Abstract
Cebranopadol is known as a highly potent analgesic. Recent studies also demonstrated that administration of cebranopadol significantly decreased cocaine self-administration and significantly reduced cue-induced cocaine-seeking behaviors in rats. However, it was unclear whether these interesting behavioral observations are related to any potential effects of cebranopadol on cocaine pharmacokinetics or cocaine-induced hyperactivity. In principle, a promising therapeutic candidate for cocaine dependence treatment may alter the cocaine pharmacokinetics and/or attenuate cocaine-induced reward and hyperactivity and, thus, decrease cocaine self-administration and reduce cue-induced cocaine-seeking behaviors. In this study, we examined possible effects of cebranopadol on cocaine pharmacokinetics and cocaine-induced hyperactivity for the first time. According to our animal data in rats, cebranopadol did not significantly alter the pharmacokinetics of cocaine. According to our more extensive locomotor activity testing data, cebranopadol itself also dose-dependently induced hyperactivity in rats at doses higher than 50 µg/kg. Cebranopadol at a low dose of 25 µg/kg (p.o.) did not induce significant hyperactivity itself, but significantly potentiated cocaine-induced hyperactivity on Days 4 to 7 after the repeated daily dosing of the drug.
Similar content being viewed by others
Introduction
Cebranopadol (CEB) is a novel, centrally acting analgesic which has been proven highly potent in various animal models of pain for preclinical studies and efficacious in multiple clinical trials1. To date, there have been 12 clinical trials including 11 completed trials (https://clinicaltrials.gov/ct2/results/details?term=cebranopadol). Cebranopadol, with a unique mode of action, is a high-affinity agonist at both μ-opioid peptide (MOP) receptor (Kd = 0.7 nM) and nociceptin opioid peptide (NOP) receptor (Kd = 0.9 nM), and can also activate κ-opioid peptide (KOP) receptor (Kd = 2.6 nM) and δ-opioid peptide (DOP) receptor (Kd = 18 nM) at higher concentrations2. Compared to classic opioid analgesics such as morphine and hydromorphone, cebranopadol is more effective and safer with a better tolerability and has less addiction potential in humans when used as an analgesic3,4,5,6.
Previous studies suggest that NOP receptor may be a potentially interesting target for treatment of drug dependence by attenuating the rewarding effects of cocaine and other drugs of abuse7,8,9. In addition, a growing body of evidence suggests that simultaneous targeting of multiple opioid receptors is necessary to reduce classic opioid side effects10,11,12. For this reason, with the characteristics of high activity at both the MOP and NOP receptors, along with multiple activation on other opioid receptors, cebranopadol has attracted much attention as a potential therapeutic candidate for treatment of cocaine dependence. Specifically, two recent studies demonstrated that administration of cebranopadol significantly decreased cocaine self-administration13 and significantly reduced cue-induced cocaine-seeking behaviors14 in rats. However, it was unclear whether these interesting behavioral observations are related to any potential effects of cebranopadol on cocaine pharmacokinetics or cocaine-induced hyperactivity. As noted in one of the reports14, these reported studies did not examine potential effects of cebranopadol on the pharmacokinetics of cocaine or its effects on cocaine-induced hyperactivity. In principle, a promising therapeutic candidate for cocaine dependence treatment may alter the pharmacokinetics (PK) of cocaine and/or attenuate cocaine-induced reward and hyperactivity (locomotor-stimulating effect)15,16 and, thus, decrease cocaine self-administration and reduce cue-induced cocaine-seeking behaviors.
As well-known, locomotor activity test is a basic unconditioned behavioral model to study the psychoactive effects of testing compounds17. Psychostimulants and opioids directly or indirectly promote neuronal activity in mesocorticolimbic dopamine (DA) system to increase extracellular DA in the brain18,19. Hence, dopaminergic systems are crucially involved in locomotor-stimulating effects and physical dependence in addiction of psychostimulants and the opiates20,21,22,23. Despite of the considerable studies on the analgesic effects of cebranopadol1,2,24,25, the actions of cebranopadol on spontaneous motor activity of rats have not been characterized extensively. De Guglielmo et al.14 did a locomotor activity test in rats administered orally of 25 µg/kg cebranopadol, and concluded that cebranopadol did not affect the locomotor activity. To the best of our knowledge, there has been no report of studies on the impact of cebranopadol on cocaine-induced hyperactivity. It is also interesting to know whether cebranopadol alters the PK profile of cocaine, as animal behavioral data are closely correlated with the PK of psychostimulants26,27,28.
In this study, we examined the possible effects of cebranopadol on cocaine pharmacokinetics and cocaine-induced hyperactivity (locomotor-stimulating effect). According to our animal data in rats, cebranopadol did not significantly alter the PK of cocaine. Further, cebranopadol significantly potentiated cocaine-induced hyperactivity at least after repeated drug administration, which is unexpected as the previous study14 revealed that cebranopadol did not affect the locomotor activity in rats. In fact, according to our more extensive locomotor activity testing data, cebranopadol itself also dose-dependently induced hyperactivity in rats at doses higher than 50 µg/kg.
Results
Influence of procedure manipulation on locomotor behavior
We carried out extensive locomotor activity tests (see Figs. 1–5) to examine whether cebranopadol has any effects on the locomotor activity of rats with or without cocaine administration. First of all, we examined possible effects of injection procedure, anesthesia, and vehicle (corn oil) on the locomotor activity in 12 rats during the first several days in order to habituate rats to the injection procedure and exclude manipulation influence. As shown in Fig. 1, curves plotted with distance moved per five minutes after anesthesia and vehicle treatment were overlapped with the corresponding control groups, showing no significant differences between the groups in the total distance moved in six hours from time 0 to 360 min (see Figs. 1 and 4A), as expected.
Baseline locomotor activity of rats (n = 12) under various testing environments/procedures. (A) Effects of anesthesia without administration of saline or vehicle. (B) Effects of anesthesia with saline administration; saline (1 ml/kg) was injected (i.p.) at t = 0 (i.e. 60 minutes after the rats were put into the chambers). (C) Effects of vehicle (corn oil) without anesthesia; vehicle oil (1 ml/kg) was administered (p.o.) 15 min before the rats were put into the testing chambers. “Nothing” means no administration of saline or vehicle. Data are plotted as mean ± SEM meters moved per five minutes. The data were analyzed by two-way ANOVA with post hoc analysis, showing no significant difference between any of the pairs.
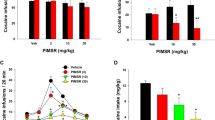
Hyperactivity in the same group of rats (n = 12) induced by cebranopadol (CEB) at various doses including 0 (vehicle), 25, 50, 75, and 100 µg/kg CEB in ascending order. Each time, vehicle or CEB was administered (p.o.) 15 min before the rats were put into the testing chambers. Data are plotted as mean ± SEM meters moved per five minutes and statistical significances were analyzed by two-way ANOVA with post hoc analysis.
Effects of 50 µg/kg cebranopadol (CEB) on cocaine-induced hyperactivity. The 12 rats were assigned randomly to two groups: testing and control groups (n = 6 per group). CEB (for the testing group) or vehicle (oil for the control group) was administered (p.o.) 15 min before the rats were put into the testing chambers; cocaine (20 mg/kg, i.p.) were administered at t = 0 (i.e. 60 minutes after the rats were put into the chambers). Prior to the locomotor activity testing with cocaine, the baseline locomotor activity data with vehicle + saline (1 ml/kg, i.p.) were determined in both groups of the rats (n = 6 + 6 = 12). Data are plotted as mean ± SEM meters moved per five minutes and statistical significances were analyzed by two-way ANOVA with post hoc analysis.
Total distances moved in 6 hours after cebranopadol (CEB) and/or cocaine (COC at a dose of 20 mg/kg, i.p.) administration in rats (n = 12). (A) Various negative control conditions; (B) dose-dependent effects of CEB on locomotor activity and effects of CEB on cocaine-induced hyperactivity. Data are presented as mean ± SEM and statistical significances were analyzed by one-way ANOVA with post hoc analysis.
Effects of 25 µg/kg cebranopadol (CEB) on cocaine-induced hyperactivity. These additional locomotor activity tests were performed on other 16 rats with daily administration of cocaine (20 mg/kg, i.p.) alone (n = 6) or cebranopadol (25 µg/kg, p.o.) + cocaine (20 mg/kg, i.p.) (n = 6) or cebranopadol (25 µg/kg, p.o.) (n = 4) alone within a week: (A) Day 1; (B) Day 2; (C) Day 3; (D) Day 4; (E) Day 5; (F) Day 6; (G) Day 7. Panel (H) shows the total distance traveled in six hours after the cocaine (or saline) administration. Prior to any drug (cocaine and/or cebranopadol) administration, all rats (n = 6 + 6 + 4 = 16) were tested for the baseline locomotor activity (with saline instead of cocaine) on Day 0 (baseline). The same baseline locomotor activity data (n = 16) were indicated in panels A to G as the common negative control. Each day, CEB or vehicle (oil) was administered (p.o.) 15 min before the rats were put into the testing chambers; cocaine (20 mg/kg, i.p.) was administered at t = 0 (i.e. 60 minutes after the rats were put into the chambers). Data are plotted as mean ± SEM meters moved per five minutes and statistical significances were analyzed by two-way ANOVA with post hoc analysis.
Cebranopadol-induced locomotor activity
Rats, administered with an ascending dose (25, 50, 75, or 100 µg/kg, p.o.) of cebranopadol, were placed in non-porous plastic chambers to observe toxicity signs and monitor locomotor activity induced by cebranopadol. It turned out that cebranopadol did not elicit locomotor activity by itself when the dose was low (25 or 50 µg/kg; see Fig. 2). However, as the dose increased, the animals became apparently more active (Fig. 2). According to the two-way analysis of variance (ANOVA) with post hoc analysis, both 75 and 100 µg/kg cebranopadol induced significant hyperactivity (p < 0.0001). These observations suggest that the rats are more active after treatment with 75 or 100 µg/kg cebranopadol than with 25 or 50 µg/kg cebranopadol during the testing process. Consistently, as shown in Fig. 4B, one-way ANOVA with post hoc analysis (on the total distances moved within six hours) also revealed a significant increase in total distance moved in six hours following the treatment with 75 and 100 µg/kg cebranopadol (p < 0.05 and p < 0.01, respectively) compared to the vehicle treatment and 50 µg/kg cebranopadol. We did not observe any adverse effects during the entire testing process.
Cebranopadol potentiated cocaine-induced hyperactivity
The dose of 50 µg/kg cebranopadol (p.o.) was chosen to investigate the effect of cebranopadol on cocaine-induced hyperactivity because it was the highest dose without inducing hyperactivity on its own, as noted above. 20 mg/kg cocaine (i.p.)-treated rats showed distinct hyperactivity (Fig. 3, p < 0.0001 vs the vehicle baseline), and they calmed down in about two hours after the cocaine injection. Two-way ANOVA with post hoc analysis revealed significantly increased locomotor activity after co-administration of 20 mg/kg cocaine (i.p.) and 50 µg/kg cebranopadol (p.o.) (Fig. 3, p < 0.0001) in comparison with the injection of 20 mg/kg cocaine (i.p.) alone. The locomotor-stimulating effect sustained for a longer period of time during which the rats were not even calmed down until four hours after the cocaine administration. One-way ANOVA with post hoc analysis indicated that pretreatment with 50 µg/kg cebranopadol (p.o.) augmented cocaine-induced hyperactivity significantly, more than two times higher compared to the treatment with cocaine alone and more than six times higher compared to the treatment with 50 µg/kg cebranopadol by analyzing total distance moved in six hours (Fig. 4B).
It should be noted that the above-discussed effects of 50 µg/kg cebranopadol on cocaine-induced hyperactivity were based on the locomotor activity testing in the same 12 rats that had been tested with cebranopadol (as reported in Table 1). A potential question was whether the previous cebranopadol administration would significantly affect the subsequent locomotor activity with cebranopadol again. Further, because previous studies13,14 demonstrated that cebranopadol at the dose of 25 µg/kg (p.o.) significantly decreased cocaine self-administration, would cebranopadol at a dose of 25 µg/kg (p.o.) also potentiate cocaine-induced hyperactivity? To address these questions, we also carried out further locomotor activity tests on other 16 rats with daily administration of cocaine (20 mg/kg, i.p.) alone or cebranopadol (25 µg/kg, p.o.) + cocaine (20 mg/kg, i.p.) or cebranopadol (25 µg/kg, p.o.) alone during a week.
As seen in Fig. 5, repeated daily dosing of 25 µg/kg cebranopadol (p.o.) within a week never induced significant hyperactivity; there was no significant difference in the locomotor activity among all the locomotor activity tests with 25 µg/kg cebranopadol or saline (baseline), according to two-way ANOVA with post hoc analysis. Further, based on the results depicted in Fig. 5A to C, 25 µg/kg cebranopadol (p.o.) did not significantly change cocaine-induced hyperactivity during the locomotor activity tests after the drug (cocaine and/or cebranopadol) administration on Days 1 to 3. There was no significant difference in the hyperactivity between the two groups (cocaine alone group and cocaine + cebranopadol group) on Days 1 to 3. However, during Days 4 to 7, compared to the hyperactivity induced by administration of cocaine (20 mg/kg, i.p.) alone, the hyperactivity induced by co-administration of cocaine (20 mg/kg, i.p.) and cebranopadol (25 µg/kg, p.o.) had a nearly equal (or even slightly lower) peak, but lasted a longer period of time (see Fig. 5D–G). The difference was significant on Days 4 to 7, based on two-way ANOVA with post hoc analysis. We also performed one-way ANOVA with post hoc analysis on the total distance moved during six hours after the drug administration, showing that the total distance associated with the co-administration of cocaine (20 mg/kg, i.p.) and cebranopadol (25 µg/kg, p.o.) was significantly longer than that associated with administration of cocaine (20 mg/kg, i.p.) alone on Days 4 to 7 (Fig. 5H).
Cebranopadol did not change the PK profile of cocaine
To investigate whether cebranopadol can significantly alter the pharmacokinetic profile of cocaine and time course in plasma or attenuate/potentiate cocaine-induced hyperactivity, we conducted PK study to measure the concentrations of cocaine and all its metabolites in whole blood after pretreatment of cebranopadol. As showed in Fig. 6, we did not detect any significant effects of cebranopadol on cocaine PK profile according to our two-way ANOVA for the time courses of the blood concentrations of each compound (cocaine or its metabolite). Therefore, the increase of cocaine-induced hyperactivity associated with the pretreatment of cebranopadol was not caused by changing the blood concentrations of cocaine and its active metabolites.
Impact of cebranopadol on the PK profile of cocaine in rats (n = 4 per group). Time-dependent concentrations of (A) Cocaine, (B) EME, (C) BZE, (D) ECG, (E) Norcocaine, and (F) NEME. Rats received nothing (for control group), 50 or 100 µg/kg cebranopadol (p.o. for testing groups) 60 min before cocaine administration (20 mg/kg, i.p.). The blood concentrations of cocaine and its metabolites were detected simultaneously. BZE, EME, NEME, and ECG are abbreviation of benzoylecgonine, ecgonine methyl ester, norecgonine methyl ester, and ecgonine, respectively. Data are plotted as mean ± SEM and statistical significances were analyzed by two-way ANOVA with post hoc analysis.
Discussion
According to the locomotor activity data, cebranopadol at a low oral dose of 25 or 50 µg/kg did not significantly affect the locomotor activity in rats. The previously reported study also observed that cebranopadol at an oral dose of 25 µg/kg did not affect general locomotor activity of the rats14. However, 25 µg/kg cebranopadol (p.o.) was the only dose tested in the previous study14. The present study revealed that cebranopadol dose-dependently stimulates locomotor activity in rats, demonstrating significant hyperactivity at an oral dose of 75 or 100 µg/kg, but without showing any visible toxicity signs during the locomotor activity tests. The observed dose-dependent hyperactivity induced by cebranopadol is actually consistent with the previously reported observation that cebranopadol had discriminative stimulus property29 to certain extent like morphine and the fact that cebranopadol is also an MOP receptor agonist (Kd = 0.7 nM) which is expected to induce hyperactivity at a sufficiently high dose30,31.
It is interesting to note that, according to our locomotor activity data, 25 µg/kg cebranopadol (p.o.) did not induce significant hyperactivity itself, but significantly potentiated cocaine-induced hyperactivity on Days 4 to 7 after the repeated daily dosing the drug. As well know, cocaine induces a uniformly dose-dependent hyperactivity after acute administration32,33. Concerning why an opioid can potentiate cocaine-induced hyperactivity, a number of preclinical studies have revealed cross-sensitization between opioids and cocaine, demonstrating that some other opioids also clearly potentiate cocaine-induced hyperactivity34,35,36,37. In general, both a psychostimulant, such as cocaine, and an opioid activate or augment the dopaminergic input into the nucleus accumbens and frontal cortex to produce psychomotor stimulant actions34,35,36. In particular, Smith et al.37 examined cross-sensitization between opioids and cocaine, and determined that the extent of cross-sensitization was mediated by an opioid’s selectivity for MOP, KOP, and DOP receptors. Likewise, their results revealed that MOP agonists morphine and buprenorphine and DOP agonist BW373U86 produced synergistic effects in cocaine locomotor activity tests. Cebranopadol may follow the same rule as it is a potent agonist of both NOP receptor (Kd = 0.9 nM) and MOP receptor (Kd = 0.7 nM).
Concerning why cebranopadol is capable of significantly decreasing cocaine self-administration and reducing cue-induced cocaine-seeking behaviors while significantly potentiating cocaine-induced hyperactivity, one possible explanation is that cebranopadol and buprenorphine have the similar effects. In fact, buprenorphine attenuates the expression of cocaine sensitization and other cocaine-related behaviors by increasing basal levels of glutamate in the nucleus accumbens, which serves to decrease the effectiveness of cocaine or cocaine-associated cues38.
We also investigated whether the alteration of pharmacokinetics (PK) of cocaine after cebranopadol administration was the possible reason for cebranopadol potentiating cocaine-induced hyperactivity. The metabolic pathways of cocaine have been well known15,39,40,41. Briefly, majority of cocaine (>90%) in rodents and primates is hydrolyzed to benzoylecgonine (BZE) and biologically inactive metabolite ecgonine methyl ester (EME) by either carboxylesterases42 or butyrylcholinesterase (BChE)16,43,44, and only minor portion (~5%) is oxidized into norcocaine (NC) by P45045,46,47. Concerning physiological effects of BZE, early investigation indicated that it was a potent vasoconstrictor, whereas more recent literature suggested that it was an inactive metabolite of cocaine48,49. NC is more toxic than cocaine itself, causing the hemodynamic changes and producing lethality49,50,51. Plasma BChE can further hydrolyze BZE to ecgonine (ECG) and NC to norecgonine methyl ester (NEME)45,47,52,53. Among all these compounds, only cocaine but not NC produced stimulatory locomotor activity51, whereas EME and ECG resulted in no specific effect26. According to our PK data, cebranopadol did not affect the peripheral processing or general accessibility of cocaine to the brain to activate motor systems after co-administration of cebranopadol and cocaine, which indicates that the behavioral differences observed after co-administration of cebranopadol and cocaine cannot be attributed to the change in the cocaine PK profile. In fact, cocaine concentration determined in blood is well correlated with the presence of cocaine concentration in the brain54. In mice, the brain/blood concentration ratios of cocaine and BZE, on the average, were approximately 7 and 0.1, respectively, between 5 and 60 min after i.p. administration of cocaine54; in human fatal cases, the brain/blood concentration ratios were 9.6 for cocaine and 0.36 for BZE55.
Further, compared to duration of the hyperactivity induced by cocaine alone, the duration of time for the hyperactivity induced by co-administration of cebranopadol and cocaine was much longer. The prolonged duration was likely related to the longer duration of action of cebranopadol. Notably, there has been no study reporting the concentration of cebranopadol in blood or brain (PK profile) and duration of action after oral administration of cebranopadol in rats. In fact, except for some professional pharmaceutical companies, it is difficult for a lab to determine the PK profile of cebranopadol administered orally, because its effective oral dose is too low to produce a concentration in plasma for a commonly used instrument to detect the compound. Nevertheless, a previous study revealed that the duration of action after intravenous (i.v.) administration of 12 µg/kg cebranopadol lasted for up to 7 hours (much longer than morphine at the equivalent effective dose) and the half-life of cebranopadol with a high i.v. dose of 160 µg/kg was 4.52 hours in rats2.
In summary, the findings in the present study suggest that cebranopadol can dose-dependently induce weak hyperactivity on its own, and that cebranopadol at a low dose of cebranopadol (25 µg/kg, p.o.) which does not induce significant hyperactivity by itself can significantly potentiate cocaine-induced hyperactivity at least after the repeated daily dosing of the drug. These findings should arouse caution that cebranopadol could have some dose-dependent rewarding/positive reinforcement effects. In consideration of the important role of dopaminergic systems involved in locomotor-stimulating effects and physical dependence in addiction of psychostimulants and the opioids, more extensive studies are needed to understand the effects of cebranopadol on dopaminergic systems. A clear understanding of the potential of rewarding and positive reinforcement effects of this compound underlying the psychological and physical behavior is required before further clinical development of the compound in treatment of cocaine-dependent patients. This knowledge will also be valuable for using cebranopadol in clinical trials as an analgesic.
Materials and methods
Animals and drugs
Male Sprague-Dawley rats were ordered from Harlan (Harlan, Indianapolis, IN), and housed initially in two rats per cage. Unless explicitly indicated otherwise, all rats were allowed ad libitum access to food and water except during testing time and maintained on a 12 h light/12 h dark cycle, with the lights on at 7:00 am at constant room temperature (21–22 °C) and humidity (45–55%)56,57. All experiments were performed during the light phase of the light/dark cycle in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The animal protocol was approved by the IACUC (Institutional Animal Care and Use Committee) at the University of Kentucky. Cebranopadol (Sigma Aldrich, USA) was freshly prepared as a fine suspension with 100% corn oil by ultrasonic and administered by gavage (p.o.). (-)-Cocaine hydrochloride was provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD) and was dissolved in sterile saline with intraperitoneal (i.p.) administration. Paraoxon and heparin were purchased from Thermo Fisher Scientific (Waltham, MA). Formic acid was from Sigma-Aldrich (St. Louis, MO).
Locomotor activity testing procedure
Cebranopadol- and cocaine-induced locomotion activity was monitored individually by using a video-tracking system (as described previously56,58) with rats weighing 260–320 grams at the beginning. Briefly, the locomotor activity tests were performed in high-density, non-porous plastic chambers measuring 50 cm (L) × 50 cm (W) × 38 cm (H) in a light- and sound-attenuating behavioral test enclosure (San Diego Instruments, San Diego, CA). Cumulative distance traveled was recorded by ANY-maze video tracking system (San Diego Instruments, San Diego, CA) to assess the locomotor activity beginning at 9:00 on testing day. The distance traveled was collected in 5-min bins. Cebranopadol or vehicle (oil) was p.o. administered 15 min under minor anesthesia with isoflurane before the rats were put into the testing chambers for 7-hour (i.e. time −60 to 360 min interval at X-axis) video track with or without administration of sterile saline (1 ml/kg, i.p.) or cocaine (20 mg/kg, i.p.) at 60 min (i.e. time 0 min point at the X-axis) after the rats were put into the testing chambers. Locomotor activity tests on the first 12 rats were proceeded as timeline in Table 1 and all injections were performed very gently. On non-testing days, all rats stayed in their home cages located in the rat-housing room. In addition to the locomotor activity tests listed in Table 1, further locomotor activity tests were performed on other 16 rats with daily administration of cocaine (20 mg/kg, i.p.) alone (n = 6) or cebranopadol (25 µg/kg, p.o.) + cocaine (20 mg/kg, i.p.) (n = 6) or cebranopadol (25 µg/kg, p.o.) (n = 4) alone within a week.
Metabolism of cocaine after treatment with cebranopadol
Rats received a dose of 1 ml/kg vehicle, 50 or 100 µg/kg cebranopadol at 60 min before cocaine administration (20 mg/kg, i.p.). Blood samples (75 µl/sample) were collected from saphenous veins into heparin-treated capillary tubes at 5, 10, 15, 20, 30, 45, 60, 90, 120, and 180 min after the cocaine injection, and mixed with 100 µl paraoxon solution (250 µM paraoxon with 10 U/ml heparin in 0.1% formic acid) immediately. Blood samples were stored at −80 °C until analysis by using our previously developed LC-MS/MS method52 for simultaneously detecting the concentrations of cocaine and its metabolites in blood samples.
Statistical analysis
All data were analyzed statistically with the Prism 8 software (GraphPad Software, San Diego, CA). All data are presented as mean ± SEM. One-way analysis of variance (ANOVA) with post hoc analysis was used to analyze differences of total distance moved in six hours in locomotion study. Two-way ANOVA with post hoc analysis was used to analyze the distance moved per 5 minutes in locomotor activity testing and the effects of cebranopadol on cocaine PK profile. Statistical significance was set at *(p < 0.05), **(p < 0.01), ***(p < 0.001), or ****(p < 0.0001).
References
Tzschentke, T. M., Linz, K., Koch, T. & Christoph, T. Cebranopadol: A Novel First-in-Class Potent Analgesic Acting via NOP and Opioid Receptors. Handb. Exp. Pharmacol. 254, 367–398, https://doi.org/10.1007/164_2019_206 (2019).
Linz, K. et al. Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. The Journal of pharmacology and experimental therapeutics 349, 535–548, https://doi.org/10.1124/jpet.114.213694 (2014).
Gohler, K. et al. Assessment of the Abuse Potential of Cebranopadol in Nondependent Recreational Opioid Users: A Phase 1 Randomized Controlled Study. J Clin Psychopharmacol 39, 46–56, https://doi.org/10.1097/jcp.0000000000000995 (2019).
Eerdekens, M. H. et al. Cancer-related chronic pain: Investigation of the novel analgesic drug candidate cebranopadol in a randomized, double-blind, noninferiority trial. European journal of pain (London, England) 23, 577–588, https://doi.org/10.1002/ejp.1331 (2019).
Tzschentke, T. M., Kogel, B. Y., Frosch, S. & Linz, K. Limited potential of cebranopadol to produce opioid-type physical dependence in rodents. Addict. Biol. 23, 1010–1019, https://doi.org/10.1111/adb.12550 (2018).
Ruzza, C., Holanda, V. A., Gavioli, E. C., Trapella, C. & Calo, G. NOP agonist action of cebranopadol counteracts its liability to promote physical dependence. Peptides 112, 101–105, https://doi.org/10.1016/j.peptides.2018.12.001 (2019).
Zhao, R. J. et al. Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport 14, 2383–2385, https://doi.org/10.1097/01.wnr.0000095707.83808.2c (2003).
Sakoori, K. & Murphy, N. P. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacology 33, 877–891, https://doi.org/10.1038/sj.npp.1301459 (2008).
Ciccocioppo, R. et al. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 172, 170–178, https://doi.org/10.1007/s00213-003-1645-1 (2004).
Bird, M. F. & Lambert, D. G. Simultaneous targeting of multiple opioid receptor types. Current opinion in supportive and palliative care 9, 98–102, https://doi.org/10.1097/spc.0000000000000129 (2015).
Azzam, A. A. H., McDonald, J. & Lambert, D. G. Hot topics in opioid pharmacology: mixed and biased opioids. British journal of anaesthesia 122, e136–e145, https://doi.org/10.1016/j.bja.2019.03.006 (2019).
Dietis, N. et al. Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile. British journal of anaesthesia 103, 38–49, https://doi.org/10.1093/bja/aep129 (2009).
Shen, Q., Deng, Y., Ciccocioppo, R. & Cannella, N. Cebranopadol, a Mixed Opioid Agonist, Reduces Cocaine Self-administration through Nociceptin Opioid and Mu Opioid Receptors. Frontiers in psychiatry 8, 234, https://doi.org/10.3389/fpsyt.2017.00234 (2017).
de Guglielmo, G. et al. Cebranopadol Blocks the Escalation of Cocaine Intake and Conditioned Reinstatement of Cocaine Seeking in Rats. The Journal of pharmacology and experimental therapeutics 362, 378–384, https://doi.org/10.1124/jpet.117.241042 (2017).
Zheng, F. & Zhan, C.-G. in Biologics to Treat Substance Use Disorders: Vaccines, Monoclonal Antibodies, and Enzymes (ed Ivan D. Montoya) 187-225 (Springer (2016).
Zheng, F. et al. A highly efficient cocaine-detoxifying enzyme obtained by computational design. Nature Commun. 5, 3457. 3410.1388/ncomms4457 (2014).
Smith, M. A., Greene-Naples, J. L., Lyle, M. A., Iordanou, J. C. & Felder, J. N. The effects of repeated opioid administration on locomotor activity: I. Opposing actions of mu and kappa receptors. The Journal of pharmacology and experimental therapeutics 330, 468–475, https://doi.org/10.1124/jpet.108.150011 (2009).
Kalivas, P. W. & Duffy, P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. The. Journal of neuroscience: the official journal of the Society for Neuroscience 13, 266–275 (1993).
Thomas, M. J., Kalivas, P. W. & Shaham, Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British journal of pharmacology 154, 327–342, https://doi.org/10.1038/bjp.2008.77 (2008).
Beninger, R. J. The role of dopamine in locomotor activity and learning. Brain research 287, 173–196, https://doi.org/10.1016/0165-0173(83)90038-3 (1983).
Kalivas, P. W. & Stewart, J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain research. Brain research reviews 16, 223–244 (1991).
Sharples, S. A., Koblinger, K., Humphreys, J. M. & Whelan, P. J. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Frontiers in neural circuits 8, 55, https://doi.org/10.3389/fncir.2014.00055 (2014).
Mogenson, G. J., Jones, D. L. & Yim, C. Y. From motivation to action: functional interface between the limbic system and the motor system. Progress in neurobiology 14, 69–97 (1980).
Schunk, S. et al. Discovery of a Potent Analgesic NOP and Opioid Receptor Agonist: Cebranopadol. ACS medicinal chemistry letters 5, 857–862, https://doi.org/10.1021/ml500117c (2014).
Rizzi, A. et al. Pharmacological characterization of cebranopadol a novel analgesic acting as mixed nociceptin/orphanin FQ and opioid receptor agonist. Pharmacology research & perspectives 4, e00247, https://doi.org/10.1002/prp2.247 (2016).
Schuelke, G. S., Konkol, R. J., Terry, L. C. & Madden, J. A. Effect of cocaine metabolites on behavior: possible neuroendocrine mechanisms. Brain research bulletin 39, 43–48, https://doi.org/10.1016/0361-9230(95)02040-3 (1996).
Lau, C. E., Falk, J. L. & King, G. R. Oral cocaine self-administration: relation of locomotor activity to pharmacokinetics. Pharmacology, biochemistry, and behavior 43, 45–51, https://doi.org/10.1016/0091-3057(92)90637-u (1992).
Falk, J. L., Ma, F. & Lau, C. E. Chronic oral cocaine self-administration: pharmacokinetics and effects on spontaneous and discriminative motor functions. The Journal of pharmacology and experimental therapeutics 257, 457–465 (1991).
Tzschentke, T. M. & Rutten, K. Mu-opioid peptide (MOP) and nociceptin/orphanin FQ peptide (NOP) receptor activation both contribute to the discriminative stimulus properties of cebranopadol in the rat. Neuropharmacology 129, 100–108, https://doi.org/10.1016/j.neuropharm.2017.11.026 (2018).
Andersen, J. M., Ripel, A., Boix, F., Normann, P. T. & Morland, J. Increased locomotor activity induced by heroin in mice: pharmacokinetic demonstration of heroin acting as a prodrug for the mediator 6-monoacetylmorphine in vivo. The Journal of pharmacology and experimental therapeutics 331, 153–161, https://doi.org/10.1124/jpet.109.152462 (2009).
Marquez, P., Baliram, R., Kieffer, B. L. & Lutfy, K. The mu opioid receptor is involved in buprenorphine-induced locomotor stimulation and conditioned place preference. Neuropharmacology 52, 1336–1341, https://doi.org/10.1016/j.neuropharm.2007.01.005 (2007).
Yeh, S. Y. & Haertzen, C. A. Cocaine-induced locomotor activity in rats. Pharmacology, biochemistry, and behavior 39, 723–727, https://doi.org/10.1016/0091-3057(91)90154-t (1991).
Cailhol, S. & Mormede, P. Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain research 842, 200–205, https://doi.org/10.1016/s0006-8993(99)01742-4 (1999).
Johnson, S. W. & North, R. A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. The Journal of neuroscience: the official journal of the Society for Neuroscience 12, 483–488 (1992).
Kuhar, M. J., Ritz, M. C. & Boja, J. W. The dopamine hypothesis of the reinforcing properties of cocaine. Trends in neurosciences 14, 299–302 (1991).
Wise, R. A. & Bozarth, M. A. A psychomotor stimulant theory of addiction. Psychological review 94, 469–492 (1987).
Smith, M. A. et al. The effects of repeated opioid administration on locomotor activity: II. Unidirectional cross-sensitization to cocaine. The Journal of pharmacology and experimental therapeutics 330, 476–486, https://doi.org/10.1124/jpet.108.150037 (2009).
Placenza, F. M., Rajabi, H. & Stewart, J. Effects of chronic buprenorphine treatment on levels of nucleus accumbens glutamate and on the expression of cocaine-induced behavioral sensitization in rats. Psychopharmacology 200, 347–355 (2008).
Zheng, F. et al. Most efficient cocaine hydrolase designed by virtual screening of transition states. Journal of the American Chemical Society 130, 12148-12155, https://doi.org/10.1021/ja803646t (2008).
Zheng, F. & Zhan, C. G. Are pharmacokinetic approaches feasible for treatment of cocaine addiction and overdose? Future medicinal chemistry 4, 125–128, https://doi.org/10.4155/fmc.11.171 (2012).
Zheng, F. & Zhan, C.-G. Enzyme therapy approaches for treatment of drug overdose and addiction. Future Med. Chem. 3, 9–13 (2011).
Yao, J., Chen, X., Zheng, F. & Zhan, C.-G. Catalytic reaction mechanism for drug metabolism in human carboxylesterase-1: Cocaine hydrolysis pathway. Mol. Pharmaceutics 15, 3871–3880 (2018).
Zhan, C.-G., Zheng, F. & Landry, D. W. Fundamental reaction mechanism for cocaine hydrolysis in human butyrylcholinesterase. J. Am. Chem. Soc. 125, 2462–2474 (2003).
Pan, Y. et al. Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc. Natl Acad. Sci. USA 102, 16656–16661, https://doi.org/10.1073/pnas.0507332102 (2005).
Zhan, M., Hou, S., Zhan, C.-G. & Zheng, F. Kinetic characterization of high-activity mutants of human butyrylcholinesterase for the cocaine metabolite norcocaine. Biochem. J. 457, 197–206 (2014).
Hou, S., Zhan, M., Zheng, X., Zhan, C.-G. & Zheng, F. Kinetic characterization of human butyrylcholinesterase mutants for hydrolysis of cocaethylene. Biochem. J. 460, 447–457 (2014).
Chen, X. et al. Metabolic enzymes of cocaine metabolite benzoylecgonine. ACS Chem. Biol. 11, 2186–2194 (2016).
Madden, J. A. & Powers, R. H. Effect of cocaine and cocaine metabolites on cerebral arteries in vitro. Life Sci 47, 1109–1114, https://doi.org/10.1016/0024-3205(90)90169-r (1990).
Morishima, H. O., Whittington, R. A., Iso, A. & Cooper, T. B. The comparative toxicity of cocaine and its metabolites in conscious rats. Anesthesiology 90, 1684–1690, https://doi.org/10.1097/00000542-199906000-00025 (1999).
Goldstein, R. A., DesLauriers, C., Burda, A. & Johnson-Arbor, K. Cocaine: history, social implications, and toxicity: a review. Seminars in diagnostic pathology 26, 10–17 (2009).
Zheng, X., Shang, L., Zhan, C.-G. & Zheng, F. In vivo characterization of toxicity of norcocaethylene and norcocaine identified as the most toxic cocaine metabolites in male mice. Drug Alcohol Depend. 204, 107410.101016/j.drugalcdep.102019.107404.107033, https://doi.org/10.1016/j.drugalcdep.2019.04.033 (2019).
Chen, X. et al. A quantitative LC-MS/MS method for simultaneous determination of cocaine and its metabolites in whole blood. J. Pharm. Biomed. Anal. 134, 243–251, https://doi.org/10.1016/j.jpba.2016.11.024 (2017).
Zheng, X. et al. Catalytic activities of cocaine hydrolases against the most toxic cocaine metabolite norcocaethylene. Org. Biomol. Chem. 18, 1968–1977 (2020).
Benuck, M., Lajtha, A. & Reith, M. E. Pharmacokinetics of systemically administered cocaine and locomotor stimulation in mice. The Journal of pharmacology and experimental therapeutics 243, 144–149 (1987).
Spiehler, V. R. & Reed, D. Brain concentrations of cocaine and benzoylecgonine in fatal cases. Journal of forensic sciences 30, 1003–1011 (1985).
Chen, X. et al. Long-acting cocaine hydrolase for addiction therapy. Proc. Natl Acad. Sci. USA 113, 422–427 (2016).
Zheng, X. et al. Effectiveness of a cocaine hydrolase for cocaine toxicity treatment in male and female rats. AAPS J. 20, 3, https://doi.org/10.1208/s12248-12017-10167-12244 (2018).
Zhang, T. et al. Clinical potential of an enzyme-based novel therapy for cocaine overdose. Sci. Rep. 7, 15303 (2017).
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH grants UH2/UH3 DA041115, R01 DA035552, R01 DA032910, R01 DA013930, and R01 DA025100).
Author information
Authors and Affiliations
Contributions
F. Zheng and C.-G. Zhan designed the research project. H. Wei and L. Shang conducted the experiments. All authors analyzed the experimental data. H. Wei, C.-G. Zhan, and F. Zheng wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, H., Shang, L., Zhan, CG. et al. Effects of Cebranopadol on Cocaine-induced Hyperactivity and Cocaine Pharmacokinetics in Rats. Sci Rep 10, 9254 (2020). https://doi.org/10.1038/s41598-020-66250-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66250-z
This article is cited by
-
Long-lasting blocking of interoceptive effects of cocaine by a highly efficient cocaine hydrolase in rats
Scientific Reports (2024)
-
Attenuated G protein signaling and minimal receptor phosphorylation as a biochemical signature of low side-effect opioid analgesics
Scientific Reports (2022)
-
The NOP Receptor System in Neurological and Psychiatric Disorders: Discrepancies, Peculiarities and Clinical Progress in Developing Targeted Therapies
CNS Drugs (2021)
-
Metabolites profiling and pharmacokinetics of troxipide and its pharmacodynamics in rats with gastric ulcer
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.