Abstract
Hyperuricemia is an abnormal metabolic condition characterized by an increase in uric acid levels in the blood. It is the cause of gout, manifested by inflammatory arthritis, pain and disability. This study examined the possible ameliorative impacts of parsley (PAR) and celery (CEL) as hypouricemic agents at biochemical, molecular and cellular levels. PAR and CEL alone or in combination were orally administered to hyperuricemic (HU) mice and control mice for 10 consecutive days. Serum levels of uric acid and blood urea nitrogen (BUN), xanthine oxidase activity, antioxidants, inflammatory (IL-1β and TNF-α) and anti-inflammatory cytokines (IL-10) were measured. mRNA expression of urate transporters and uric acid excretion genes in renal tissues were examined using qRT-PCR (quantitative real time PCR). Normal histology and immunoreactivity of transforming growth factor-beta 1 (TGF-β1) in kidneys was examined. Administration of PAR and CEL significantly reduced serum BUN and uric acids in HU mice, ameliorated changes in malondialdehyde, catalase, and reduced glutathione, glutathione peroxidase (GPX), IL-1β, TNF-α and IL-10 in hyperuricemic mice. Both effectively normalized the alterations in mURAT-1, mGLUT-9, mOAT-1 and mOAT-3 expression, as well as changes in TGF-β1 immunoreactivity. Interestingly, combined administration of PAR and CEL mitigated all examined measurements synergistically, and improved renal dysfunction in the hyperuricemic mice. The study concluded that PAR and CEL can potentially reduce damaging cellular, molecular and biochemical effects of hyperuricemia both individually and in combination.
Similar content being viewed by others
Introduction
Hyperuricemia (HU) is defined as an increase in the levels of uric acid over normal ranges (6 mg/dL in females and 7 mg/dL in males)1,2. HU is associated with meat and seafood ingestion, hypertension and obesity2,3,4. Advanced HU is associated with gout5. Gout results in deposition of urate in soft tissues and joints, and arthritis in men over 40 years old5. Uric acid (UA) is the end product of the catabolism of purine compounds in the liver. UA is excreted mainly by the kidneys and to a lesser extent by the gastrointestinal tract6,7. It is degraded by gut microbiota (one third) in a process known as intestinal uricolysis8. The remaining two thirds depends on interchange between UA secretion and reabsorption in the kidney tubules8,9,10. Treatment of gout mainly depends on allopurinol (ALP). ALP is an inhibitor of xanthine oxidase and stimulates renal excretion of UA10,11. Other anti-inflammatory drugs (indomethacin) can be used, but these may cause side effects11. Therefore, identifying safe herbal medications is the goal for both patients and physicians.
The use of organic drugs and therapies is cost-effective12. The positive and promising effects of medicinal herbs on renal diseases, infertility, liver disorders and diabetes are clearly established and are accepted by patients and clinicians as a safe medication for these disorders13,14,15,16,17. Plants of medical importance contain flavonoids and other phenolic compounds that have strong antioxidant effects, and have been investigated in many studies15,16,17. Medicinal plants have fewer side effects compared to manufactured drugs and are often used as alternative medicine to counter the side effects of synthetic therapies18,19.
Parsley (Petroselinum crispum, PAR) is used as a spice, garnish or fragrance for cuisine across the world. Parsley is the most well-known antioxidant-rich herb that reduces inflammation, especially in the joints20. The leafy part is rich in polyphenols and has high antioxidant activity21,22. Parsley prevents cancer cells from differentiation and growth23, and is a safe, natural remedy to reduce glucose levels in diabetic individuals24.
Celery (Apium graveolens L, CEL) is known in the Middle East as Karafs. Celery seeds, leaves, and essential oil extracts are widely used in medicine. Phytochemical compounds extracted from celery include carbohydrates and phenols such as flavonoids, alkaloids and steroids19. Limonene, flavonoids, selinene, and frocoumarin glycosides are the main compounds present in celery, making it one of the most commonly used plants in traditional medicine25. Celery can prevent cardiovascular diseases, jaundice, liver diseases, and rheumatoid associated diseases26. Research on rats has shown that ethanolic extracts of celery leaves increases spermatogenesis27, improves fertility28 and has antifungal and anti-inflammatory properties29. Moreover, its seeds have therapeutic use in the treatment of bronchitis, fever, chronic skin disorders, and tumors26,27,28,29. Recent reports have confirmed celery’s lowering effect on UA levels30. However, the exact descriptive mechanism of such effects is still unclear. Therefore, this study aimed to investigate the anti-hyperuricemic activity of parsley and celery at cellular and molecular levels on experimental hyperuricemia induced by oxonate in mice.
Materials and Methods
Chemicals and kits
ALP, ethidium bromide, PO and agarose were purchased from Sigma-Aldrich (St. Louis, MO, USA). Reverse transcriptase enzymes and 100 bp DNA ladder were from MBI (Fermentas, Thermo Fisher Scientific, USA). Qiazol and Oligo dT primers were from QIAGEN (Valencia, CA, USA). The kits for MDA, catalase, GSH, GPx were from Biodiagnostic Co. (Dokki, Giza, Egypt). The kits for blood urea nitrogen (BUN), uric acid, creatinine, GOT and GPT were from EGY-CHEM for lab technology (Badr City, Egypt). Serum creatinine was measured using ELISA kit from Mybiosource (Cat. No. MBS763433, San Diego, CA 92195-3308, USA). Mouse IL-1 beta (Catalog No. E-EL-M0037), mouse TNF-alpha (Catalog No. E-EL-M0049), IL-10 ELISA kit (Catalog No. E-EL-M0046) and XO kits (Catalog No. E-BC-K024) were from Elabscience Biotechnology Inc., Memorial Drive, Suite 216, Houston, Texas 77079, USA.
Preparation of parsley aqueous extract
Parsley was purchased from the local market in the Taif area, Saudi Arabia. Its identity was confirmed by a botanist (Prof. Yassin Alsudani) at the College of Science, Taif University. The leaves were washed in desalinated water and dried at room temperature in the dark. Parsley powders were diluted with distilled water (1:1 w/v) then given to the mice once daily at a dose of 7 g/kg bw. The remaining ground parsley was stored at −20 °C20,31.
Preparation of celery aqueous extract
Fresh celery was identified by Prof. Yassin Alsudani (College of Science, Taif University). The whole plant was purchased from local Taif markets, KSA and kept for dryness in the dark. It was then ground into a fine powder. Two hundred grams of the powder was soaked in 70% ethanol for 2 days at 40 °C with gentle shaking. After centrifugation at 7000 × g, at 20 °C for 10 min, the supernatant was filtered. The solvent was removed using a rotary evaporator (Rotavapor R-300/R-300 Pro, https://www.buchi.com/rotavapor-r-300). The residue yield was 16% (w/w) and this was kept at −20 °C until use30.
Animals, experimental design and samples collection
Swiss male mice were bought from the College of Pharmacy, King Abdel-Aziz University, Jeddah, Saudi Arabia. The Ethical Committee Office of Turabah University College, Taif University, Saudi Arabia, approved all procedures and in vivo animal use for this study. 56 male mice (7/group), aged 10 weeks and weighing 30–35 g were used. Mice were handled manually for one week to overcome handling stress prior to the onset of experiments. The animals were maintained in a dark/light cycle with free access to food and water. Group 1 was used as a control group and given free access to food and water. Group 2 was a positive HU group, injected PO intraperitoneally (250 mg/kg bw, daily at 8:00 am). The PO dosage and timing were determined as stated previously20. Group 3 was administered PO with an oral dose of allopurinol (ALP; 5 mg/kg bw daily, one hour after PO administration) for 10 days32. Group 4 was administered parsley at 7 g/kg bw orally as stated previously31. Group 5 was administered celery at 500 mg/kg bw orally as stated30. Groups 6 and 7 were administered PO at 8.00am, followed by PAR for group 6 and CEL for group 7 one hour later (9:00 am) for 10 days. Group 8 was administered PO at 8:00 am, followed by a combination of PAR and CEL at 9:00 am for 10 consecutive days. To overcome diethyl ether inhalation side effects, mice were fasted overnight then anaesthetized over 2 minutes using diethyl ether-soaked cotton in a 50 ml Falcon tube. Quickly, blood samples were taken from the eyes and the mice were then decapitated to collect further samples. Blood serum was stored at −20 °C; renal and hepatic tissue samples were preserved in Qiazol in anticipation of RNA extraction and gene expression analysis; and further kidney tissue samples were separated out for histopathology analysis and stored in 10% buffered neutral formalin.
Xanthine Oxidase activity
The kit used depends on the catalysis of hypoxanthine to form xanthine and superoxide anion free radicals. In the presence of chromogenic agent and electronic receptors, it will form a purplish-red substance that can be measured at the OD value of 530 nm. For liver tissues, homogenate in 1:9 normal saline was placed on ice, centrifuged for 10 minutes and the supernatant used for XO assay. The measurement unit for serum is U/l and for liver is U/g protein tissue. The protocol used for XO is a partially modified version of the method used by Haidari et al.31
Biochemical assays
Serum levels of the biomarkers specific to liver and kidney (GPT, GOT, uric acid, creatinine, BUN), cytokines (IL-1β, TNF-α and IL-10) and antioxidants (MDA, GSH, GPX and catalase) were measured using specific calorimetric commercial kits, following the relevant instruction manuals.
Gene expression and quantitative real time PCR (qRT-PCR)
Total RNA was extracted from the kidney and liver tissues. RNA integrity was confirmed as stated previously33. Total RNA was denatured in Bio-Rad T100 Thermal Cycle at 70 °C for 5 minutes and reverse transcribed33. For qRT-PCR analysis, specific primers (Table 1) were designed using real-time Taqman primer design tool (https://www.genscript.com/tools/real-time-pcr-taqman-primer-design-tool). Each PCR reaction consisted of 1.5 μl of 1 μg/μl cDNA, 10 μl SYBR Green PCR Master Mix (Quanti Tect SYBR Green PCR Kit, Qiagen), 1 μM of each forward and reverse primer for each gene, and nuclease-free water to a final volume of 20 μl. Reactions were run and analyzed on an Applied Bio-system 7500 Fast Real time PCR Detection system. qRT-PCR conditions were: first denatured at 95 °C for 10 minutes, followed by 40 cycles at 95 °C for 15 seconds (second denaturation), then 60 °C for 60 seconds (annealing and extension stage). Variations in gene expression and intensity of examined genes were calculated from the obtained cycle threshold (CT) values provided by the real-time PCR instrumentation using the comparative CT method to beta-actin as a reference.
Histological and immunohistochemistry analyses of kidney tissue
Kidney slices were dehydrated, embedded in paraffin and sectioned at 4 µm. Slides were stained with hematoxylin and eosin (H&E). Morphological changes were examined using a microscope (Nikon Eclipse 80i, Japan) and images were captured with a digital camera (Canon, SX620 HS - 20 MP, Japan). For immunohistochemistry, paraffin-embedded renal sections were deparaffinized, rehydrated and immersed in 2% H2O2 for 15 minutes (to inhibit peroxidase activity). Sections were then washed in phosphate buffer saline. Bovine serum albumin (5%) was used to block nonspecific binding sites. TGF-β1 polyclonal antibody was added for kidney slides in a dilution of 1:350 overnight at 4 °C. Slides were then incubated with secondary antibody after washing in PBS, developed with 3,3-diaminobezidine tetrahydrochloride then counterstained with hematoxylin. The percentage of positive immunoreactive cells for TGF-β1 was shown as the ratio of positively stained cells to the total cell number in the same field.
Statistical analysis
Data are expressed as means ± standard error for values collected from 7 mice per group. One-way ANOVA was used to analyze the data. The probability level P < 0.05 and the individual comparisons were obtained using Duncan’s multiple range tests for SPSS software version 12.5 for Windows (SPSS, IBM, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Ethical statement
All procedures used in this study were carried out based on the NIH Guide for the care and use of laboratory animals. All precautions were followed to minimize animal suffering throughout the experiments.
Results
Administration of parsley and celery ameliorated liver and kidney dysfunction in hyperuricemic mice
Administration of PO increased serum levels of GOT, GPT, uric acid and BUN in hyperuricemic (HU) mice compared to normal control mice. ALP, PAR and CEL treated HU mice exhibited decreased altered parameters (Table 2). Co-administration of PAR and CEL showed a greater ameliorative effect (P < 0.05) on normalization of assayed parameters (Table 2) compared with PAR or CEL alone in HU mice.
Administration of parsley and celery decreased serum and hepatic Xanthine Oxidase (XO) activity in hyperuricemic mice
As shown in Table 3, serum and liver activities of XO were increased in HU mice and were normalized significantly (P < 0.05) in the ALP, PAR and CEL administered group compared to oxonate administered mice. Co-administration of both PAR and CEL induced additive inhibition in XO activity compared to ALP treated HU mice.
Administration of parsley and celery ameliorated disorders in cytokine levels in hyperuricemic mice
Table 4 shows the changes in serum levels of IL-1β and TNF-α inflammatory cytokines, and IL-10 anti-inflammatory cytokine. Hyperuricemia induced a state of inflammation and significantly increased IL-1β and TNF-α levels (P < 0.05), while decreasing serum levels of IL-10. PAR and CEL administration ameliorated these effects. Co-administration of PAR and CEL induced a greater (P < 0.05) inhibitory effect on IL-1β and TNF-α, and a stimulatory effect on secretion of IL-10 (Table 4).
Antioxidant activities of parsley and celery against oxidative stress associated with hyperuricemia in mice
Hyperuricemia increased tissue degradation by increasing MDA levels in the HU group (Table 5). These increases in MDA were normalized by PAR and CEL treatment. Hyperuricemia decreased catalase, and GSH and GPX levels but these returned to nearly control levels after PAR and CEL administration (Table 5). Co-administration of PAR and CEL to HU mice induced an additive ameliorative effect on the changes induced in measured antioxidants (Table 5).
Impacts of PAR and CEL on mRNA expression of genes associated with renal hyperuricemia
We examined mRNA expression of mOAT-1, mOAT-3, mURTA-1 and mGlut9 genes responsible for urate excretion and reabsorption in the kidney. Figure 1 shows significant oxonate down-regulation in mRNA expression of mOAT-1 and mOAT-3, and significant (p < 0.05) up-regulation in mURAT-1 and mGlut-9 mRNA expression in HU mice kidneys compared with the control group. The alteration in mRNA expression of urate transporter-related genes was consistent with the elevation of serum uric acid and BUN levels reported in Table 2. PAR and CEL administration alone showed significant down-regulation in mURAT-1 and mGlut-9 and up-regulation in mOAT-1 and mOAT-3 mRNA expression (Figs. 1 and 2). The additive synergistic effect on altered genes was clear when both PAR and CEL were co-administered for the HU group.
Ameliorative Effects of parsley and celery on changes of mRNA expression of OAT-1 and OAT-3 in HU mice using quantitative real time PCR. Graphic presentation of renal mRNA of OAT-1 and OAT-3 in different groups of mice after normalization with beta actin. *p < 0.05 vs control group; #P < 0.05 vs HUR group and $P < 0.05 vs either HU + Parsley or HU + Celery groups.
Ameliorative Effects of parsley and celery on mRNA expression of mURAT-1 and mGlut-9 in HU mice using real time PCR. Graphic presentation of renal mRNA of mURAT-1 and mGlut-9 in different groups of mice after normalization with beta actin. *p < 0.05 vs control group; #P < 0.05 vs HUR group and $P < 0.05 vs either HU + parsley or HU + celery groups.
Impacts of PAR and CEL on mRNA expression of liver genes associated with uric acid metabolism
We examined mRNA expression of mice PNP and mice guanine Gda genes responsible for uric acid metabolism in the liver. As shown in Fig. 3, oxonate administration induced significant up-regulation in mPNP and mGda mRNA expression in HU mice (p < 0.05) compared to the control group. PAR and CEL regulated the alteration reported in HU groups. There was an additive synergistic effect for PAR and CEL when administered together to HU mice (Fig. 3).
Ameliorative Effects of parsley and celery on mRNA expression of mPNP and mGda HU mice using real time PCR. Graphic presentation of liver mRNA of mPNP and mGda in different groups of mice after normalization with beta actin. *p < 0.05 vs control group; #P < 0.05 vs HUR group and $P < 0.05 vs either HU + parsley or HU + celery groups.
Impacts of parsley and celery on renal histology and TGF-β1 immunoreactivity in hyperuricemic mice
Kidney histology
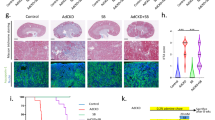
Control mice kidneys showed normal glomerular and tubular structure (Fig. 4A), whereas HU group kidneys showed a dense eosinophilic mass occluding the tubular lumina as well as leukocytic infiltration (Fig. 4B). Shrunken glomerular tufts, and periglomerular and interstitial (*) round cells infiltration were also observed. HU group kidneys treated with allopurinol showed normal glomerular architecture with normal tubular histology (Fig. 4C). Kidneys of parsley administered mice showed normal renal tissue with a normal tubular and glomerular picture (Fig. 4D). Kidneys of celery administered mice showed the normal histological picture of both glomerular and tubular sections (Fig. 4E). Kidneys of the HU group treated with parsley alone showed restoration of the normal picture with mild perivascular round cells infiltration (Fig. 4F). Kidneys of the HU group treated with celery showed restoration of glomerular and tubular tissue histology (Fig. 4G). Kidneys of the HU group treated with celery and parsley showed a normal histological picture of both glomerular and tubular tissue (Fig. 4H).
(A) Kidney of control group showing normal glomerular (arrow) and tubular (*) structure. (B) Kidney of HU group showed occlusion of tubular lumina by dense cell infiltration (arrow) and a shrinkage of glomerular tufts with periglomrular and interstitial (*) round cells infiltration. (C) Kidney of HU group treated with allopurinol showed normal glomerular architecture (arrow) with normal tubular histology (*). (D) Kidney of parsley administered mice showed normal renal tissue with normal tubular (arrow) and glomerular picture (*). Kidney of celery administered group showed the normal histological picture of both glomerular (arrow) and tubular (*) sections (F) Kidney of HU group treated with parsley alone showed restoration of normal picture with normal glomerular (thick arrow) and tubular (*) structure and mild perivascular round cells infiltration (thin arrow). (G) Kidney of HU group treated with celery showed restoration of glomerular (arrow) and tubular (*) tissue histology. H. Kidney of HU group treated with celery and parsley showed normal histological picture of both glomerular (arrow) and tubular (*) tissue with absence of urate crystals. Scale bar = 50 μm.
Immunoreactivity of renal TGF-β1
Kidneys of the control group showed an absence of TGF-β1 expression in renal tissue (Fig. 5A). Kidneys of the HU group showed high intensity and immunoreactivity for TGF-β1 in renal tubular tissue (Fig. 5B). Kidneys of the HU group treated with allopurinol showed no marked expression of TGF-β1 in renal tissue (Fig. 5C). Kidneys of parsley administered HU mice showed an absence of expression of TGF-β1 in renal tubular tissue (Fig. 5D). Kidneys of the celery group showed an absence of TGF-β1 expression in tubular tissue (Fig. 5E). Kidneys of the HU group treated with parsley alone showed no observed reactivity for TGF-β1 in renal tissue (Fig. 5F). Kidneys of the HU group treated with celery showed glomerular and tubular tissue with no TGF-β1 expression (Fig. 5G). Kidneys of the HU group treated with celery and parsley together showed more restoration in renal cells without expression of TGF-β1 (Fig. 5H). Table 6, shows particularly high intensity scores for TGF-β1 expression in the PAR and CEL administered HU groups.
(A) Kidney of control group showed absence of expression of TGF-β1 in renal tissue. (B) Kidney of HU group showed increased expression of TGF-β1 in renal tubular tissue. (C) Kidney of HU group treated with allopurinol showed no marked expression of TGF-β in renal tissue. (D) Kidney of parsley administered mice showed absence of expression of TGF-β1 in renal tubular tissue. (E) Kidney of celery group showed absence of TGF-β1 expression in tubular tissue. (F) Kidney of HU group treated with parsley alone showed no observed reactivity for TGF-β1 in renal tissue. (G) Kidney of HU group treated with celery showed glomerular and tubular tissue with no TGF-β1 expression. H. Kidney of HU group treated with celery and parsley together showed restoration of normal picture without expression of TGF-β1 in renal tissue. Scale bar = 50 μm.
Discussion
The results suggested that parsley and celery are safe herbal remedies that can be used either alone or in combination30,31 to lower the effects on serum levels of uric acid and xanthine oxidase activity in hyperuricemic mice. Hyperuricemia increases the production of oxygen free radicals, induces lipid peroxidation, and up-regulates inflammatory and down-regulates anti-inflammatory cytokine expression and secretion32,34,35. Herbal plants can increase antioxidant content in experimental animals and rodents24,36. The major functions of most flavonoids present in medicinal plants are their ability to scavenge free radicals and increase antioxidant activities37,38. As shown in previous reports, medicinal plants increased total antioxidant capacity, suppressed reactive oxygen species (ROS) and prevented damage induced by oxidative stress39,40. Here, parsley and celery showed the potency to improve and increase antioxidant activities, eliminate tissue destruction and reduce inflammatory effects of hyperuricemia.
A negative correlation between the levels of antioxidants and XO activity has been confirmed in patients with acute herbicide poisoning41. XO is the key enzyme responsible for catalytic synthesis of uric acid from xanthine and hypoxanthine42, and is responsible for ROS generation43. Consequently, higher amounts of ROS are generated alongside uric acid production43.
Therefore, the suppressive effects of parsley and celery on experimental hyperuricemia may be attributed to inhibition of oxidative stress. Deposition of urate crystals in the kidney and joints stimulates inflamed cells to produce IL-1β, which promotes the release of a series of inflammatory cytokines (TNF-α and IL-6)44, causing a state of general inflammation45. Furthermore, patients with hyperuricemia exhibit decreased levels of the anti-inflammatory cytokine, IL-1046. Clinical trials have also shown that gout is associated with elevated IL-1β47. These alterations in cytokine levels were ameliorated by PAR and CEL administration either alone or in combination; the combination effect was more effective.
In this study, parsley and celery reduced inflammatory cytokines (IL-1β and TNFα), enhanced serum antioxidant activities and eliminated pathological changes in the kidney. The results suggest that the effect of parsley and celery on IL-1β and TNF-α may be through the modulation of oxidative stress and the enhancement of antioxidant activities. Celery contains furocoumarins, flavonoids (apigenin), phenolic compounds and tannins48. The hyperuricemic and xanthine oxidase inhibitory activity of celery was investigated to a lesser extent. Lin et al.49 reported in vitro studies that apigenin interacts with XO in its active site.
Several transporter genes play critical roles in urate secretion and excretion during hyperuricemia. URAT1, a renal urate anion exchanger and an integral membrane protein found primarily in kidney, transports urate across the proximal convoluted tubules50,51. Its expression depends on the uric acid levels in the blood. mGlut-9 is another urate transporter that regulates urate transport through the proximal tubules52. OAT-1 and OAT-3 are localized in the proximal convoluted tubules (in the basolateral membrane)53. OAT-1 plays a role in the uptake and secretion of urate53. OAT-3 participates in the cellular uptake of urate and in urate secretion. URAT1, OAT-1 and OAT-3 have recently been considered the ideal targets for hyperuricemia treatment54. This study is the first to show that parsley and celery have the potential to regulate urate excretion associated genes (URAT1, GLUT-9, OAT-1 and OAT-3), either alone or in combination. PO administration significantly up-regulated mURAT1 and mGlut-9 expression, and down-regulated mOAT-1 and mOAT-3 expressions in mouse kidneys. Oxonate-induced urate reabsorption and reduced urate secretion is counteracted by parsley and celery, which, when co-administered, reduced the effect of disorders associated with hyperuricemia. Both PAR and CEL effectively cured hyperuricemia through: control of xanthine oxidase activity, control of inflammatory cytokines, increase in antioxidant activities and decrease in oxidative stress. Further, the genes responsible for urate transporter expression were controlled. These effects are illustrated in Fig. 6.
Conclusion
The effects of experimentally induced hyperuricemia were controlled and regulated through administering parsley and/or celery. These herbs are a safe and effective treatment and their effect is heightened when co-administered. Figure 6 shows the cellular, biochemical and molecular effects of the treatment.
Data availability
The data of the current study are available on reasonable request.
References
Li, L. et al. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 15, 122, https://doi.org/10.1186/1471-2369-15-122 (2014).
Lapi, F., Azoulay, L., Yin, H., Nessim, S. J. & Suissa, S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. Bmj 346, e8525, https://doi.org/10.1136/bmj.e8525 (2013).
Choi, H. K., Atkinson, K., Karlson, E. W., Willett, W. & Curhan, G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 350, 1093–1103, https://doi.org/10.1056/NEJMoa035700 (2004).
Zhu, J. N., Qi, X. Y., Tan, Y. & Lyu, X. H. Dietary Factors Associated with Hyperuricemia and Glycolipid Metabolism Disorder in Middle-aged and Elderly People. Sichuan Da Xue Xue Bao Yi Xue Ban. 47, 68–72 (2016).
Weaver, A. L. Epidemiology of gout. Cleve Clin. J. Med. 75(Suppl 5), S9–12, https://doi.org/10.3949/ccjm.75.suppl_5.s9 (2008).
de Oliveira, E. P. & Burini, R. C. High plasma uric acid concentration: causes and consequences. Diabetol. Metab. Syndr. 4, 12, https://doi.org/10.1186/1758-5996-4-12 (2012).
Rock, K. L., Kataoka, H. & Lai, J. J. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 9, 13–23, https://doi.org/10.1038/nrrheum.2012.143 (2013).
Maiuolo, J., Oppedisano, F., Gratteri, S., Muscoli, C. & Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 213, 8–14, https://doi.org/10.1016/j.ijcard.2015.08.109 (2016).
Bobulescu, I. A. & Moe, O. W. Renal transport of uric acid: evolving concepts and uncertainties. Adv. Chronic Kidney Dis. 19, 358–371, https://doi.org/10.1053/j.ackd.2012.07.009 (2012).
Jalal, D. I., Chonchol, M., Chen, W. & Targher, G. Uric acid as a target of therapy in CKD. Am. J. Kidney Dis. 61, 134–146, https://doi.org/10.1053/j.ajkd.2012.07.021 (2013).
Terkeltaub, R. Update on gout: new therapeutic strategies and options. Nat. Rev. Rheumatol. 6, 30–38, https://doi.org/10.1038/nrrheum.2009.236 (2010).
Al-Asmari, A. K., Athar, M. T. & Kadasah, S. G. An Updated Phytopharmacological Review on Medicinal Plant of Arab Region: Apium graveolens Linn. Pharmacogn. Rev. 11, 13–18, https://doi.org/10.4103/phrev.phrev_35_16 (2017).
Kooti, W. et al. The effect of hydro-alcoholic extract of celery on male rats in fertility control and sex ratio of rat offspring. 16, 43–49 (2014).
Popovic, M., Kaurinovic, B., Trivic, S., Mimica-Dukic, N. & Bursac, M. Effect of celery (Apium graveolens) extracts on some biochemical parameters of oxidative stress in mice treated with carbon tetrachloride. Phytother. Res. 20, 531–537, https://doi.org/10.1002/ptr.1871 (2006).
Lone, Z. A., Lone, Y., Khan, S. S., Wani, A. A. & Reshi, M. I. J. J. O. M. P. R. Hepatoprotective medicinal plants used Gond. Bhill tribals Dist. Raisen Madhya Pradesh, India. 9, 400–406 (2015).
Wu, S. Y. et al. An emerging translational model to screen potential medicinal plants for nephrolithiasis, an independent risk factor for chronic kidney disease. Evid. Based Complement. Altern. Med. 2014, 972958, https://doi.org/10.1155/2014/972958 (2014).
Kooti, W., Farokhipour, M., Asadzadeh, Z., Ashtary-Larky, D. & Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron. Physician 8, 1832–1842, https://doi.org/10.19082/1832 (2016).
Asadi-Samani, M., Kooti, W., Aslani, E. & Shirzad, H. A Systematic Review of Iran’s Medicinal Plants With Anticancer Effects. J. Evid. Based Complementary Altern. Med. 21, 143–153, https://doi.org/10.1177/2156587215600873 (2016).
Gauri, M., Ali, S. J. & Khan, M. S. J. I. A. I. M. A Rev. Apium graveolens Spec. Ref. Unani medicine. 2, 131–136 (2015).
Rahmat, A., Ahmad, N. S. S., Ramli, N. S. J. O. P. & Medicine, E. Parsley (Petroselinum crispum) supplementation attenuates serum uric acid level and improves liver and kidney structures in oxonate-induced hyperuricemic rats. 1–9 (2018).
Marin, I., Sayas-Barbera, E., Viuda-Martos, M., Navarro, C. & Sendra, E. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Organic Fennel, Parsley, and Lavender from Spain. Foods 5, https://doi.org/10.3390/foods5010018 (2016).
Wong, P. Y. & Kitts, D. D. J. Fc Stud. dual Antioxid. Antibact. Prop. parsley cilantro extracts. 97, 505–515 (2006).
Mafuvadze, B. et al. Apigenin prevents development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Sprague-Dawley rats. Cancer Prev. Res. 4, 1316–1324, https://doi.org/10.1158/1940-6207.Capr-10-0382 (2011).
Farzaei, M. H., Abbasabadi, Z., Ardekani, M. R., Rahimi, R. & Farzaei, F. Parsley: a review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 33, 815–826, https://doi.org/10.1016/s0254-6272(14)60018-2 (2013).
Kooti, W. et al. The effects of hydro-alcoholic extract of celery on lipid profile of rats fed a high fat diet. 8, 325-330 (2014).
Sowbhagya, H., Srinivas, P. & Krishnamurthy, N. J. F. C. Effect of enzymes on extraction of volatiles from celery seeds. 120, 230-234 (2010).
Kooti, W., Mansouri, E., Ghasemiboroon, M., Harizi, M. & Amirzargar, A. Protective effects of celery (Apium Graveolens) on testis and cauda epididymal spermatozoa in rat. Iran. J. Reprod. Med. 12, 365–366 (2014).
Marzouni, H. Z., Daraei, N., Sharafi-Ahvazi, N., Kalani, N. & Kooti, W. J. W. J. P. P. S. The effects of aqueous extract of celery leaves (Apium graveolens) on fertility in female rats. 5, 1710-1714 (2016).
Mencherini, T. et al. An extract of Apium graveolens var. dulce leaves: structure of the major constituent, apiin, and its anti-inflammatory properties. J. Pharm. pharmacology 59, 891–897, https://doi.org/10.1211/jpp.59.6.0016 (2007).
Dolati, K. et al. Inhibitory Effects of Apium graveolens on Xanthine Oxidase Activity and Serum Uric Acid Levels in Hyperuricemic Mice. Preventive Nutr. food Sci. 23, 127–133, https://doi.org/10.3746/pnf.2018.23.2.127 (2018).
Haidari, F., Keshavarz, S. A., Mohammad Shahi, M., Mahboob, S. A. & Rashidi, M. R. Effects of Parsley (Petroselinum crispum) and its Flavonol Constituents, Kaempferol and Quercetin, on Serum Uric Acid Levels, Biomarkers of Oxidative Stress and Liver Xanthine Oxidoreductase Aactivity inOxonate-Induced Hyperuricemic Rats. Iran. J. Pharm. research: IJPR 10, 811–819 (2011).
Billiet, L., Doaty, S., Katz, J. D. & Velasquez, M. T. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol. 2014, 852954, https://doi.org/10.1155/2014/852954 (2014).
Saad, D. Y., Soliman, M. M., Baiomy, A. A., Yassin, M. H. & El-Sawy, H. B. Effects of Karela (Bitter Melon; Momordica charantia) on genes of lipids and carbohydrates metabolism in experimental hypercholesterolemia: biochemical, molecular and histopathological study. BMC Complement. Altern. Med. 17, 319, https://doi.org/10.1186/s12906-017-1833-x (2017).
Balakumar, P., Sharma, R., Kalia, A. & Singh, M. J. C. H. R. Hyperuricemia: is it a risk factor for vascular endothelial dysfunction and associated cardiovascular disorders? 5, 1-6 (2009).
Kanellis, J. et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. 41, 1287–1293 (2003).
Kolarovic, J., Popovic, M., Zlinska, J., Trivic, S. & Vojnovic, M. Antioxidant activities of celery and parsley juices in rats treated with doxorubicin. Molecules 15, 6193–6204, https://doi.org/10.3390/molecules15096193 (2010).
Nickavar, B., Kamalinejad, M. & Izadpanah, H. In vitro free radical scavenging activity of five Salvia species. Pak. J. Pharm. Sci. 20, 291–294 (2007).
Yao, Y., Sang, W., Zhou, M. & Ren, G. Phenolic composition and antioxidant activities of 11 celery cultivars. J. Food Sci. 75, C9–13, https://doi.org/10.1111/j.1750-3841.2009.01392.x (2010).
Nguyen, M. T. et al. Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 27, 1414–1421, https://doi.org/10.1248/bpb.27.1414 (2004).
Zummah, A. & Martha, R. D. J. M. O. T. Antihyperurisemic Activity of Aqueous Celery Infusion by Xanthine Oxidase Enzyme Inhibition. 23, 131-136.
Zhang, J., Lv, G. & Zhao, Y. The significance of serum xanthine oxidase and oxidation markers in acute paraquat poisoning in humans. Clin. Biochem. 44, 221–225, https://doi.org/10.1016/j.clinbiochem.2010.09.006 (2011).
Ramallo, I. A., Zacchino, S. A., Furlan, R. L. J. P. A. A. I. J. O. P. C. & Techniques, B. A rapid TLC autographic method for the detection of xanthine oxidase inhibitors and superoxide scavengers. 17, 15-19 (2006).
Oguz, N., Kirca, M., Cetin, A. & Yesilkaya, A. Effect of uric acid on inflammatory COX-2 and ROS pathways in vascular smooth muscle cells. J. Recept. Signal. Transduct. Res. 37, 500–505, https://doi.org/10.1080/10799893.2017.1360350 (2017).
Sabina, E. P. & Rasool, M. An in vivo and in vitro potential of Indian ayurvedic herbal formulation Triphala on experimental gouty arthritis in mice. Vasc. Pharmacol. 48, 14–20, https://doi.org/10.1016/j.vph.2007.11.001 (2008).
Jeong, J. H. et al. CD14(+) Cells with the Phenotype of Infiltrated Monocytes Consist of Distinct Populations Characterized by Anti-inflammatory as well as Pro-inflammatory Activity in Gouty Arthritis. Front. Immunol. 8, 1260, https://doi.org/10.3389/fimmu.2017.01260 (2017).
Li, S. et al. Antigouty arthritis and antihyperuricemia properties of celery seed extracts in rodent models. Mol. Med. Rep. 20, 4623–4633, https://doi.org/10.3892/mmr.2019.10708 (2019).
Zeng, M. et al. IL-37 inhibits the production of pro-inflammatory cytokines in MSU crystal-induced inflammatory response. Clin. Rheumatol. 35, 2251–2258, https://doi.org/10.1007/s10067-015-3109-5 (2016).
Kooti, W., Daraei, N. J. J. O. E.-b. C. & Medicine, A. A review of the antioxidant activity of celery (Apium graveolens L). 22, 1029-1034 (2017).
Lin, C. M., Chen, C. S., Chen, C. T., Liang, Y. C. & Lin, J. K. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 294, 167–172, https://doi.org/10.1016/s0006-291x(02)00442-4 (2002).
Li, S. et al. Antigouty arthritis and antihyperuricemia properties of celery seed extracts in rodent models. Mol Med Rep, https://doi.org/10.3892/mmr.2019.10708 (2019).
Enomoto, A. et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417, 447–452, https://doi.org/10.1038/nature742 (2002).
Vitart, V. et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 40, 437–442, https://doi.org/10.1038/ng.106 (2008).
Ichida, K. et al. Urate transport via human PAH transporter hOAT1 and its gene structure. Kidney Int. 63, 143–155, https://doi.org/10.1046/j.1523-1755.2003.00710.x (2003).
So, A. & Thorens, B. Uric acid transport and disease. J. Clin. Invest. 120, 1791–1799, https://doi.org/10.1172/jci42344 (2010).
Acknowledgements
All authors are greatly appreciate Dr. Morgan G., for his help in revising and language editing for the current paper. The study was self-supported using authors’ expenses.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to finish this finished work: M.M.S. and M.A.N. were responsible for the conception and design of the experiments; F.A., M.A.N. and M.M.S. undertook the experiments; W.A.M. and M.M.S. analyzed the data; W.A.M. undertook the biochemical assays; M.A.N. performed the histopathology; M.M.S. was responsible for the gene expression; and A.A. and F.A. undertook the data interpretation. M.M.S. wrote and interpret all data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soliman, M.M., Nassan, M.A., Aldhahrani, A. et al. Molecular and Histopathological Study on the Ameliorative Impacts of Petroselinum Crispum and Apium Graveolens against Experimental Hyperuricemia. Sci Rep 10, 9512 (2020). https://doi.org/10.1038/s41598-020-66205-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66205-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.