) or pluripotent stem cells. Furthermore, the number of transplantable cells is limited because Il2rg-KO mice and X-SCID rats have a small body size. X-SCID rabbits can be transplanted with a similar or equal number of cells to that of humans. Safety tests including those related to regenerative medicine and cell therapy are potentially applicable because they are considered advantageous for optimising the dosage of transplanted cells and for confirming the characteristics of grafted cells at the transplant site. Accordingly, rabbits are potentially applicable as a link between mice and rats and other animal species including pigs and monkeys, and as an excellent tool for studies on organ and cell transplantation, cancer, and haematopoietic system bridging to humans.
Methods
Animals
Japanese White (JW) rabbits were obtained from KITAYAMA LABES Japan (Nagano, Japan) and housed under conditions of 30–70% humidity and a 12:12-h light/dark cycle. They were fed with pellets which had been sterilised via gamma irradiation (50 kGy/box) and supplied with water treated using a reverse osmosis system (Edstrom Japan, Co., LTD., Saitama, Japan). All X-SCID rabbits were subcutaneously injected twice per day with 10 mg/kg enrofloxacin from six weeks of age.
All animal care and experimental protocols conformed to the Guidelines for Animal Experiments of Osaka University and were approved by the Animal Experimental Committee of Osaka University. All of the experiments using recombinant DNA were approved by the Recombinant DNA Committees of Osaka University and were performed in accordance with our institutional guidelines.
Construction of the CRISPR/Cas9 vector and gRNA design
A Cas9 expression plasmid (RDB13130) was used herein. To generate a Cas9 mRNA expression vector, plasmids were linearized with Nhe1 (New England Biolabs, Ipswich, MA, USA), and extracted with NucleoSpin Gel and PCR Clean-up kits (Macherey-Nagel, Duren, Germany) in accordance with the manufacturer’s instructions. After purification of the linearized DNA, Cas9 mRNA was transcribed in vitro using a mMESSAGE mMACHINE T7 Ultra Kit (Thermo Fisher Scientific, Waltham, MA, USA) and was purified using a MEGAClear kit (Thermo Fisher Scientific) and resuspended in RNase-free water, in accordance with the manufacturer’s instructions.
gRNA was designed using CRISPR DESIGN software (http:crispr.mit.edu/). gRNAs were transcribed in vitro using a MEGAshortscript T7 Transcription Kit (Thermo Fisher Scientific) in accordance with the manufacturer’s instructions from synthetic double-stranded DNA containing a T7 promoter, 20-bp target sequences, and gRNA tail sequences (Integrated DNA Technologies, San Diego, CA, USA). The gRNA DNA template was synthesised in vitro using CUGAR 7 in vitro Transcription Kit (NIPPON GENE, Tokyo, Japan) in accordance with the manufacturer’s instructions and transcribed. mRNA was purified using a MEGAClear kit (Thermo Fisher Scientific) in accordance with the manufacturer’s instructions and resuspended in RNase-free water47,48.
Embryo microinjection and transfer
Sexually matured JW female rabbits (n = 39, KITAYAMA LABES), as donors, were super-ovulated via subcutaneous injection of follicle-stimulating hormone (FSH, Antrin R10, Kyoritsu Seiyaku, Tokyo, Japan) six times per day. Seventy-two hours after FSH injection, the donor females were artificially inseminated, and a single dose of 75 IU human chorionic gonadotropin (hCG, GONATROPIN1000, ASKA Pharmaceutical, Tokyo, Japan) was immediately intramuscularly administered to induce ovulation. Simultaneously, 75 IU of hCG was injected to sexually mature recipient female rabbits to induce pseudopregnancy. Nineteen to 21 h after hCG injection, fertilised eggs were harvested from donor females and pronuclear-stage embryos were selected and cultured in a modified medium (Medium199 [1×] Hanks‘ Salts [Thermo Fisher Scientific]) supplemented with 5% FBS (Biological Industries, Cromwell, CT, USA) before the microinjections. Using a micromanipulator (Narishige, Tokyo, Japan), 200 ng/uL Cas9 mRNA, 100 ng/ul gRNA, 15 ng/ul pExo1 or 100 ng/ul Cas9 mRNA, 50 ng/ul gRNA, 15 ng/ul pExo1 were microinjected into the male pronuclei of embryos. These embryos were cultured in a modified medium (Medium199 [1×] Earle’s Salts; Thermo Fisher Scientific) supplemented with 20% FBS (Biological Industries) after the microinjections. Surviving embryos were sorted and twenty to thirty embryos were transplanted into the oviduct of recipient rabbits. A part of the tail of few-day old pups tail was sampled and genome DNA was extracted using the KAPA Express Extract Kit (Roche Sequencing and Life Science Kapa Biosystems, Wilmingto, MA, USA). PCR was performed using the following conditions: 94 °C for 1 min, 35 cycles of 98 °C for 10 s, 61 °C for 15 s, and 68 °C for 30 s, and final extension at 72 °C for 1 min (Table S3). The PCR products were then directly sequenced using the BigDye Terminator v3.1 cycle sequence mix and Applied Biosystems 3130 DNA Sequencer (Thermo Fisher Scientific) standard protocol.
Physiological characteristics
Body weights of rabbits (F2 offspring X-SCID [n = 5] and WT [n = 5]) were assessed once a week. Rabbits were monitored throughout the observation period and mortalities were recorded daily (F2 offspring X-SCID [n = 5] and WT [n = 5]). Blood parameters were analysed in 20-week-old, X-SCID (n = 4) and control WT (n = 4) F2 offspring. Approximately 600 μL peripheral blood was sampled from the ear vein. Blood parameters including the blood cell count and a differential WBC count were measured using ADVIA2120 (Siemens Healthineers Headquarters, Erlangen, Germany).
Fluorescence activated cell sorting (FACS) analysis of T and B lymphocytes
Flow cytometric analyses were performed for 17–25-week-old, Il2rg-/Y (n = 4–5) and control WT (n = 5–6) F2 offspring. To enumerate T and B lymphocytes in whole blood, approximately 2 mL of peripheral blood was sampled from the ear vein in a heparin-coated syringe. Lymphocytes from blood were separated using a Ficoll-Paque PREMIUM (GE Healthcare UK Ltd., Bucks, UK) gradient via centrifugation for 40 min at 400 × g. Cells were filtered with a 40-μm cell strainer (BD Biosciences, San Diego, CA, USA) and washed with PBS to eliminate debris. The remaining pellets were resuspended in 1 ml of PBS and enumerated with a haemocytometer. Anti-human CD3 (F7.2.38, Dako Denmark A/S, Glostrup, Denmark), anti-rabbit IgM (MCA812GA, Bio-Rad, Hercules, CA, USA) were used as primary antibodies. Mouse IgG1 antibodies (Biolegend, San Diego, CA, USA) were used as isotype-matched controls. For intracellular protein staining, a BD Cytofix/Cytoperm (BD Biosciences) kit was used in accordance with the manufacturer’s instructions.
Approximately 2.5–5.0 × 105 cells per sample were incubated with the primary antibodies in PBS for 30 min on ice and washed twice via centrifugation at 400 × g for 5 min. Thereafter, cells were incubated with Alexa Fluor 647-conjugated secondary antibodies (1:200; Thermo Fisher Scientific) under the same conditions. After washing, the cells were resuspended with 500 μL PBS before FACS analysis. Thereafter, 10,000 live cells were used for flow cytometry analysis using FACSVerse (BD Biosciences). The data were analysed using the BD FACSDiva Software (BD Biosciences).
RT-PCR analysis
Total RNA was extracted from the spleen of 36-week-old F2 offspring using QIAzol reagent (Qiagen, Valencia, CA, USA). Reverse transcription was performed using the SuperScript III First-Strand Synthesis System for RT-PCR (Thermo Fisher Scientific) in accordance with the manufacturer’s protocol, and cDNA was used as a template for PCR. The cycling conditions were as follows: 98 °C for 10 s, followed by 40 cycles at 60 °C for 15 s and 68 °C for 30 s (Table S3). PCR products were analysed via electrophoresis on 2.0% agarose gels. Gapdh was used as an internal control refer to previous study49.
Allogenic skin transplantation
The rabbits were anaesthetised via isoflurane inhalation 4–5% and maintained at 2–3%. The dorsae of the rabbits were shaved, and the surgical area was disinfected with Povidone-Iodine and washed with 70% ethanol using sterile cotton, and finally dried with sterile gauze. The surgical procedure was carried out under aseptic conditions.
The skin graft donor site was located on the dorsa and a 1.5 × 1.5-cm2 area was marked and harvested using scissors and a scalpel. Immediately thereafter, the grafts stored in HBSS (Thermo Fisher Scientific) at 4 °C until grafting. The recipient rabbits were inflicted with a full-thickness excision skin wound of 1.5 × 1.5 cm2 on the dorsa, using a scalpel and scissors. The skin graft was grafted at the dorsal wound in recipient rabbits, using 5–0 PROLENE (Johnson & Johnson, New Brunswick, NJ, USA) sutures, with one stitch at each of four corners and 3–4 stitches on each side. The skin graft sites were covered with a transparent dressing (Tegaderm, 3 M, St. Paul, MN, USA), and sterile gauze, elastic bandage, and surgical tape were applied to seal the wound. Upon regular inspection of the wounds, the bandages were changed50.
Stitches and bandages were removed on day 14, and the skin grafts were inspected on days 0, 1, 3, 5, 7, 10, 12, 14, and 21 after surgery. Two X-SCID rabbits were inspected up to day 56. Graft rejection was detected through the macroscopic appearance of graft necrosis, while graft necrosis was evaluated using a scale based on colour changes and scarring. At 21–56 days after transplantation, the animals were euthanised with pentobarbital. Graft skin tissues were fixed in 10% formalin solution, embedded in paraffin, and sectioned. The sections were stained with haematoxylin and eosin.
Immunofluorescence staining
The grafted skin tissues were embedded in Tissue-Tek® Optimal Cutting Temperature compound (Sakura Fineteck, Tokyo, Japan) and sectioned at 10-μm thickness. The sections were washed thrice with Tris-buffered saline (TBS, TaKaRa Bio, Shiga, Japan) for 10 min and blocked for 1 h in TBS (TaKaRa Bio) containing 5% donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and 0.3% Triton X-100 (FUJIFILM Holdings Corporation, Tokyo, Japan). The sections were then probed overnight at 4 °C with anti-CD3 (CD3-12; Abcam, Cambridge, UK, USA) and anti-CD45 (L12/201; Bio-Rad) antibodies in TBS containing 1% donkey serum and 0.3% Triton X-100. Subsequently, samples were again washed thrice with TBS for 5 min and were labelled for 1 hour at room temperature (20 °C–28 °C) with secondary antibodies conjugated with Alexa Flour 488 (Thermo Fisher Scientific). The sections were counterstained with Hoechst 33342 (Thermo Fisher Scientific) to visualise the nuclei and imaged using a fluorescence microscope (Axio Observer. D1, Carl Zeiss, Jena, Germany).
Histochemical staining
Haematoxylin and eosin staining, tissues were fixed with 10% formaldehyde neutral buffered solution and embedded in paraffin and then 3–5-μm-thick sections were cut and stained with haematoxylin and eosin after deparaffinisation and hydration. The sections were imaged using a microscope equipped with a NanoZoomer-XR C12000 system (Hamamatsu Photonics, Hamamatsu, Japan) and an Axio Observer.D1 system. Grocott staining was performed by KAC Co., LTD. (Kyoto, Japan).
Statistical analyses
All data are expressed as mean ± S.D. values. Statistical analyses were performed using the Welch’s t test. p values of <0.05 were considered to indicate statistical significance. All of statistical analyses were performed using the GraphPad Prism8 software (GraphPad Software Inc., San Diego, CA, USA).
References
Flanagan, S. P. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 8, 295–309 (1966).
Szadvari, I., Krizanova, O. & Babula, P. Athymic nude mice as an experimental model for cancer treatment. Physiol. Res. 65, S441–S453 (2016).
Bosma, G. C., Custer, R. P. & Bosma, M. J. A severe combined immunodeficiency mutation in the mouse. Nature 301, 527–30 (1983).
McCune, J., Namikawa, R., Shih, C., Rabin, L. & Kaneshima, H. Pseudotypes in HIV-infected mice. Science (80-.). 250, 1152–1154 (1990).
Ohbo, B. K. et al. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood 87, 956-967 (1996)
Allenspach, E., Rawlings, D. J. & Scharenberg, A. M. X-Linked Severe Combined Immunodeficiency. GeneReviews® (1993).
Mashimo, T. et al. Generation of Knockout Rats with X-Linked Severe Combined Immunodeficiency (X-SCID) Using Zinc-Finger Nucleases. PLoS ONE 5, (2010)
Sato, K. et al. Generation of a Nonhuman Primate Model of Severe Combined Immunodeficiency Using Highly Efficient Genome Editing. Cell Stem Cell 19, 127-138 (2016)
Suzuki, S. et al. Il2rg Gene-Targeted Severe Combined Immunodeficiency Pigs. Cell Stem Cell 10, 753-758 (2012)
Felsburg, P. J., Somberg, R. L., Hartnett, B. J., Henthorn, P. S. & Carding, S. R. Canine X-linked severe combined immunodeficiency. A model for investigating the requirement for the common gamma chain (gamma c) in human lymphocyte development and function. Immunol. Res. 17, 63–73 (1998).
Graur, D., Duret, L. & Gouyt, M. Phylogenetic position of the order Lagomorpha (rabbits, hares and allies). Nature 379, 333–335 (1996).
Peng, X., Knouse, J. A. & Hernon, K. M. Rabbit Models for Studying Human Infectious Diseases. Comp. Med. 65, 499–507 (2015).
Tomozawa, Y. et al. Anti-tumor Effects of Sorafenib Administered at Different Time Points in Combination with Transarterial Embolization in a Rabbit VX2 Liver Tumor Model. Cardiovasc. Intervent. Radiol. 40, 1763–1768 (2017).
Panfil, A. R., Al-saleem, J. J. & Green, P. L. Virology: Research and Treatment. Virol. (Auckl). 49–59 https://doi.org/10.4137/VRT.S12140.TYPE (2013).
Ghivizzani, S. C. et al. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc. Natl. Acad. Sci. 95, 4613–4618 (2002).
Wu, Y., Zhang, C., Wu, J., Han, Y. & Wu, C. Angiogenesis and bone regeneration by mesenchymal stem cell transplantation with danshen in a rabbit model of avascular necrotic femoral head. Exp. Ther. Med. 163–171 https://doi.org/10.3892/etm.2019.7556 (2019).
Klokkevold, P. R., Nishimura, R. D., Adachi, M. & Caputo, A. Osseointegration enhanced by chemical etching of the titanium surface. A torque removal study in the rabbit. Clin. Oral Implants Res. 8, 442–7 (1997).
Hayashi, R. et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 531, 376-380 (2016)
Taylor, W. R. & Vaney, D. I. Diverse Synaptic Mechanisms Generate Direction Selectivity in the Rabbit Retina. J. Neurosci. 22, 7712–7720 (2018).
Ytrehus, K., Liu, Y. & Downey, J. M. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am. J. Physiol. Circ. Physiol. 266, H1145–H1152 (2017).
Peuster, M. A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal---results 6-18 months after implantation into New Zealand white rabbits. Heart 86, 563–569 (2001).
Buja, L. M., Kita, T., Goldstein, J. L., Watanabe, Y. & Brown, M. S. Cellular pathology of progressive atherosclerosis in the WHHL rabbit. An animal model of familial hypercholesterolemia. Arterioscler. An Off. J. Am. Hear. Assoc. Inc. 3, 87–101 (2011).
Fan, J. et al. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 146, 104–119 (2015).
Fan, J. et al. Principles and Applications of Rabbit Models for Atherosclerosis Research. J. Atheroscler. Thromb. 25, 213–220 (2018).
Fan, J. & Watanabe, T. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol. Ther. 99, 261–282 (2003).
Spieker-Polet, H., Sethupathi, P., Yam, P. C. & Knight, K. L. Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc. Natl. Acad. Sci. 92, 9348–9352 (2006).
Sui, T. et al. CRISPR/Cas9-mediated mutation of PHEX in rabbit recapitulates human X-linked hypophosphatemia (XLH). Hum. Mol. Genet. 25, 2661–2671 (2016).
Yang, D. et al. Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J. Mol. Cell Biol. 6, 97–99 (2014).
HONDA, A. et al. Single-step generation of rabbits carrying a targeted allele of the tyrosinase gene using CRISPR/Cas9. Exp. Anim. 64, 31–37 (2014).
Yan, Q. et al. Generation of multi-gene knockout rabbits using the Cas9/gRNA system. Cell Regeneration. 3, 3:12 (2014)
Song, J. et al. Production of immunodeficient rabbits by multiplex embryo transfer and multiplex gene targeting. Sci. Rep. 7, 1–9 (2017).
Puck, J. M., Nussbaum, R. L. & Conley, M. E. Carrier detection in X-linked severe combined immunodeficiency based on patterns of X chromosome inactivation. J. Clin. Invest. 79, 1395–1400 (1987).
Niu, Y. et al. Generation of Gene-Modified Cynomolgus Monkey via Cas9/RNA-Mediated Gene Targeting in One-Cell Embryos. Cell 156, 836-843 (2014)
Shin, H. Y. et al. CRISPR/Cas9 targeting events cause complex deletions and insertions at 17 sites in the mouse genome. Nat. Commun. 8, 1–10 (2017).
Cao, X. et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity 2, 223-238 (1995)
Wang MM. et al. Induction of immune tolerance and altered cytokine expression in skin transplantation recipients. Kaohsiung J Med Sci. 34, 330–334 (2018).
Samstein B. et al. Toll-like receptor-4 and allograft responses. Transplantation 168, 628–632 (2003).
Zhou, L., Xiao, Q., Bi, J., Wang, Z. & Li, Y. RabGTD: A comprehensive database of rabbit genome and transcriptome. Database 2018, 1–8 (2018).
Klempnauer J1, Steiniger B, Marquarding E, Vogt P, Lipecz A, Wonigeit K, G. E. Effects of the RT1.C Region in Rat Allotransplantation. 713–5 doi:3274851 (1987).
C. K. CHAI. Effects of Inbreeding in Rabbits. Journal of Heredity 60, 64-70, (1969)
Rigatti, L. H., Toptan, T., Newsome, J. T., Moore, P. S. & Chang, Y. Identification and Characterization of Novel Rat Polyomavirus 2 in a Colony of X-SCID Rats by P-PIT assay. mSphere 1, 1–13 (2016).
Song, J. et al. Bacterial and pneumocystis infections in the lungs of gene-knockout rabbits with severe combined immunodeficiency. Front. Immunol. 9, 1–9 (2018).
Lavoie, M. C. et al. Pneumocystis carinii infection in transgenic B cell-deficient mice. J. Infect. Dis. 173, 1034–1037 (1996).
Sanchez, C. A. et al. Exploring transplacental transmission of Pneumocystis oryctolagi in first-time pregnant and multiparous rabbit does. Med. Mycol. 45, 701–707 (2007).
Cere, N., Polack, B. & Coudert, P. Obtaining a Pneumocystis-free rabbit breeding stock (Oryctolagus cuniculus). J. Eukaryot. Microbiol. 44, 19S-20S (1997).
Samata, B. et al. X-linked severe combined immunodeficiency (X-SCID) rats for xeno-transplantation and behavioral evaluation. J. Neurosci. Methods 243, 68–77 (2015).
Yoshimi, K., Kaneko, T., Voigt, B. & Mashimo, T. Allele-specific genome editing and correction of disease-associated phenotypes in rats using the CRISPR-Cas platform. Nat. Commun. 5, 1–9 (2014).
Yoshimi, K. et al. SsODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nat. Commun. 7, 1–10 (2016).
Sobajima, S. et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 5, 14–23 (2005).
Ding, J. & Tredget, E. E. Transplanting Human Skin Grafts onto Nude Mice to Model Skin Scars. In Methods Mol. Biol. 65–80. https://doi.org/10.1007/978-1-4939-7113-8_5 (2017).
Acknowledgements
This work was supported in part by the grant (JP19bm0404058h0001) from the Japan Agency for Medical Research and Development (AMED). We thank Y. Kobayashi, K. Suzuki, Y. Kudo and staffs of Osaka university of institute of large laboratory animal sciences for their excellent technical assistance.
Author information
Authors and Affiliations
Contributions
R.H. and T.M. conceived and designed the study. Y.H. collected and analysed the data and drafted the manuscript. T.M. and Y.K. developed the CRISPR/Cas9 constructs and gRNA. N.T. and Y.O. performed microinjection of CRISPR/Cas into rabbit embryos. Y.H., Y.K., T.O., and S.A. for PCR and sequencing analyses. Y.H. and T.O. performed experiments and performed FACS analyses. Y.H. and M.T. measured the body weight and performed skin grafting. M.K. and Y.H. conducted the histological analyses. K.N. assisted in designing the study and data interpretation. All authors have read and approved the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hashikawa, Y., Hayashi, R., Tajima, M. et al. Generation of knockout rabbits with X-linked severe combined immunodeficiency (X-SCID) using CRISPR/Cas9. Sci Rep 10, 9957 (2020). https://doi.org/10.1038/s41598-020-66780-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66780-6
This article is cited by
-
The preclinical and clinical progress of cell sheet engineering in regenerative medicine
Stem Cell Research & Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.