Abstract
The master sex determinant in rainbow trout (Oncorhynchus mykiss), sexually dimorphic on the Y chromosome (sdY), is strongly but not perfectly associated with male phenotype in several other species from the family Salmonidae. Currently, the cause and implications of discordance for sdY-predicted genotypic sex and phenotypic sex in these species is unclear. Using an established multiplex PCR test for exons 2 and 3 of sdY, we demonstrated that sdY-predicted genotypic sex was discordant with histologically evidenced phenotypic sex in 4% of 176 Tasmanian Atlantic salmon. All discordant individuals were phenotypic females presenting a male genotype. Using real-time qPCR assays that we developed and validated for exons 2, 3 and 4 of sdY, all genotype-phenotype discordant females were confirmed to possess sdY, albeit at a reduced number of copies when compared to phenotypic males. The real-time qPCR assays also demonstrated reduced levels of sdY in 30% of phenotypic females that the established multiplex PCR-based test indicated to be devoid of sdY. These findings suggest sdY may be reduced in copy number or mosaicked in the genomic DNA of sdY-positive phenotypic female Atlantic salmon and highlight the importance of understanding the effects of reduced sdY copies on the development of phenotypic sex.
Similar content being viewed by others
Introduction
Many members of the family Salmonidae (comprised of three sub-families: Coregoninae, Thymalline and Salmoninae) are of high societal and economic importance for their value in aquaculture, wild stock fisheries and recreational sports fisheries1,2. With particular reference to Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss), they also present as important scientific models in the fields of toxicology, immunology, physiology, nutrition and genetics3,4,5.
The development of a reliable DNA-based method for identifying genotypic sex in salmonids has been of high interest to facilitate management of native populations and production of farmed stocks6,7,8,9. Initial efforts resulted in the discovery of several markers tightly linked to the sex determining locus on the Y chromosome within the Oncorhynchus genera of the Salmonidae. These include OtY1 in chinook salmon (Oncorhynchus tshawytscha)10, GH-Y in chinook11, coho (Oncorhynchus kisutch), masu (Oncorhynchus masou)12, chum (Oncorhynchus keta) and pink salmon (Oncorhynchus gorbuscha)13, and OmyY1 in rainbow trout14. However, variable rates of genotype-phenotype discordance using these sex markers have been reported14,15,16,17,18,19,20. This is likely attributed to the fact that these markers are non-functional sequences residing close to the sex determination locus, and thus are not directly selected for20.
Recently the master sex determining gene sexually dimorphic on the Y chromosome (sdY) was discovered in rainbow trout, and its presence in genomic DNA (gDNA) was demonstrated to align 100% with male phenotype in this species (425 individuals studied)21. sdY is also strongly associated with male phenotype in several other species from all three subfamilies of the family Salmonidae, suggesting a conserved function for this gene as the master sex determinant9. However, cases where sdY is not perfectly associated with male phenotype have been reported in populations of lake char (Salvelinus namaycush), chinook salmon9,22, sockeye salmon (Oncorhynchus nerka)23 and Atlantic salmon24,25,26,27. In addition, in European whitefish (Coregonus lavaretus) and lake whitefish (Coregonus clupeaformis), both phenotypic males and females possess sdY9. The cause and significance for these cases of sdY-predicted genotype-phenotype discordance remain unclear, however some studies have suggested phenotype miscalls, sex reversal and/or loss of gene function in select individuals could be attributed22,27. For European whitefish and lake whitefish (members of the Coregoninae), sdY may act through a dosage mechanism to trigger testicular differentiation9. Indeed, variations in sequence copy number have been reported for multiple non-functional markers on the Y chromosome in salmonids20. Thus, quantitative approaches may provide new insights in the association of sdY with phenotypic sex in salmonids.
Studies in the Tasmanian Atlantic salmon population (originated from the River Philip in Nova Scotia, Canada) have consistently identified individuals whose phenotypic sex does not concur with sdY predictions24,27. This makes the Tasmanian population ideal for studying the association of sdY copy number with phenotypic sex. In the present study, we determined the rate of concordance for sdY-predicted genotypic and phenotypic sex in 176 Tasmanian Atlantic salmon using a multiplex PCR-based test adapted from Eisbrenner, et al.27. This method for determining genotypic sex in Atlantic salmon has also been used for studies in sexual maturation28,29,30,31, sex differentiation32, farmed stock introgression33,34,35 and fisheries management36. Phenotypic sex for all fish was determined by histology. We then examined the abundance of sdY in gDNA for all fish using real-time quantitative PCR (qPCR) assays for exons 2, 3 and 4 of sdY. Our findings confirm that a proportion of phenotypic females possess sdY in the Tasmanian Atlantic salmon population. However, sdY appears to be reduced in copy number in these individuals compared to phenotypic males. No phenotypic males lacking sdY were observed in this study.
Results
Histological assessment of phenotypic sex
104 males and 72 females were identified in the population sampled. Two distinct male phenotypes were observed. The gonads of 33 males were comprised of seminiferous tubules predominantly filled with cysts of primary and secondary spermatocytes and spermatids. Spermatozoa typically filled the lumen of the seminiferous tubules, and sertoli cells and type A and B spermatogonias were adjacent to the seminiferous epithelium (Fig. 1a). Gonads from the remaining 71 males were characterised by cysts of type A and B spermatogonias and sertoli cells (Fig. 1b). For the majority of females sampled, the gonad was predominantly comprised of oocytes in the early perinucleolus stage, with chromatin nucleolar oocytes, oocytes in meiotic prophase and oogonias also present (Fig. 1c). However, in four females perinucleolar oocytes occupied a much smaller proportion of the gonad (0–50%), which was instead comprised mainly of cysts of oogonias and oocytes in meiotic prophase. Large amounts of stromal tissue were also identified in these gonads (Fig. 1d,e).
Histological assessment of phenotypic sex. Gonadal phenotypes observed amongst the 176 Tasmanian Atlantic salmon used in this study. (a) testis with cells at all spermatogenic stages; (b) testis in resting state, comprised of type A and type B spermatogonias, and sertoli cells; (c) ovary almost entirely comprised of perinucleolar oocytes; (d) ovary comprised equally by perinucleolar oocytes and cysts of oogonias and oocytes in meiotic prophase; (e) ovary dominated by stromal tissue and cysts of oogonias and oocytes in meiotic prophase. Sga, type A spermatogonia; Sgb, type B spermatogonia; Sci, primary spermatocytes; Scii, secondary spermatocytes; Sd, spermatids; Sz, spermatozoa; St, sertoli cell; O, oogonias; M, oocytes in meiotic prophase; Cno, chromatin nucleolar oocyte; Po, perinucleolar oocyte; S, stromal cells.
Genotype-phenotype discordance identified using multiplex PCR-based test
Male genotype was demonstrated for all phenotypic males in the population studied. Female genotype was evident in 65 of the 72 phenotypic females studied, the remaining seven had a male genotype. Five of the 65 genotype-phenotype concordant females amplified PCR products for fabp6b and exon 3 of sdY, but not exon 2 (Table 1). The remaining 60 genotype-phenotype concordant females amplified PCR products for fabp6b, but not exon 2 or exon 3 of sdY. No other genotypes were observed. Of the seven phenotypic females with a male genotype, two had ovaries comprised mainly by cysts of primary oocytes in prophase of meiosis I and stromal tissue (Fig. 1d,e). All of the phenotypic females that amplified PCR products for fabp6b and exon 3 of sdY, but not exon 2, had ovaries dominated by perinucleolar oocytes.
Reduced sdY copies detected in genotype-phenotype discordant individuals
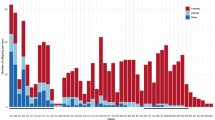
All gDNA samples from phenotypic males presented levels of exons 2, 3 and 4 of sdY within the linear dynamic range (LDR) of the real-time qPCR assays (Fig. 2a). A single phenotypic female sample had exon 2 in levels within the LDR, whereas four phenotypic females presented levels for exon 3 and exon 4 within the LDR. Exons 2, 3 and 4 of sdY were also detected in levels below the LDR in a considerable number of phenotypic females (Fig. 2b). The four phenotypic females presenting levels of exon 2, and/or exons 3 and 4 of sdY within the LDR were identified as four of the seven individuals for which genotype-phenotype was discordant. These exons were also detected in the remaining three individuals, but at levels below the LDR (Fig. 3). The mean number of copies/10 ng gDNA for exons 2, 3 and 4 of sdY in phenotypic males with a male genotype was 2,314.43 ± 789.13, 2880.99 ± 971.95 and 2725.83 ± 971.95, respectively. This was significantly higher than the mean of phenotypic females with a male genotype (excluding levels below the LDR), which was 55.20, 86.80 ± 33.59 and 75.42 ± 26.59 for exons 2, 3 and 4, respectively (exon 2: n = 104, t(103) = 29.20, p < 2.2e-16, one sample t-test; exon 3: n = 108, t(105.5) = −28.73, p < 2.2e-16; exon 4: n = 108, t(105.99) = −28.13, p < 2.2e-16, Welch’s t-test). The number of copies for exons 2, 3 and 4 of sdY were not significantly different between the two male phenotypes observed (exon 2: n = 104, t(102) = −0.26, p = 0.79, exon 3: n = 104, t(102) = −0.09, p = 0.93, exon 4: n = 104, t(102) = −0.07, p = 0.94; independent t-test).
Quantification of sdY by real-time qPCR. Number of sdY copies detected within the linear dynamic range (LDR) of the real-time qPCR assay in phenotypic males ( ) and phenotypic females (
) and phenotypic females ( ), and outside of the LDR in phenotypic females (
), and outside of the LDR in phenotypic females ( ).
).  indicates a phenotypic female with no sdY copies detected. (a) data for all 176 Tasmanian Atlantic salmon used in this study; (b) magnified view of individuals with low numbers of target copies.
indicates a phenotypic female with no sdY copies detected. (a) data for all 176 Tasmanian Atlantic salmon used in this study; (b) magnified view of individuals with low numbers of target copies.
sdY gene structure, real-time qPCR assay amplicon localisation and results of the real-time qPCR assays for phenotypic females with an sdY-positive genotype (as determined by the multiplex PCR-based test). Structure of sdY in Atlantic salmon according to the gene sequence provided by http://www.ncbi.nlm.nih.gov (GenBank accession number: KT223110). The size and position of each exon (▪) and intron (▪▪▪) is indicated. The 5′ untranslated region (UTR) spans from positions 1–100, and the 3′UTR spans from positions 4395–4565. Amplicon locations for the real-time qPCR assays ( are indicated. Data presented as copies/10 ng DNA. < LDR, below the linear dynamic range of the real-time qPCR assay; (+), amplified in the multiplex PCR-based test; (−), did not amplify in the multiplex PCR-based test.
are indicated. Data presented as copies/10 ng DNA. < LDR, below the linear dynamic range of the real-time qPCR assay; (+), amplified in the multiplex PCR-based test; (−), did not amplify in the multiplex PCR-based test.
In the five phenotypic females that had a female genotype characterised by absence of exon 2 but presence of exon 3 of sdY (as determined by the multiplex PCR-based test), at least one of the three exons investigated using real-time qPCR was present in gDNA at levels below the LDR (Fig. 3). Exon 2 was detected in two of these individuals by real-time qPCR despite not being detected by the multiplex PCR based test (Fig. 4). Low levels of predominantly exon 3 and/or exon 4 of sdY were also detected by real-time qPCR in several phenotypic females with a genotype devoid of sdY (as determined by the multiplex PCR-based test) (Fig. 4). None of the sdY exons were detected by real-time qPCR in the two genotype-phenotype concordant females whose ovaries were comprised mainly of cysts of oogonias and oocytes in meiotic prophase, and stromal tissue.
Combinations of sdY exons amplified in phenotypic females by real-time qPCR. Proportion of genotype-phenotype concordant females where exons 2, 3 and 4 ( ), 2 and 4 (
), 2 and 4 ( ), 3 and 4 (
), 3 and 4 ( ), 3 (
), 3 ( ), 4 (
), 4 ( ) or no exons (
) or no exons ( ) of sdY were detected by real-time qPCR. Number of individuals observed is indicated above the respective bar.
) of sdY were detected by real-time qPCR. Number of individuals observed is indicated above the respective bar.
Discussion
Using the multiplex PCR-based test, genotypic sex was concordant with phenotypic sex in 96% of the 176 fish studied. This rate of concordance is in agreement with findings from previous studies in the Tasmanian Atlantic salmon population, which used a larger sample size (up to 3,176 fish)24,27. In the present study, all genotype-phenotype discordant fish observed were phenotypic females with a male genotype. These findings contrast previous studies in the Tasmanian population, which have reported phenotypic males with a female genotype as the dominant form of genotype-phenotype discordance27. Therefore, the absence of phenotypic males with a female genotype in the present study is intriguing. sdY positive phenotypic females have been previously documented in Atlantic salmon from Tasmania27 and the Faroe Islands26, as well as in populations of north American sockeye salmon23 and chinook salmon9,22. In chinook salmon, similar findings to the present study were reported, whereby all phenotypic males investigated possessed the sdY gene as did a proportion (~2–22%) of phenotypic females. Alternatively, phenotypic males lacking sdY have been reported in north American sockeye salmon populations23, lake char9 and Norwegian Atlantic salmon25 in addition to the Tasmanian Atlantic salmon population27
Of the 65 genotype-phenotype concordant females observed in the present study, five amplified the product for exon 3 primers but did not for exon 2 using the multiplex PCR-based test. This phenomenon of an ‘incomplete sdY gene’ has been previously reported in Atlantic salmon from the Tasmanian population27, and is thought to be a result of instability in the 5’ end of sdY sequence37. Such an explanation may also provide insights in the occurrence of phenotypic female chinook salmon which possess exons 2, 3 and 4 of sdY, but not exon 122. However, in the present study we found that some of the phenotypic females with a genotype characterised by an incomplete sdY gene in fact had very low intensity bands for exon 2 that were below the detection limit of Image Lab Software (Bio-Rad, USA). Considering these observations, we suspect that at least for some individuals the phenomenon of an incomplete sdY gene (as determined by the multiplex PCR-based test) can rather be attributed to a combination of low target amounts in template gDNA and the inclusion of ~7 times more primer for exon 3 than exon 2 in the multiplex PCR-based test used. In support of this, the band for exon 2 was much less intense than the band for exon 3 even when a male genotype was assigned. Reduced target amounts of the priming sites on exon 2 may suggest sdY is present at a reduced copy number in some phenotypic females in the Tasmanian Atlantic salmon population.
Variable copy numbers for sdY within salmonid populations have not previously been reported despite the use of real-time qPCR assays for detecting the presence of this gene in chinook salmon22 and Atlantic salmon26. However, variations in copy number for other Y- chromosome markers have been reported by several studies. In Chinook salmon, PCR-based tests for OtY1 and GH-Y have produced low intensity target bands in both phenotypic males and females16,18. Devlin, et al.18 also demonstrated reduced signal intensity for OtY8 (an 8 kb repeat containing the OtY1 sequence) by southern blot analysis in these individuals, further suggesting OtY1 was reduced in copy number. Alternatively, Muttray, et al.20 observed a single phenotypic male coho salmon and two phenotypic male pink salmon with increased signal intensity for GH-Y via southern blot analysis relative to other phenotypic males from the studied populations, suggesting GH-Y occurred in a higher copy number in these individuals. These results suggest the Y chromosome in salmonids is subject to multiple forms of sequence copy number at multiple loci20.
To test for variations in copy number of sdY within the Tasmanian Atlantic salmon population, we developed real-time qPCR assays for exons 2, 3 and 4 of sdY. An assay for exon 1 was not developed because this exon is considered to be comprised of unstable sequence37. The real-time qPCR assays confirmed the presence of sdY in all phenotypic males studied as well as the seven phenotypic females for which a male genotype was assigned by the multiplex PCR-based test. However, the number of target copies detected by each of the real-time qPCR assays was significantly less in phenotypic females with a male genotype compared to phenotypic males. The highest number of copies for each exon observed amongst phenotypic females was more than an order of magnitude lower than the mean number of copies detected in phenotypic males, and more than four times lower than the lowest number detected in a phenotypic male. These findings indicate sdY may occur at a reduced copy number in the genome of phenotypic female Atlantic salmon for which a male genotype has been assigned (using the multiplex PCR-based test) compared to phenotypic males.
Of the five phenotypic females that had a female genotype characterised by the presence of exon 3 but absence of exon 2 using the multiplex PCR-based test, two were demonstrated to in fact possess exon 2 using the real-time qPCR assay. In one of these five individuals, the real-time qPCR assays detected exons 2, 3 and 4. Alternatively, only exons 3 and 4 amplified in two other individuals, exons 2 and 4 in another, and only exon 3 in one other individual. However, the quantities of exons 2, 3 and 4 in template gDNA from these five individuals was below the LDR for the real-time qPCR assays. Failure to detect exon 2, exon 3, and exons 2 and 4, respectively, in four of these five individuals therefore most likely reflects the limitations of the real-time qPCR assays for reliably detecting very low quantities of target rather than these exons not being present in gDNA. Indeed, exon 3 was detected using the multiplex PCR-based test in the individual whom it was not detected using the real-time qPCR assay. Thus, results from the real-time qPCR assays confirm that the occurrence of individuals with a genotype characterised by an incomplete sdY gene is at least in some cases an artefact of the multiplex PCR-based test when low copies of target sequence are present in template gDNA. The real-time qPCR assays also detected the presence of sdY at levels below the LDR in 30% of genotype-phenotype concordant females that did not amplify any sdY products in the multiplex PCR-based test. Only a single exon of sdY was detected by real-time qPCR in the majority of these individuals (none amplified all three of the sdY exons tested). Also, a high proportion of the exons detected amplified only in a single well of the triplicate reactions.
Results from the real-time qPCR assays in the present study suggest sdY is present in a considerable proportion of phenotypic female Atlantic salmon from the Tasmanian population. To confirm these observations, DNA extractions and real-time qPCR runs were repeated for a number of samples from phenotypic females where target sdY sequence was detected by real-time qPCR. Repeated procedures were completed in the absence of samples from phenotypic males to mitigate sdY contamination. Comparable results were obtained for some but not all repeated samples (Supplementary Table S1), suggesting sdY cannot be consistently detected using the current methodology when present in a reduced quantity in gDNA. Devlin, et al.18 reported similar findings for the repeatability of detecting OtY1 and GH-Y at reduced levels in Chinook salmon. Thus, it’s possible that more phenotypic females from the present study in fact possessed sdY in a reduced number of copies but were not detected. Alternatively, all samples from phenotypic males (sdY present in high number of copies) showed a high level of repeatability when re-extracted and re-assayed (Supplementary Table S2). A reduced number of sdY copies and variable repeatability for the analysis in phenotypic females may suggest a ‘mosaic’ presence of sdY in the muscle cells of these individuals. In gDNA isolated from semen and sperm cells from infertile human males, a copy number less than one was detected for Sex-determining Region Y (SRY) using real-time qPCR, indicative of Y chromosome mosaicism38. Y chromosome mosaicism has also been demonstrated in blood cells from an aborted human foetus using fluorescence in-situ hybridisation39. In this study, some cells were completely devoid of signal for a Y chromosomal marker, whereas other cells showed varying signal intensities39. Considering ~3074 copies of the Atlantic salmon genome (GenBank accession number: GCA_000233375.4) are present in 10 ng gDNA, our findings suggest sdY is present in approximately a single copy in the male genome (mean copy number of 0.75, 0.94 and 0.89 determined for exons 2, 3 and 4, respectively, in phenotypic males with a male genotype). The sdY copy number estimate in phenotypic females with a male genotype is less than 0.1 (mean copy number of 0.02, 0.03, and 0.02 for exons 2, 3 and 4, respectively, excluding levels below the LDR). These estimates are in agreement with a previous study that reported male Atlantic salmon possess a single copy of the chromosomal region containing sdY24, and indicate that sdY is present in a single copy within this chromosomal region. These estimates also further suggest a mosaic presence of sdY in the muscle cells of some phenotypic females. Such a phenomenon would also explain the variability in amounts of sdY recovered from repeated gDNA extractions on the same sample. Understanding the mechanisms by which sdY mosaicism is acquired, and its effects on the development of phenotypic sex, both in parent and offspring, will be important to further our understanding of the association of sdY with phenotypic sex in Atlantic salmon.
The complete concordance of high number of sdY copies and male phenotype observed in the present study suggests sdY may be required above a threshold amount in gDNA to promote testicular development. A dosage-dependent mechanism for sdY has also been suggested in members of the Coregoninae family, following the observation that all phenotypic females and males possessed sdY, as determined by a PCR-based test9. However, the occurrence of phenotypic males with a female genotype described previously in Tasmanian27 and Norwegian Atlantic salmon populations25 suggests there could be alternative factors influencing sex determination in addition to sdY in this species. In support of this, we confirmed the occurrence of phenotypic males which lack sdY in the Tasmanian population using the real-time qPCR assays developed in this study and archived fin samples from known sdY-negative phenotypic male Tasmanian Atlantic salmon (Supplementary Table S3). These findings demonstrate that sdY quantification in gDNA does not explain genotypic discordance in phenotypic males, thus preventing the sole use of sdY-based quantitative methods to reliably indicate genotypic sex in Atlantic salmon. Future studies investigating the association of genotypic and phenotypic sex in salmonids should continue to assign phenotypic sex with histological evidence where possible. Additionally, observations of phenotypic sex at developmental stages prior to sexual maturity, including during the sex differentiation period, may provide new insights. For instance, genotype-phenotype discordance due to sex reversal could be evident during these stages.
Methods
Sampling
All procedures conducted during this trial were in accordance with approval by the Deakin University Animal Ethics Committee (Permit No. B29-2017), and were compliant with the guidelines outlined in the Australian code for the care and use of animals for scientific purposes (2013). This study used 176 (39.72 g ± 1.03) mixed-sex, freshwater acclimated juvenile Atlantic salmon from Salmon Enterprises of Tasmania Pty. Ltd. (SALTAS), Wayatinah, Tasmania. All fish were randomly selected for use in this study from a population that was being maintained under commercial conditions. Sampling was conducted over three consecutive days. Each fish was euthanized by a lethal dose of AQUI-S (AQUI-S, New Zealand). Once euthanized, mass was recorded for each fish followed by collection of dorsal muscle tissue and a single gonad. Muscle tissue was immediately placed in 500 µl of RNAlater (Merck, Germany) and stored at ambient temperature for 5–7 days, then −80 °C for 12 months until time of analysis. The gonad was fixed in Bouin’s solution (Merck, Germany) for 5–7 days, then stored in 70% ethanol until time of processing.
Histology
Fixed gonads were dehydrated by sequential immersion in 70%, 90%, and 100% ethanol, cleared with Xylene, embedded in Histosec wax (Merck, Germany) and sectioned with a microtome. For each sample, three 4 µm cross-sections were stained with hematoxylin and eosin then examined under a light microscope (10-400×). Paraffin processing, embedding, cutting and staining was performed by the Melbourne Histology Platform, The University of Melbourne (Parkville, Australia). Histological descriptions of gonad morphology in salmonids were used to determine phenotypic sex for each sample40,41.
Genomic DNA extraction
Muscle tissue was thawed, blotted dry of excess RNAlater on a Kimwipe (Kimberly-Clark Professional, USA) and immersed in 1X phosphate-buffered saline solution (Bio-Rad, USA) for 2 hours prior to gDNA extraction. Following, gDNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Germany) following the manufacturer’s specifications. All extractions were normalised to 5 ng/µl using a spectrophotometer (NanoDrop, Thermo Fisher Scientifc, USA) and quality checked by visualisation on 1% 1X TAE agarose gel with GelRed (Gene Target Solutions, Australia).
Conventional PCR-based assay
A multiplex PCR-based test for identifying the genotypic sex of Atlantic salmon was adapted from Eisbrenner, et al.27. The multiplex consisted of primer pairs for exons 2 and 3 of sdY to determine genotypic sex, and primer pairs for fabp6b to verify gDNA quality was sufficient for PCR. Primer sequences and amplicon sizes are provided in Supplementary Table S4. Each 25 μl reaction contained 50 ng gDNA, 1.25 units iTaq DNA polymerase (Bio-Rad, USA), 1X iTaq DNA buffer (Bio-Rad, USA), 1.5 mM MgCl2 (Bio-Rad, USA), 200 μm dNTP mix (Bio-Rad, USA), 3.45% dimethyl sulfoxide (Merck, Germany), 170 nm exon 2 forward and reverse primers, 1.15 μm exon 3 forward and reverse primers, 400 nm fabp6b forward and reverse primers and nuclease-free water (Ambion, USA). All reactions were loaded in triplicate into a hard shell 96 clear-well PCR plate (Bio-Rad, USA), covered with a clear adhesive PCR plate seal (Bio-Rad, USA) and run on a Mastercycler ep gradient S (Eppendorf, Germany). Thermal cycling conditions were as reported by Eisbrenner, et al.27. Three no template control (NTC) reactions were included in each PCR run. PCR products for each reaction were visualised on a 2% 1X TAE agarose gel with GelRed alongside a 50 bp DNA ladder (Thermo Fisher Scientific, USA). Amplicon presence was determined using Image Lab Software (Bio-Rad, USA) with band detection sensitivity set to 100%. Positive amplification of the target for each sample was given when a band was detected in one or more of the triplicate reactions. Results denoting male and female genotype for this PCR assay are outlined by Eisbrenner, et al27. Briefly, male genotype was indicated when products for all 3 primer pairs were amplified. Female genotype was denoted by all other results provided fabp6b primers produced an amplicon.
Real-time qPCR assays
Real-time qPCR assays were developed to determine the quantities of exons 2, 3 and 4 of sdY in Atlantic salmon gDNA, and are reported in accordance with the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE)42. An MIQE checklist is provided in Supplementary Table S542. Primer and hydrolysis probes specific to exons 2, 3 and 4 of Atlantic salmon sdY were designed using PrimerQuest Tool (Integrated DNA Technologies, USA) according to the gene sequence provided by http://www.ncbi.nlm.nih.gov for sdY in Atlantic salmon (GenBank accession number: KT223110). sdY gene structure and amplicon localisation for each assay is described in Fig. 3. Primer specificity was confirmed using primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Primer and probe sequences, and amplicon sizes are provided in Supplementary Table S6. Each 20 µl reaction contained 10 ng gDNA, 200 nm forward and reverse primer, 200 nm hydrolysis probe, 1X SsoAdvanced universal probes Supermix (Bio-Rad, USA) and nuclease-free water. All reactions were loaded in triplicate into a hard shell 96 clear-well PCR plate, covered with a clear adhesive PCR plate seal and run on a CFX Connect Real-Time PCR Detection System (Bio-Rad, USA). Threshold was manually set to 150 relative fluorescence units, which was within the exponential phase of each run, and Quantification cycle (Cq) was determined using CFX Manager software 3.1 (Bio-Rad, USA). Positive amplification of the target for each sample was given when amplification was detected in one or more of the triplicate reactions. Thermal cycling conditions were 95 °C for 3 minutes, followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 60 seconds. Three NTC reactions were also included in each real-time qPCR run, and showed no amplification throughout the study except for a single replicate in one exon 4 run (Cq 38.76). PCR efficiency for exon 2, 3 and 4 assays were determined by four five-fold serial dilutions of pooled gDNA from two sdY-positive samples, and was 94.6%, 94.3% and 92.8% respectively. Target amplicon size and sequence was verified by visualising real-time qPCR products for each assay on 2% 1X TAE agarose gel with GelRed alongside a 50 bp ladder and Sanger sequencing, respectively. However, the amplicon sequence for the exon 3 assay was unable to be identified due to being too small for Sanger sequencing.
Absolute quantification
A 397 bp gBlocks Gene Fragment (Integrated DNA Technologies, USA) containing the target amplicons for exon 2, 3 and 4 real-time qPCR assays was designed for use as a standard curve in each assay. The dried gBlocks Gene Fragment pellet was re-suspended with nuclease-free water to obtain a concentration of 8.55 ng/μl, as determined by measurement with a spectrophotometer. The total number of copies/μl for each target in this solution was determined as 1.96 ×1010 using the following formula: number of copies/μl = (6.02 ×1023 x DNA (g/μl))/(DNA length (bp) x 660)43. This formula assumes that the average weight of a base pair is 660 Da44,45. To determine the linear dynamic range (LDR) for each assay, a ten-point standard curve was constructed by performing 10-fold serial dilutions of the gBlocks Gene Fragment from 1.96 × 109 copies/μl to 1.96 × 100 copies/μl. All standards were run in triplicate following the conditions outlined above, and the LDR was defined between the lowest and highest concentration for which all 3 replicates amplified accurately with less than 1 cycle of deviation42. The LDR for exon 2, 3 and 4 assays was 3.93 × 109 to 3.93 × 101 copies/reaction (Supplementary Data S1). For each assay, a six-point standard curve was constructed by performing 10-fold serial dilutions from 1.96 ×106 copies/μl to 1.96 × 101 copies/μl. The standard curve was run six to nine times for each assay in runs containing gDNA samples. For each run, new serial dilutions were performed from a single use aliquot of a 1.96 × 109 copies/μl stock solution.
To address the significant effects minor run-to-run variations in standard preparation and loading can have on number of copies estimates, a master standard curve approach was used46,47. For each assay, highly fitted master standard curves were produced by plotting the mean Cq of all replicates for each standard (independent of run) against the log-transformed known number of copies/reaction48,49 (Supplementary Data S2). The equations of the master standard curves were then used to transform Cq values to number of copies/reaction for gDNA samples. Data was normalised to template concentrations as determined by spectrophotometer, and presented as number of copies/10 ng gDNA. PCR efficiencies calculated from the master standard curves (92.1%, 91.0% and 89.3% for exon 2, 3 and 4 assays, respectively) were similar to those determined from gDNA, suggesting they provide accurate number of copy estimates. Intra-assay coefficient of variation as a percentage (c.v %) for exon 2, 3 and 4 assays was 13.92, 6.29 and 11.06, respectively, whilst inter-assay c.v % was 2.86, 4.54 and 7.52.
Statistical analysis
All statistical analysis was performed through R 3.3.3 (www.r-project.org). Data was checked visually for homogeneity of variance by examining the residuals plotted against the predicted values. Quantile-quantile plots were used to see if the data was normally distributed. A two-tailed Welch’s t-test (variances were not treated as equal) was used to compare the mean number of copies for exon 3 and exon 4 in phenotypic males and phenotypic females (with levels within the LDR). For exon 2, a one-tailed one sample t-test was used to test if the mean for phenotypic males was greater than 55.20 (the number of copies/10 ng gDNA in the single phenotypic female with levels within the LDR). A two-tailed independent t-test (variances were treated as equal) was used to test for differences in the means of phenotypic males grouped according to reproductive developmental stage. Statistical significance was considered at a level of p < 0.05.
Change history
15 July 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Davidson, W. S. Understanding salmonid biology from the Atlantic salmon genome. Genome 56, 548–550 (2013).
Davidson, W., Huang, T.-K., Fujiki, K., Von Schalburg, K. & Koop, B. The sex determining loci and sex chromosomes in the family salmonidae. Sex. Dev. 3, 78–87 (2009).
Thorgaard, G. H. et al. Status and opportunities for genomics research with rainbow trout. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 133, 609–646 (2002).
Powers, D. A. Fish as model systems. Science 246, 352–358 (1989).
Forné, I., Abián, J. & Cerdà, J. Fish proteome analysis: model organisms and non‐sequenced species. Proteomics 10, 858–872 (2010).
Quéméré, E. et al. An improved PCR-based method for faster sex determination in brown trout (Salmo trutta) and Atlantic salmon (Salmo salar). Conserv. Genet. Resour. 6, 825–827 (2014).
King, R. A. & Stevens, J. R. An improved genetic sex test for Atlantic salmon (Salmo salar L.). Conserv. Genet. Resour. 1–3 (2019).
Araneda, C., Lam, N. & Iturra, P. Development and Application of Sex‐Linked Markers in Salmonidae in Sex Control in Aquaculture (ed. Wang, H-P., Piferrer, F., Chen, S-L. &Shen, Z-G.) 281–295 (Wiley, 2018).
Yano, A. et al. The sexually dimorphic on the Y‐chromosome gene (sdY) is a conserved male‐specific Y‐chromosome sequence in many salmonids. Evol. Appl. 6, 486–496 (2013).
Devlin, R. H., McNeil, B. K., Groves, T. D. D. & Donaldson, E. M. Isolation of a Y-chromosomal DNA probe capable of determining genetic sex in chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. 48, 1606–1612 (1991).
Du, S. J. & Devlin, R. H. & HEW, C. L. Genomic structure of growth hormone genes in chinook salmon (Oncorhynchus tshawytscha): presence of two functional genes, GH-I and GH-II, and a male-specific pseudogene, GH-Ψ. DNA Cell Biol. 12, 739–751 (1993).
Nakayama, I., Biagi, C., Koide, N. & Devlin, R. Identification of a sex-linked GH pseudogene in one of two species of Japanese salmon (Oncorhynchus masou and O. rhodurus). Aquaculture 173, 65–72 (1999).
Devlin, R., Biagi, C. & Smailus, D. Genetic mapping of Y-chromosomal DNA markers in Pacific salmon. Genetica 111, 43–58 (2001).
Brunelli, J. P., Wertzler, K. J., Sundin, K. & Thorgaard, G. H. Y-specific sequences and polymorphisms in rainbow trout and Chinook salmon. Genome 51, 739–748 (2008).
Nagler, J. J., Bouma, J., Thorgaard, G. H. & Dauble, D. D. High incidence of a male-specific genetic marker in phenotypic female chinook salmon from the Columbia River. Environ. Health Perspect. 109, 67–69 (2001).
Williamson, K. S. & May, B. Incidence of Phenotypic Female Chinook Salmon Positive for the Male Y-Chromosome-Specific Marker OtY1 in the Central Valley, California. J. Aquat. Anim. Health 14, 176–183, 10.1577/1548-8667(2002)014##0176:IOPFCS$$2.0.CO;2 (2002).
Metcalf, V. & Gemmell, N. Sexual genotype markers absent from small numbers of male New Zealand Oncorhynchus tshawytscha. J. Fish Biol. 68, 136–143 (2006).
Devlin, R. H. et al. Variation of Y-chromosome DNA markers in Chinook salmon (Oncorhynchus tshawytscha) populations. Can. J. Fish. Aquat. Sci. 62, 1386–1399, https://doi.org/10.1139/f05-048 (2005).
Zhang, Q. et al. Sex identification by male-specific growth hormone pseudogene (GH-ψ) in Oncorhynchus masou complex and a related hybrid. Genetica 111, 111–118 (2001).
Muttray, A. et al. Deletion and copy number variation of Y-chromosomal regions in coho salmon, chum salmon, and pink salmon populations. Trans. Am. Fish. Soc. 146, 240–251 (2017).
Yano, A. et al. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr. Biol. 22, 1423–1428 (2012).
Cavileer, T. D., Hunter, S. S., Olsen, J., Wenburg, J. & Nagler, J. J. A sex-determining gene (sdY) assay shows discordance between phenotypic and genotypic sex in wild populations of Chinook salmon. Trans. Am. Fish. Soc. 144, 423–430 (2015).
Larson, W. A., McKinney, G. J., Seeb, J. E. & Seeb, L. W. Identification and characterization of sex-associated loci in sockeye salmon using genotyping-by-sequencing and comparison with a sex-determining assay based on the sdY gene. J. Hered. 107, 559–566 (2016).
Kijas, J. et al. Evolution of Sex Determination Loci in Atlantic Salmon. Sci. Rep. 8, 5664 (2018).
Perry, W. B. et al. Evolutionary drivers of kype size in Atlantic salmon (Salmo salar): domestication, age and genetics. R. Soc. Open Sci. 6, 190021 (2019).
Eysturskarð, J., Dam, M., í Kongsstovu, S. K., Jacobsen, Á. & Petersen, P. E. Rapid sex identification of Atlantic salmon (Salmo salar L.) by real‐time PCR. Aquacult. Res. 48, 2618–2620 (2017).
Eisbrenner, W. et al. Evidence for multiple sex-determining loci in Tasmanian Atlantic salmon (Salmo salar). Heredity 113, 86–92 (2014).
Ayllon, F. et al. The vgll3 locus controls age at maturity in wild and domesticated Atlantic salmon (Salmo salar L.) males. PLoS Genet. 11, e1005628 (2015).
Ayllon, F. et al. The influence of vgll3 genotypes on sea age at maturity is altered in farmed mowi strain Atlantic salmon. BMC Genet. 20, 44 (2019).
Gutierrez, A. P., Yáñez, J. M., Fukui, S., Swift, B. & Davidson, W. S. Genome-wide association study (GWAS) for growth rate and age at sexual maturation in Atlantic salmon (Salmo salar). PLoS One 10, e0119730 (2015).
Kleppe, L. et al. Sex steroid production associated with puberty is absent in germ cell-free salmon. Sci. Rep. 7, 12584 (2017).
Lubieniecki, K. P. et al. Expression analysis of sex-determining pathway genes during development in male and female Atlantic salmon (Salmo salar). Physiol. Genomics 47, 581–587 (2015).
Glover, K. et al. Genetic screening of farmed Atlantic salmon escapees demonstrates that triploid fish display reduced migration to freshwater. Biol. Invasions 18, 1287–1294 (2016).
Wargelius, A. et al. Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci. Rep. 6, 21284 (2016).
Gabián, M. & Morán, P. A genetic tool for evaluating male‐mediated stock introgression in Atlantic salmon. Aquat. Conserv.: Mar. Freshwat. Ecosyst. 29, 142–147 (2019).
Harvey, A. C., Tang, Y., Wennevik, V., Skaala, Ø. & Glover, K. A. Timing is everything: Fishing‐season placement may represent the most important angling‐induced evolutionary pressure on Atlantic salmon populations. Ecol. Evol. 7, 7490–7502 (2017).
Lubieniecki, K. P. et al. Genomic instability of the sex-determining locus in Atlantic salmon (Salmo salar). G3 5, 2513–2522 (2015).
Kumari, A., Yadav, S. K., Misro, M. M., Ahmad, J. & Ali, S. Copy number variation and microdeletions of the Y chromosome linked genes and loci across different categories of Indian infertile males. Sci. Rep. 5, 17780 (2015).
Bashamboo, A. et al. Fate of SRY, PABY, DYS1, DYZ3 and DYZ1 loci in Indian patients harbouring sex chromosomal anomalies. Mol. Human Reprod. 11, 117–127 (2005).
Dziewulska, K. & Domagala, J. Histology of salmonid testes during maturation. Reprod. Biol. 3, 47–61 (2003).
Foyle, T. A histological description of gonadal development and sex differentiation in the coho salmon (Oncorhynchus kisutch) for both untreated and oestradiol immersed fry. J. Fish Biol. 42, 699–712 (1993).
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009).
Kon, N. F. et al. Spatial distribution of toxic Alexandrium tamiyavanichii (Dinophyceae) in the southeastern South China Sea-Sulu Sea: A molecular-based assessment using real-time quantitative PCR (qPCR) assay. Harmful Algae 50, 8–20 (2015).
Whelan, J. A., Russell, N. B. & Whelan, M. A. A method for the absolute quantification of cDNA using real-time PCR. J. Immunol. Methods 278, 261–269 (2003).
Lee, C., Kim, J., Shin, S. G. & Hwang, S. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 123, 273–280 (2006).
Sivaganesan, M., Haugland, R. A., Chern, E. C. & Shanks, O. C. Improved strategies and optimization of calibration models for real-time PCR absolute quantification. Water Res. 44, 4726–4735 (2010).
Sivaganesan, M., Seifring, S., Varma, M., Haugland, R. A. & Shanks, O. C. A Bayesian method for calculating real-time quantitative PCR calibration curves using absolute plasmid DNA standards. BMC Bioinformatics 9, 120 (2008).
Ros-García, A., Nicolás, A., García-Pérez, A. L., Juste, R. A. & Hurtado, A. Development and evaluation of a real-time PCR assay for the quantitative detection of Theileria annulata in cattle. Parasites Vectors 5, 171 (2012).
Gokduman, K., Avsaroglu, M. D., Cakiris, A., Ustek, D. & Gurakan, G. C. Recombinant plasmid-based quantitative Real-Time PCR analysis of Salmonella enterica serotypes and its application to milk samples. J. Microbiol. Methods 122, 50–58 (2016).
Acknowledgements
This work was supported by Deakin University LES Strategic Growth Funds (PG00510), and FRDC on behalf of the Australian Government: project 2018-21 Sex reversal and sex differentiation in Atlantic salmon (Salmo salar). The authors thank Kate Darton (SALTAS) and Aaron Wingrove (Tassal Operations) for their assistance with sample collection, Jade Tramontana for assistance with gel electrophoresis and Ryan Nai (Deakin University) for support with qPCR assays.
Author information
Authors and Affiliations
Contributions
M.S.B. and L.O.B.A. conceived and designed the experiment. M.S.B. conducted the experiment and analysis. M.S.B. and L.O.B.A. drafted the manuscript. M.S.B., B.S.E. and L.O.B.A. revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brown, M.S., Evans, B.S. & Afonso, L.O.B. Discordance for genotypic sex in phenotypic female Atlantic salmon (Salmo salar) is related to a reduced sdY copy number. Sci Rep 10, 9651 (2020). https://doi.org/10.1038/s41598-020-66406-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66406-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.