Abstract
In the era of intensity-modulated radiotherapy (IMRT), it is important to analyse the prognostic value of deficient mismatch repair (dMMR) in nasopharyngeal carcinoma (NPC). In this study, in pretreatment biopsies of 69 patients with stage II–IVa NPC, the expression levels of MMR proteins, including MLH1, MSH2, MSH6 and PMS2, were assessed by immunohistochemistry (IHC). The median follow-up time was 37.5 months (3.1–87.4 months). 50.7% of cases (35/69) showed preserved expression of all 4 MMR proteins, which was interpreted as proficient mismatch repair (pMMR). Only 1.5% of cases (1/69) lost expression of all 4 MMR proteins, 26.1% of cases (18/69) have PMS2 loss alone and 21.7% of cases (15/69) lost expression of both PMS2 and MLH1. Thus, 49.3% of cases (34/69) lost expression of one or more MMR proteins, which was interpreted as dMMR. There was no significant difference (P > 0.05) in terms of sex, age, clinical stage, T category, N category or therapy regimens between the dMMR and pMMR groups. The multivariate Cox regression analysis revealed that dMMR was an independent significant prognostic factor for distant metastasis-free survival (DMFS) (dMMR vs pMMR: P = 0.01, HR = 0.25, 95% CI: 0.09~0.75). Therefore, NPC patients with dMMR had significantly superior DMFS compared with patients with pMMR. It can be expected that dMMR will become a new independent prognostic factor for NPC.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is a kind of common head and neck cancers, occurring frequently in southern China and Southeast Asia. NPC has strong invasiveness and early cervical lymph node metastasis. Seventy percent of NPC patients have locally advanced nonmetastatic stage III-IVa disease at diagnosis. Radiotherapy remains the mainstay of treatment, which can cause many types of DNA and gene damage1,2,3. Mismatch repair (MMR) proteins play an important role in not only safeguarding genetic stability during replication, but also responding to and repairing cellular DNA damage4,5,6,7,8,9. In recent years, deficient MMR (dMMR) has become one of the highlights in tumour pathogenesis, disease screening, diagnosis, guiding drug use and judging prognosis, especially in colorectal cancer. Although two previous studies10,11 reported that dMMR was a rare event in NPC, it’s prognostic value was not analysed. In this study, we tried to explore the prognostic value of dMMR in NPC patients.
Methods and Materials
Patients
NPC patients were treated with intensity-modulated radiotherapy (IMRT) and were analysed retrospectively between January 1, 2012, and December 31, 2017, at the Department of Radiation Oncology of Weifang People’s Hospital (Weifang, China). This study was approved by the ethics review board of the Weifang People’s Hospital (No. KY2019003), and a waiver of informed consent was obtained due to the retrospective nature of the study. All experiments were performed in accordance with the relevant guidelines and regulations.
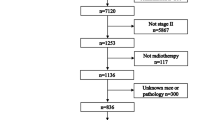
The inclusion criteria were as follows: (1) newly diagnosed and histologically confirmed NPC; (2) received radical IMRT during the course of treatment; (3) stage II–IVa according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system for NPC; and (4) no history of head and neck radiotherapy, other malignant tumours or severe illnesses. The exclusion criteria were as follows: (1) patients with adenocarcinoma or adenoid cystic carcinoma pathology; (2) radiotherapy not completed; (3) women who were pregnant or lactating; and (4) the biopsy sample was insufficient or severe artefacts were present. A total of 81 patients were reviewed, 12 patients were excluded, including 2 patients with keratinized squamous cell carcinoma, and 69 patients were included in the analysis. The median age was 53 years (10–82 years). All patients completed a pretreatment evaluation to identify the clinical stage, which included a complete medical history, a physical examination, haematology and biochemistry profiles, electronic fibre nasopharyngoscopy, a magnetic resonance imaging (MRI) scan of the neck and nasopharynx, chest radiography and abdominal sonography or a chest and abdominal computed tomography (CT) scan, and a whole-body bone scan using single-photon emission computed tomography; additionally, some patients underwent a PET/CT examination. All patients were restaged according to the 8th edition of the AJCC staging system12. The quality of the HE-stained sections and wax blocks was evaluated by 1 intermediate and 1 senior pathologist, and all histological pathologies were reviewed by them according to the World Health Organization (WHO) Classification of Head and Neck Tumours 4th Edition13, disagreement was resolved by discussion and consensus, and where required through discussion with a pathologist with content expertise.
Treatment
All patients were treated with the IMRT technique, and 52 (75.4%) patients were treated with additional chemotherapy. Cisplatin-based two-drug regimens were administered to 20 (29.0%) patients as asynchronous combination chemoradiotherapy (ACCRT, including induced chemotherapy or adjuvant chemotherapy). Cisplatin alone was administered to 32 (46.4%) patients as concurrent chemotherapy (CCRT).
Target volumes and organs at risk were delineated according to the consensus14.The median dose of the gross tumour volume of the nasopharynx (GTVnx) was 70.0 Gy (69.0 Gy–73.0 Gy), and the median dose of the gross tumour volume of the positive cervical lymph node (GTVnd) was 68.0 Gy (66.0 Gy–70.0 Gy). The median dose of high-risk regions (CTV1) was 60.9 Gy (60.0 Gy–62.0 Gy), and the median dose of low-risk regions (CTV2) was 54.4 Gy (50.0 Gy–56.0 Gy).
Follow-up
Primary lesions, enlarged cervical lymph nodes and acute hematologic responses were closely observed during the treatment period. The follow-up strategy was the same as previously described15. All events were measured from the date of the histological diagnosis16. The last follow-up date was November 21, 2018. Local recurrence included primary and regional nodal recurrence. The median follow-up time was 37.5 months (3.1–87.4 months). Three patients were lost to follow up, and the follow-up rate was 95.7%.
Immunohistochemistry (IHC) staining and evaluation
The tumour tissue MMR status was detected by IHC. The formalin-fixed, paraffin-embedded (FFPE) tumour tissue blocks were obtained for IHC examination using primary antibodies against MLH1 (ES05 clone, MXB, Fuzhou, China), MSH2 (RED2 clone, ZSGB-BIO, Beijing, China), MSH6 (EP49 clone, ZSGB-BIO), and PMS2 (EP51 clone, ZSGB-BIO) according to the immunohistochemical standard operating procedure (SOP). The normal IHC staining patterns for MLH1, MSH2, MSH6 and PMS2 MMR proteins were nuclear. Samples with >25% of tumour cells stained were interpreted as expression preserved of the MMR protein17. dMMR was interpreted as expression loss for at least one MMR protein18.
Statistical analysis
All data were analysed using SPSS statistical software (version 22.0; IBM, Armonk, NY). The chi-squared and Fisher’s exact probability tests were used to analyse differences between qualitative data. Survival curves were plotted according to the Kaplan-Meier method and were compared using the log-rank test. A Cox proportional hazards model was used to identify significant prognostic factors. Forward LR method was use to selected variables. Statistical significance was set at P < 0.05 (two-sided).
Results
Expression of MMR proteins
The normal IHC staining patterns for all four MMR proteins were nuclear (Fig. 1). Of 69 patients with stage II-IVa NPC, 35 (50.7%) patients showed preserved PMS2 expression, 53 (76.8%) patients showed preserved MLH1 expression, and 68 (98.6%) patients showed preserved MSH2 or MSH6 expression. For all 4 MMR proteins, 35 (50.7%) cases showed all expression preserved, which was interpreted as proficient mismatch repair (pMMR); only 1 (1.5%) cases showed loss of all expression. Eighteen (26.1%) cases were appraised as PMS2 expression loss only, while 15 (21.7%) cases showed expression loss of both PMS2 and MLH1. A total of 34 (49.3%) cases showed expression loss for at least one MMR protein, which was interpreted as dMMR (Table 1).
Baseline characteristics and distribution of MMR
Of 69 patients with NPC, there was no significant difference (P > 0.05) in terms of sex, age, clinical stage, T category, N category or therapy regimens between the dMMR and pMMR groups. Histological pathology type was not analysed because only non-keratinizing squamous cell carcinoma was included (Table 2).
Univariate analysis of prognostic factors
Kaplan-Meier survival curves of NPC patients were analysed by log-rank test according to sex, age, clinical stage, T category, N-category, therapy regimens and MMR status (Table 2). The analysis revealed that clinical stage (P = 0.01) or T category (P = 0.02) for overall survival (OS), age (P = 0.02) or T category (P = 0.03) for progression-free survival (PFS), age (P = 0.04) for local recurrence-free survival (LRFS), and age (P = 0.04) or T category (P = 0.04) or MMR status (P = 0.02, Fig. 2a) for distant metastasis-free survival (DMFS) were identified as significant prognostic factors(Table 2). In addition, the influence of N category or MMR status (Fig. 2b) on OS (P = 0.06), and clinical stage on PFS (P = 0.05) were the same as the cutoff values (Table 2).
Kaplan-Meier survival curve of NPC patients according to MMR status. (a) Distant metastasis-free survival (DMFS); (b) Overall survival (OS); (c) Local recurrence-free survival (LRFS); (d) progression-free survival (PFS); NPC: nasopharyngeal carcinoma; MMR: mismatch repair; dMMR: deficient MMR; pMMR: proficient MMR.
Multivariate analysis of prognostic factors
The factors brought into the Cox proportional hazards regression model included age (<53 years vs. ≥53 years), clinical stage (II or III vs. IVa), T category (T1-2 vs. T3-4), N category (N0-2 vs. N3), MMR status (dMMR vs. pMMR) and therapy regimens (IMRT vs. CCRT or ACCRT). The multivariate analysis revealed that clinical stage for OS (stage III vs. IVa, P = 0.04, HR = 0.37, 95% CI: 0.14~0.97) (Fig. 3a, Table 3), age for LRFS (<53 vs. ≥53, P = 0.04, HR = 0.10, 95% CI: 0.01~0.92) (Fig. 3b, Table 3), age (<53 vs. ≥53, P = 0.01, HR = 0.25, 95% CI: 0.09~0.72) (Fig. 3c, Table 3) or T category (T1-2 vs. T3-4, P = 0.04, HR = 0.11, 95% CI: 0.02~0.86) (Fig. 3d, Table 3) for PFS, and MMR status (dMMR vs. pMMR, P = 0.01, HR = 0.25, 95% CI: 0.09~0.75) for DMFS (Fig. 3e, Table 3) were independent significant prognostic factors. The prognostic value of T category for DMFS was marginally statistically significant (T1-2 vs. T3-4, P = 0.05, HR = 0.13, 95% CI: 0.02~1.02) (Fig. 3f, Table 3).
Cox proportional hazards regression model survival curve of nasopharyngeal carcinoma patients (Factors brought into the Cox proportional hazards regression model included age, clinical stage, T category, N category, MMR status and therapy strategy). (a) Overall survival (OS) according to clinical stage; (b) Local recurrence-free survival (LRFS) according to age; (c,d) progression-free survival (PFS) according to age or T category, respectively; (e,f) Distant metastasis-free survival (DMFS) according to MMR status or T category, respectively. NPC: nasopharyngeal carcinoma; MMR: mismatch repair; dMMR: deficient MMR; pMMR: proficient MMR.
Discussion
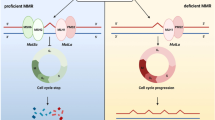
In living cells, MMR proteins play an important role in not only safeguarding genetic stability by excising DNA mismatches introduced by DNA polymerase during replication, but also responding to and repairing cellular DNA damage4,5,6,7,8. For a start, in human cells, MMR proteins are recruited immediately to the sites of various types of DNA damage including single-strand breaks (SSBs), double-strand breaks (DSBs) and pyrimidine dimers4,5,6,7, for which recruitment is mediated by protein-protein interactions in nucleotide-excision-repair dependent or function domain of MMR protein dependent manners4. Then, the recruitment leads to degradation of licensing factor Cdt1 (a G1-specific cell-cycle regulatory protein) in the G1 phase and efficient repair of DNA damage6, and apoptosis is activated via the mitochondria and p53-independent mechanisms7. Furthermore, MMR proteins are involved in the responses to the genotoxicity of γ-radiation in human cells, including thymidine kinase gene mutation, micronucleus formation or apoptosis, through inducing the expression of p53 and delaying the cell cycle8. In addition, MMR can play a role in the repair of SSBs and DSBs via homologous recombination and nonhomologous end joining19,20,21.
MMR proteins bind to mismatch errors DNA as heterodimer complexes: MSH2 binds to and cooperates with MSH6, while MLH1 binds to and cooperates with PMS2 as heterodimers. Furthermore, MSH2 and MLH1 are the dominant proteins for each pair. Hence, the loss of dominant MMR protein expression due to a pathogenic mutation or methylation is usually related to the degradation of the corresponding non-dominant partner: MSH6 is degraded if MSH2 is mutated, and PMS2 is degraded if MLH1 is mutated. However, the opposite is not true because of the compensatory effects of other MMR proteins. MSH2 expression is preserved if MSH6 is lost, and MLH1 expression is preserved if PMS2 is lost22,23. This law was also observed in our studies. Furthermore, in our study, the expression of MSH2 and MSH6 was loss in only one patient (1.5%), and all the MLH1/PMS2-double preserved patients (50.7%) were the same as the pMMR patients, 26.1% of the patients showed the loss of PMS2 alone. Thus, loss of PMS2 expression may be the dominant factor for the dMMR in NPC. The multivariate Cox regression analysis confirmed this hypothesis (data was not shown).
The expression of MMR proteins and the rate of dMMR were variable in different cancers or variable according to interpretation criteria24,25,26,27,28,29,30. Previous studies reported that 14.6–26.0% of patients in colorectal cancer24,25 and 16.0–45.0% in endometrial cancer26,27,28,29,30 were dMMR, 3.7–6.0% of patients in colorectal cancer24,25 and 0.0–4.2% in endometrial cancer26,27,28,29,30 showed the simultaneous loss of MSH2 and MSH6, 78.4–89.6% of patients in colorectal cancer24,25 and 70.2–92.6% in endometrial cancer26,27,28,29,30 showed MLH1/PMS2-double preserved, and 3.9–4.2% of patients in colorectal cancer24,25 and 0.5–4.9% in endometrial cancer26,27,28,29,30 showed the loss of PMS2 alone. Some studies have demonstrated that normal expression is defined as the presence of the nuclear staining of tumour cells regardless of the proportion or intensity25,26,27,28,29,30,31,32. However, other researchers believe that the intensity of staining and the proportion of positive cells must be considered comprehensively17,23,24,33. The latter approach was used in our study, and the proportion of NPC patients with dMMR was 49.3%, which was higher than the proportion in two previous reports (approximately 2%)10,11 on NPC. The interpretation criteria may be the major reason. Moreover, NPC is characterized by a geographical feature: the patients in the two previous studies were from southern China or the Philippines, while the patients in the current study were from northern China.
In our study, the multivariate analysis using the Cox regression model revealed that in patients with stage II-IVa NPC, clinical stage for OS (Fig. 3a, Table 3), age for LRFS (Fig. 3b, Table 3) or for PFS (Fig. 3c, Table 3), T categoryfor PFS (Fig. 3d, Table 3) were independent prognostic factors, consistent with previous reports16,34,35.
MMR-defective cell lines are more resistant to cell death induced by several DNA-damaging agents, including methylation agents, cisplatin and UV radiation, whereas they are more sensitive to cell death caused by interstrand crosslinking agents4. Radiotherapy can cause many types of DNA and gene damage, including loss of heterozygosity, homozygosity deletion1, DSBs2,3. Misrepair of radiation -induced DNA damage underlies genomic instability and increased radiosensitivity36; thus, MMR-defective cells may underlie increased radiosensitivity.
Previous studies have reported that compared with patients with pMMR tumours, colorectal cancer patients with dMMR tumours had significantly superior OS and PFS24,37, and endometrial cancer patients with dMMR tumours had significantly superior PFS38. In this study, there was no significant difference (P > 0.05) between the dMMR and pMMR groups in sex, age, clinical stage, T category, N category or therapy regimens. Univariate and multivariate analyses of the prognostic factors showed that patients with dMMR tumours had significantly superior DMFS compared with patients with pMMR tumours (dMMR vs. pMMR, P = 0.01, HR = 0.25, 95% CI: 0.09~0.75) (Fig. 3e, Table 3). Therefore, dMMR promises to be a potential prognostic biomarker for NPC. The better prognosis of dMMR NPC patients might result from a stronger immunologic response driven by abundant tumour infiltrating lymphocytes (TILs) in the tumour microenvironment39. Immunotherapy has been approved for the treatment of patients with microsatellite instability-high (MSI-H) or dMMR solid tumours in 12 different tumour types, although NPC was not included in the 12 cancers40. In addition, several studies confirmed that dMMR cases had reduced levels of vascular endothelial growth factor (VEGF) compared to pMMR cases, which might partly explain why patients with dMMR tumours had more favourable prognosis39.
In the IMRT era, distant metastasis predominates as the pattern of disease relapse in patients with stage II-IVa NPC, accounting for approximately 70% of cancer-specific mortality41. In this study, we found that dMMR was indicative of a favourable DMFS in patients with stage II-IVa NPC. It can be expected that dMMR will become a new independent prognostic factor of NPC. However, prospective clinical studies are needed to further investigate the prognostic value of dMMR in patients with advanced NPC and the role of dMMR in NPC immunotherapy. Thus, NPC should be screened for dMMR.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Yamamoto, N. et al. Genetic effects of X-ray and carbon ion irradiation in head and neck carcinoma cell lines. The Bulletin of Tokyo Dental College. 48, 177–185 (2007).
Siva, S. et al. Radiotherapy for Non-Small Cell Lung Cancer Induces DNA Damage Response in Both Irradiated and Out-of-field Normal Tissues. Clinical cancer research: an official journal of the American Association for Cancer Research. 22, 4817–4826 (2016).
Bonner, W. M. et al. GammaH2AX and cancer. Nat Rev Cancer. 8, 957–967 (2008).
Hong, Z. et al. Recruitment of mismatch repair proteins to the site of DNA damage in human cells. Journal of cell science. 121, 3146–3154 (2008).
Liberti, S. E. et al. Bi-directional routing of DNA mismatch repair protein human exonuclease 1 to replication foci and DNA double strand breaks. DNA repair. 10, 73–86 (2011).
Tanaka, M. et al. Mismatch repair proteins recruited to ultraviolet light-damaged sites lead to degradation of licensing factor Cdt1 in the G1 phase. Cell cycle (Georgetown, Tex). 16, 673–684 (2017).
Narine, K. A. et al. Defining the DNA mismatch repair-dependent apoptotic pathway in primary cells: evidence for p53-independence and involvement of centrosomal caspase 2. DNA repair. 9, 161–168 (2010).
Yamamoto, A., Sakamoto, Y., Masumura, K., Honma, M. & Nohmi, T. Involvement of mismatch repair proteins in adaptive responses induced by N-methyl-N’-nitro-N-nitrosoguanidine against γ-induced genotoxicity in human cells. Mutation research. 713, 56–63 (2011).
Klein, H. L. et al. Guidelines for DNA recombination and repair studies: Mechanistic assays of DNA repair processes. Microb Cell. 6, 65–101 (2019).
Zhao, L. et al. Mismatch repair status and high expression of PD-L1 in nasopharyngeal carcinoma. Cancer Manag Res. 11, 1631–1640 (2019).
Chang, A. M. V., Chiosea, S. I., Altman, A., Pagdanganan, H. A. & Ma, C. Programmed Death-Ligand 1 Expression, Microsatellite Instability, Epstein-Barr Virus, and Human Papillomavirus in Nasopharyngeal Carcinomas of Patients from the Philippines. Head Neck Pathol. 11, 203–211 (2017).
Amin, M. et al. AJCC Cancer Staging Manual(Eighth ed.)107–108 (Springer, 2017).
A K. EI-Naggar, J K.C. Chan, J R. Grandis, T T & Pieter J. Slootweg. WHO Classification of Head and Neck Tumors(4th ed.)67–69 (IARC, 2017).
Working Committee on Clinical staging of Nasopharyngeal carcinoma in China. 2010 expert consensus on target area and dose design guidelines for intensity modulation radiotherapy for nasopharyngeal carcinoma. Chin J Radiat Oncol. 20, 267–269 (2011).
Sun, Y. et al. Promising treatment outcomes of intensity-modulated radiation therapy for nasopharyngeal carcinoma patients with N0 disease according to the seventh edition of the AJCC staging system. BMC Cancer. 12, 68 (2012).
Pan, J. J. et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 122, 546–558 (2016).
Chen, G. T. et al. Relationship between mismatch repair gene expression and microsatellite instability in gastric cancer. Chin J Exp Surg. 23, 1420–1421 (2006).
Svensson, M. C. et al. Expression of PD-L1 and PD-1 in Chemoradiotherapy-Naive Esophageal and Gastric Adenocarcinoma: Relationship With Mismatch Repair Status and Survival. Front Oncol. 9, 136 (2019).
Sugawara, N., Pâques, F., Colaiácovo, M. & Haber, J. E. Role of Saccharomyces cerevisiae Msh2 and Msh3 repair proteins in double-strand break-induced recombination. Proceedings of the National Academy of Sciences of the United States of America. 94, 9214–9219 (1997).
Evans, E., Sugawara, N., Haber, J. E. & Alani, E. The Saccharomyces cerevisiae Msh2 mismatch repair protein localizes to recombination intermediates in vivo. Molecular cell. 5, 789–799 (2000).
Eichmiller, R. et al. Coordination of Rad1-Rad10 interactions with Msh2-Msh3, Saw1 and RPA is essential for functional 3’ non-homologous tail removal. Nucleic acids research. 46, 5075–5096 (2018).
De’ Angelis, G. L. et al. Microsatellite instability in colorectal cancer. Acta Biomed. 89, 97–101 (2018).
Yozu, M., Kumarasinghe, M. P., Brown, I. S., Gill, A. J. & Rosty, C. Australasian Gastrointestinal Pathology Society (AGPS) consensus guidelines for universal defective mismatch repair testing in colorectal carcinoma. Pathology. 51, 233–239 (2019).
Gong, Q. et al. Mismatch repair-deficient status associates with favorable prognosis of Eastern Chinese population with sporadic colorectal cancer. Oncol Lett. 15, 7007–7013 (2018).
Shia, J. et al. Immunohistochemistry as first-line screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: a 2-antibody panel may be as predictive as a 4-antibody panel. Am J Surg Pathol. 33, 1639–1645 (2009).
Nelson, G. S. et al. MMR deficiency is common in high-grade endometrioid carcinomas and is associated with an unfavorable outcome. Gynecol Oncol. 131, 309–314 (2013).
Kato, M. et al. DNA mismatch repair-related protein loss as a prognostic factor in endometrial cancers. J Gynecol Oncol. 26, 40–45 (2015).
Grzankowski, K. S., Shimizu, D. M., Kimata, C., Black, M. & Terada, K. Y. Clinical and pathologic features of young endometrial cancer patients with loss of mismatch repair expression. Gynecol Oncol. 126, 408–412 (2012).
Shih, K. K. et al. Clinicopathologic significance of DNA mismatch repair protein defects and endometrial cancer in women 40years of age and younger. Gynecol Oncol. 123, 88–94 (2011).
Garg, K. et al. Endometrial carcinomas in women aged 40 years and younger: tumours associated with loss of DNA mismatch repair proteins comprise a distinct clinicopathologic subset. Am J Surg Pathol. 33, 1869–1877 (2009).
Smyth, E. C. et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 3, 1197–1203 (2017).
Shi, Y. & Zheng, J. Significance and Strategy of systematic screening for unstable Microsatellite Colorectal Cancer. Chin. J Pathol. 44, 9–14 (2015).
Son, B. H. et al. Significance of mismatch repair protein expression in the chemotherapeutic response of sporadic invasive ductal carcinoma of the breast. Breast J. 10, 20–26 (2004).
Han, L. et al. Prognostic factors of 305 nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Chin J Cancer. 29, 145–150 (2010).
Liu, X. et al. Changes in Disease Failure Risk of Nasopharyngeal Carcinoma over Time: Analysis of 749 Patients with Long-Term Follow-Up. J Cancer. 8, 455–459 (2017).
Alsbeih, G., Brock, W. & Story, M. Misrepair of DNA double-strand breaks in patient with unidentified chromosomal fragility syndrome and family history of radiosensitivity. International journal of radiation biology. 90, 53–59 (2014).
Zhao, P., Li, L., Jiang, X. & Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 12, 54 (2019).
McMeekin, D. S. et al. Clinicopathologic Significance of Mismatch Repair Defects in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 34, 3062–3068 (2016).
Zhang, C. M. et al. Role of Deficient Mismatch Repair in the Personalized Management of Colorectal Cancer. Int J Environ Res Public Health. 13, 892 (2016).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 357, 409–413 (2017).
Zhang, Y. et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med. 381, 1124–1135 (2019).
Acknowledgements
This work was supported by Doctor Sang Mao-zhong, Liu Jie, Jiang Ying-xiao, Guo Ying-hua, Li Jian-wen, Wang Pei-he, Li Yong, Li Yanxiang, and Yin Dandan of Weifang People’s Hospital. This work was supported by the Science and Technology Development Project of Weifang City (China)(Grant NO. 2019YX003).
Author information
Authors and Affiliations
Contributions
Chen F.M.: undertook the collection of data, responsibility for the first draft of the manuscript, and the statistical analyses; Zhang Y.X. and Li X.F.: pathology review; Gao J.F. and Ma H.: immunohistochemical standard operating procedure; Wang X.L., Li C., Zhang Y.N., Zhang Y.T., Kan H.X., Li H. and Zhang S.G.: undertook the collection of data and follow-up; Li Y.: IMRT treatment Image and Plan archiving and recovery; Hao F.R.: original idea for the research, database construction, TNM staging, revised the draft of the manuscript, provided statistical advice for the data from the inception of the study and undertook the statistical analyses and agreed to be accountable for all aspects of the work; Wang M.C.: organized and coordinated all aspects of work. All authors contributed to the drafting or critical review of the manuscript and provided final approval.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Fm., Zhang, Yx., Li, Xf. et al. The Prognostic Value of Deficient Mismatch Repair in Stage II–IVa Nasopharyngeal Carcinoma in the Era of IMRT. Sci Rep 10, 9690 (2020). https://doi.org/10.1038/s41598-020-66678-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66678-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.