Abstract
Abiotic synthesis of biomolecules is an essential step for the chemical origin of life. Many attempts have succeeded in synthesizing biomolecules, including amino acids and nucleobases (e.g., via spark discharge, impact shock, and hydrothermal heating), from reduced compounds that may have been limited in their availabilities on Hadean Earth and Noachian Mars. On the other hand, formation of amino-acids and nucleobases from CO2 and N2 (i.e., the most abundant C and N sources on Earth during the Hadean) has been limited via spark discharge. Here, we demonstrate the synthesis of amino acids by laboratory impact-induced reactions among simple inorganic mixtures: Fe, Ni, Mg2SiO4, H2O, CO2, and N2, by coupling the reduction of CO2, N2, and H2O with the oxidation of metallic Fe and Ni. These chemical processes simulated the possible reactions at impacts of Fe-bearing meteorites/asteroids on oceans with a CO2 and N2 atmosphere. The results indicate that hypervelocity impact was a source of amino acids on the Earth during the Hadean and potentially on Mars during the Noachian. Amino acids formed during such events could more readily polymerize in the next step of the chemical evolution, as impact events locally form amino acids at the impact sites.
Similar content being viewed by others
Introduction
The composition of early Earth’s atmosphere has been a subject of discussion. The atmosphere was once regarded as strongly reduced, composed mostly of CH4, NH3, and H21. Since approximately 40 years ago, a CO2-N2 dominated neutral atmosphere which was equilibrated to oxidized silicate mantle/crust, has been favored2,3,4,5. More recently, addition of reduced species to the atmosphere by the late veneer and the late heavy bombardment (LHB) have been proposed6,7,8,9,10,11. Although such local events might have provided reduced volatiles, the quantity of reduced species provided to the atmosphere remained unclear.
Initiated by Miller’s 1953 experiment, the formation of amino acids and nucleobases by spark discharge from reduced reactants (e.g., CO, CH4, and H2) has long been investigated as a potential source of the building blocks of life12,13. The yields of amino acids and other organic compounds are significantly sensitive to the amounts of reduced compounds13. The formation of amino acids from non-reduced species (i.e., CO2, N2, and H2O) by spark discharge has been proposed14,15. The formation reported in Plankensteiner et al.14 is coupled by the oxidation of Cu electrode, which is not available in natural spark discharge in the atmosphere. Spark-discharge with carbonate buffer reported by Cleaves et al.15 might have worked as a source of prebiotic amino acid, although ascorbate hydrolysis used in the work was challenged by a subsequent spark discharge study as a source of amino acid contamination16. Therefore, an experimentally-supported geological event that synthesizes amino acids is limited in spark discharge on neutral ocean. For other methods, such as shock-heating of the atmosphere, photochemical reactions, and proton irradiation, studies have reported that the formation of amino acids were limited to experiments using reduced C and N sources12,13,17,18,19,20,21,22. Formation of nucleobases is more significantly limited to experiments using reduced C and N sources21,23,24,25. They have not, however, been established using CO2 and N2, which were major terrestrial sources of C and N during the Hadean. A previous work reported amino acid formation by laser irradiation to CO2-N2-H2O without reductants26. However, it is not clear whether the detected amino acids were products or contaminants.
Several studies propose a high CO2 partial pressure for the early Hadean2,3,27. This suggestion leads to the possibility that concentrations of dissolved inorganic carbon, e.g., HCO3−, in the Hadean oceans were most likely much higher than of those in today’s oceans. Several sources of NH3 during the Hadean have been proposed. Most of these previous studies investigated the reduction of nitrogen oxides by Fe-sulfides, metallic Fe, or Fe2+ in rocks or ocean28,29,30,31. Even considering these sources, models suggest that there were significantly low NH3 concentrations in the oceans (e.g., 10−5 mol/L)32. Formation of organic compounds around hydrothermal vents is a popular model for an endogenous organic source. However, the formation of amino acids has only been demonstrated in experiments using fluid analogues containing reduced C and reduced N compounds (e.g., formaldehyde, NH3, and HCN) in extremely high concentrations19,33. Amino-acid synthesis has not been shown using non-reduced compounds, which were common in hydrothermal fluids34. Furthermore, the chemical equilibrium at high temperatures that are characteristic of hydrothermal vents has been found to favor amino-acid degradation rather than synthesis34.
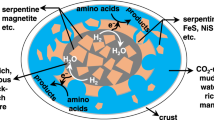
Lunar crater records suggest intense impacts of meteorites and asteroids on the Hadean Earth35. Hypervelocity impacts of meteorites and asteroids on water generated post-impact vapor plumes and induced reactions between projectiles, water, and the atmosphere9,36. Nakazawa et al.37 and Nakazawa38 proposed that post-impact reactions, which were generated by Fe-bearing projectiles, formed organic compounds essential for life. Both experimental and theoretical studies have indicated that impacts of Fe-bearing asteroids and meteorites reduced the surrounding atmosphere and formed a variety of reduced compounds (e.g., CO, NH3, and HCN)8,10,37,39. Furthermore, other studies have demonstrated the formation of amino acids and nucleobases in impact-induced reactions between Fe-bearing meteorite analogues, H2O, NH3, HCO3− or solid C, and gaseous N220,21. These experiments are demonstrations of the synthesis of amino acids using non-reduced carbon sources (i.e., solid C and HCO3−) and a reduced nitrogen source (i.e., NH3). Metallic Fe worked as a reductant and a catalyst in organic synthesis in these experiments. An catalytic effect by metallic Fe-Ni on reactions between organic compounds, on impact, was suggested by a previous work40. Among cataloged meteorites, >85% were found to contain significant amounts of metallic Fe41. For example, H chondrite, which is the most common type of ordinary chondrite, has been found to contain ~10 vol% of metals42. The impact synthesis model is consistent with a model recently proposed by Benner and co-workers in which large single impactor reduced surrounding materials and formed organic compounds43.
We conducted laboratory shock-recovery experiments to investigate impact-induced reactions between a meteorite analogue, oceanic components, and the atmosphere. The experimental setup and analytical procedures are described elsewhere21. Mixed powders of forsterite (Mg2SiO4; 200 mg), metallic Fe (100 mg), and metallic Ni (10 mg) were used as an ordinary chondrite (OC) analogue whereas mixed powders of metallic Fe (300 mg) and metallic Ni (10 mg) were used as an iron meteorite (IM) analogue (Table S1). The impact velocity was ~0.9 km/s, which generated a shockwave of ~7 GPa for 0.7 μsec. The temperature during the shock compression was ~300 °C. Post-shock temperature was estimated to be ~1500 °C44. NaHCO3 was used as the C source. Dissolved HCO3− and CO2 were generated from NaHCO3 before and during the shock-induced reactions, and these represent the C species in the ocean and atmosphere, respectively. Carbon in NaHCO3 was labeled using 13C to distinguish products from potential contaminants. Gaseous N2 was used as a N source to simulate atmospheric N for all experiments. NH3 was mixed with the starting materials in several experiments for comparison. 13C-labeled amino acids in the products were analyzed by liquid chromatography tandem mass spectrometry.
13C-labeled amino acids (glycine and alanine) were synthesized in the shock-recovery experiment from the IM analogue, H2O, bicarbonate and N2 (Fig. 1). Glycine was also formed using the OC analogue in place of the IM analogue (Fig. S1). The molar conversion rates from gaseous nitrogen to amino acids were ~1 × 10−4% in both experiments. Six kinds of amino acids (glycine, alanine, β-alanine, α-amino butyric acid, β-amino-iso-butyric acid, and sarcosine) were formed in the IM and OC experiments containing 2 mol/L NH3 (Figs. 2, S2 and S3). Molar conversion rates from gaseous nitrogen N to amino acids were ~1 × 10−3% in both the IM and OC experiments. In the starting materials, N speciation was 87 mol% NH3 and 13 mol% N2. The conversion rates of these experiments were one order of magnitude higher than that of the NH3-free experiments, in which all N in the starting materials was N2. In the experiments containing 20 mmol/L NH3, in which 94 mol% of N in the starting materials was N2, the conversion rate was comparable to the NH3-free experiments.
Multiple reaction monitoring (MRM) chromatogram of derivatized standards and the derivatized product of NH3-free experiment with the iron meteorite (IM) analogue. (a) 12C-glycine (Gly) (mass-to-charge ratio (m/z) = 246 > 171) and product, 13C-glycine (13C-Gly) (m/z = 248 > 171). (b) 12C-alanine (Ala), 12C-β-alanine (β-Ala), and sarcosine (Sar) (m/z = 260 > 171) and product.
Amounts of amino acids (glycine, Gly; alanine, Ala; β-alanine, β-Ala; α-amino butyric acid, α-ABA; β-amino-iso-butyric acid, β-AIBA; sarcosine, Sar) produced in the iron meteorite (IM) and ordinary chondrite (OC) experiments (NH3-free, 20 mmol/L NH3 added, 2 mol/L NH3 added). Experiments were conducted three times for each condition and 1σ errors are shown.
More amino acids formed in the IM analogue experiment than in the experiment that used the OC analogue. The IM analogue contained three times more metallic Fe than the OC analogue and did not contain silicates. In the experiments using the IM analogue, metallic Fe was still the major mineral component after impact, although part of the Fe was oxidized to form FeCO3, i.e., siderite (Fig. 3a,b). Partial oxidation of Fe and the formation of siderite were also found in the experiments using the OC analogue (Fig. 3c,d). The effects of NH3 concentrations were negligible on siderite formation.
A part of NaHCO3 was dissolved in water and was presented as HCO3− in the starting materials. A previous ab initio molecular dynamics calculation showed the formation of formic acid (HCOOH) from HCO3− and H with metallic Fe oxidation after several picoseconds under high pressure and high temperature conditions generated by a 5 km/s meteorite impact45. In the present experiments, the duration of shock compression was >100 times longer than the calculation, and further HCO3− reduction most likely formed formaldehyde (H2CO). Formaldehyde condenses to form larger aldehydes in alkaline solution46 and formation of various amino acids from aldehydes and ammonia have been well known47,48. In this type of amino acid synthesis, smaller amino acids form in larger yields and therefore larger amino acids would not be formed in detectable amounts in this small-scale simulation. More amino acids might have been formed if the starting martials were larger enough like natural impacts.
The formation of NH3 in impact-induced reactions with metallic Fe has been shown in both laboratory and numerical simulations37,39. In the cooling period, amino acids would be formed from aldehydes with the involvement of NH3 since amino acids are unstable at high temperatures, typically >300 °C (see Supplementary Information). Higher yields of simpler amino-acids are consistent with this formation via mono-carbon compounds such as formaldehyde (i.e., higher yields of glycine than alanine; Fig. 2). Similar yields of glycine in NH3-free experiments and 20 mM NH3 experiment suggest that NH3 formed during the impact-induced reactions worked similarly to the ammonia in the starting material. Thus, the 10−5 mol/L oceanic ammonia concentration suggested in a previous work may not provide a significant difference in amino acid synthesis from an NH3-free ocean in such a type of impact synthesis.
Abiotic syntheses of various organic compounds, including amino acids, have been successful in previous impact experiments when reduced forms of C and N were used as starting materials17,21. However, such reduced species were not the most likely major components of the early Earth’s atmosphere and ocean, and their availability is unclear. Formation of amino acids from non-reduced C and N sources, which were abundant on Earth during the Hadean, proposes an additional source other than the reactions associated with spark discharge (Figs. 2a and S1).
In natural meteorite/asteroid impacts, small projectiles less than 20 m tend to be broken in air and do not impact on the ground surface with hypervelocity (i.e., more than several km/sec)49. Projectiles larger than 100 m radius are expected to impact the ground surface with an initial velocity (i.e., mostly more than 11 km/sec) and these impactors are expected to be crashed significantly by the impact and react with terrestrial materials. Projectiles having 20 to 100 m radius have various impact velocities. Further, temperature profile of materials in an impactor is not homogeneous through the impact process; it differs significantly depending on its location in the impactor50. The temperature and pressure profile of the present impact would be applicable to some part of materials in the middle scale projectiles having 20 to 100 m radius.
Furthermore, the organic synthesis coupled with meteoritic Fe oxidation in impacts would be applicable to larger impactors. Larger impacts tend to be by iron-rich meteorites51. There was an incomplete oxidation of Fe followed by a limited formation of reactive reduced species (e.g., NH3, CO, H2, and formaldehyde) in the laboratory simulation due to limited impact conditions. In natural hypervelocity impacts, on the other hand, far greater amounts of shock heating are expected and this might have reduced more nitrogen and carbon. The organic synthesis might have been more significant, yielding a variety of amino acids in higher yields. This is supported by the higher yields and larger variations of amino acids in a higher ammonia concentration experiment that yielded six kinds of amino acids (Fig. 2).
The amounts of extraterrestrial objects accreted during the LHB period has been estimated as 4 × 1023 g52, and the contents of metallic Fe in OCs (e.g., H chondrites) are ~10 wt%42. Therefore, a large amount of metallic Fe was provided by impacts. In the present study, reduction of Fe, followed by the formation of reduced species, initiated the formation of amino acids. The conversion of the consumed Fe to amino acid was 2 × 10−5 wt% and N2 to amino acid was 1 × 10−4 atm% in the present NH3-free OC analogue experiment. The amount of amino acid production by the Hadean impacts would not be small, considering the huge Fe flux during the late Hadean (~4 × 1022 g, assuming 10 wt% of total LHB amount) and almost infinite amounts of CO2 and N2 (e.g., 1021 g on the modern Earth or might have been half of the modern level) in atmosphere of the Earth at that time3,27,53. It is difficult to precisely estimate the amounts of amino acid products generated via natural impacts with the present experiments due to technical difficulties in the demonstration of large impacts, which are expected to yield more amino acids. Alternatively, the present yield would be a baseline amount along with variation.
Extraterrestrial delivery is another source of the building blocks of life on prebiotic Earth54,55,56. Many amino acids have been detected from meteorites57,58. However, the amounts of the delivery remained unclear, because the composition of the building blocks of life in different carriers, particularly micrometeorites that are regarded as the largest carrier, remained unclear52,59,60. Further investigations regarding both endogenous formation and exogenous delivery are indispensable for understanding the availability of the building blocks of life on prebiotic Earth.
Since impact events occur locally, produced organic compounds distribute locally around the site of the impacts. This may have advantage in subsequent chemical evolution, since enrichment is one of the most significant problem in chemical evolution of polymerization38,43,61.
Impact-induced amino-acid formation might have been possible on Noachian Mars. The high intensity of crater distribution on Martian Noachian highland surface suggests the presence of intense impacts on Mars before 3.7 billion years ago62,63. A considerable amount of geological and geophysical evidence indicates a wide distribution of liquid water on Noachian Mars63,64 (see Supplementary Information). Atmospheric modeling combined with geological data has suggested that the major atmospheric components of Noachian Mars were CO2 and N2 with minor amounts of reduced species (see Supplementary Information). Therefore, the results of the present study further suggest that Noachian impact events synthesized abiotic organic matter including amino acids on Mars. Complex organic matter found by Mars Science Laboratory in ~3.5 Ga sediments support this implication65. Thus, chemical evolution might have been promoted and accomplished to the formation of monomers of catalytic biopolymers even on Noachian Mars.
Materials and Methods
Materials
Meteorite analogues were composed of metallic Fe (99.9 wt%, powder <45 μm in diameter; Wako), metallic Ni (99.95 wt%, sponge; Wako), and natural forsterite (Mg2SiO4) from Myanmar, which were ground and heat-treated at 450 °C for 6 h prior to use. These powders were mixed using an agate mortar and pestle. NaH13CO3 was prepared with commercial 13C-labeled CO2 (Cambridge Isotope Lab) and NaOH (Wako). Meteorite analogue material was contained in a sample container with purified water (distilled and further purified with MilliQ; 18.2 MΩ), NaH13CO3, and gaseous N (>99.995%). Details of the sample alignments are presented elsewhere21. All glassware for the experiments were washed and heated at 450 °C for 6 h prior to use. Sample containers were washed with water, methanol, and dichloromethane repeatedly before use.
Shock-recovery experiments
The shock recovery experiments were conducted at the National Institute for Materials Science, Japan, using a 5 m long single stage propellant gun. Shockwaves were generated by the impacts between a 1 km/sec accelerated SUS304 disk and the sample container made of SUS304L. Detailed temperature and pressure profiles were similar to those reported in a previous study44.
Sample analysis
After impact, the sample container was trimmed and washed repeatedly with water, methanol, and dichloromethane. The container was then cooled with liquid nitrogen, and holes were made as a means of accessing the sample cavity. The frozen container was immersed with water and soluble organic compounds were extracted after the ice melted. The supernatant was dried and analyzed using ultrahigh performance liquid chromatography tandem mass spectrometry (Shimadzu LCMS-8040; UHPLC/MSMS) after derivatization with AccQ-Tag reagent (Waters). Details of the UHPLC/MSMS conditions are described elsewhere21. The mineral composition was evaluated via a powder X-ray diffractometer (Philips) as described elsewhere21.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
References
Urey, H. C. On the early chemical history of the earth and the origin of life. Proc. Natl. Acad. Sci. 38, 351–363 (1952).
Abe, Y. & Matsui, T. Evolution of an impact-generated H2O–CO2 atmosphere and formation of a hot proto-ocean on Earth. J. Atmos. Sci. 45, 3081–3101 (1988).
Kasting, J. F. Bolide impacts and the oxidation state of carbon in the Earth’s early atmosphere. Orig. Life Evol. Biosph. 20, 199–231 (1990).
Trail, D., Watson, E. B. & Tailby, N. D. The oxidation state of Hadean magmas and implications for early Earth’s atmosphere. Nature 480, 79–82 (2011).
Kasting, J. F. in Earth’s Early Atmosphere and Surface Environment Vol. 504 Geological Society of America Special Papers (ed G. H. Shaw) 19–28 (2014).
Sekine, Y. et al. An experimental study on Fischer-Tropsch catalysis: implications for impact phenomena and nebular chemistry. Meteorit. Planet. Sci. 41, 715–729 (2006).
Hashimoto, G. L., Abe, Y. & Sugita, S. The chemical composition of the early terrestrial atmosphere: formation of a reducing atmosphere from CI-like material. J. Geophys. Res. Planets 112, 12 (2007).
Schaefer, L. & Fegley, B. Chemistry of atmospheres formed during accretion of the Earth and other terrestrial planets. Icarus 208, 438–448 (2010).
Kurosawa, K. et al. Hydrogen cyanide production due to mid-size impacts in a redox-neutral N2-rich atmosphere. Orig. Life Evol. Biosph. 43, 221–245 (2013).
Furukawa, Y., Samejima, T., Nakazawa, H. & Kakegawa, T. Experimental investigation of reduced volatile formation by high-temperature interactions among meteorite constituent materials, water, and nitrogen. Icarus 231, 77–82 (2014).
Yang, X., Gaillard, F. & Scaillet, B. A relatively reduced Hadean continental crust and implications for the early atmosphere and crustal rheology. Earth Planet. Sci. Lett. 393, 210–219 (2014).
Miller, S. L. A production of amino acids under possible primitive earth conditions. Science 117, 528–529 (1953).
Schlesinger, G. & Miller, S. L. Prebiotic synthesis in atmospheres containing CH4, CO, and CO2. 1. Amino acids. J. Mol. Evol. 19, 376–382 (1983).
Plankensteiner, K., Reiner, H., Schranz, B. & Rode, B. M. Prebiotic formation of amino acids in a neutral atmosphere by electric discharge. Angew. Chem. Int. Ed. 43, 1886–1888 (2004).
Cleaves, H. J., Chalmers, J. H., Lazcano, A., Miller, S. L. & Bada, J. L. A reassessment of prebiotic organic synthesis in neutral planetary atmospheres. Orig. Life Evol. Biosph. 38, 105–115 (2008).
Kuwahara, H. et al. The use of ascorbate as an oxidation inhibitor in prebiotic amino acid synthesis: a cautionary note. Orig. Life Evol. Biosph. 42, 533–541 (2012).
Barnun, A., Barnun, N., Bauer, S. H. & Sagan, C. Shock synthesis of amino acids in simulated primitive environments. Science 168, 470–472 (1970).
Kobayashi, K. et al. In Space Life Sciences: Life in the Solar System: Prebiotic Chemistry, Chirality and Space Biology Vol. 27 Advances in Space Research (eds F. Raulin et al.) 207–215 (Elsevier Science Bv, 2001).
Huber, C. & Wächtershäuser, G. α-hydroxy and α-amino acids under possible Hadean, volcanic origin-of-life conditions. Science 314, 630–632 (2006).
Furukawa, Y., Sekine, T., Oba, M., Kakegawa, T. & Nakazawa, H. Biomolecule formation by oceanic impacts on early Earth. Nat. Geosci. 2, 62–66 (2009).
Furukawa, Y., Nakazawa, H., Sekine, T., Kobayashi, T. & Kakegawa, T. Nucleobase and amino acid formation through impacts of meteorites on the early ocean. Earth Planet. Sci. Lett. 429, 216–222 (2015).
Botta, L. et al. Prebiotic synthesis of carboxylic acids, amino acids and nucleic acid bases from formamide under photochemical conditions. Eur. Phys. J. 132 (2017).
Saladino, R. et al. Meteorite-catalyzed syntheses of nucleosides and of other prebiotic compounds from formamide under proton irradiation. Proc. Natl. Acad. Sci. USA 112, E2746–E2755 (2015).
Ferus, M. et al. High-energy chemistry of formamide: A unified mechanism of nucleobase formation. Proc. Natl. Acad. Sci. USA 112, 657–662 (2015).
Ferus, M. et al. Formation of nucleobases in a Miller–Urey reducing atmosphere. Proc. Natl. Acad. Sci. USA 114, 4306–4311 (2017).
Civiš, S. et al. Amino acid formation induced by high-power laser in CO2/CO-N2-H2O gas mixtures. Chem. Phys. Lett. 386, 169–173 (2004).
Kasting, J. F. Earth’s early atmosphere. Science 259, 920–926 (1993).
Summers, D. P. & Chang, S. Prebiotic ammonia from reduction of nitrite by iron (II) on the early Earth. Nature 365, 630–633 (1993).
Brandes, J. A. et al. Abiotic nitrogen reduction on the early Earth. Nature 395, 365–367 (1998).
Smirnov, A., Hausner, D., Laffers, R., Strongin, D. R. & Schoonen, M. A. A. Abiotic ammonium formation in the presence of Ni-Fe metals and alloys and its implications for the Hadean nitrogen cycle. Geochem. Trans. 9 (2008).
Summers, D. P., Basa, R. C. B., Khare, B. & Rodoni, D. Abiotic nitrogen fixation on terrestrial planets: reduction of NO to ammonia by FeS. Astrobiology 12, 107–114 (2012).
Summers, D. Sources and sinks for ammonia and nitrite on the early Earth and the reaction of nitrite with ammonia. Orig. Life Evol. Biosph. 29, 33–46, https://doi.org/10.1023/A:1006517823004 (1999).
Marshall, W. L. Hydrothermal synthesis of amino acids. Geochim. Cosmochim. Acta 58, 2099–2106 (1994).
Aubrey, A. D., Cleaves, H. J. & Bada, J. L. The role of submarine hydrothermal systems in the synthesis of amino acids. Orig. Life Evol. Biosph. 39, 91–108 (2009).
Culler, T. S., Becker, T. A., Muller, R. A. & Renne, P. R. Lunar impact history from 40Ar/39Ar dating of glass spherules. Science 287, 1785–1788 (2000).
Sugita, S. & Schultz, P. H. Interactions between impact-induced vapor clouds and the ambient atmosphere: 1. Spectroscopic observations using diatomic molecular emission. J. Geophys. Res. Planets 108, 11 (2003).
Nakazawa, H., Sekine, T., Kakegawa, T. & Nakazawa, S. High yield shock synthesis of ammonia from iron, water and nitrogen available on the early Earth. Earth Planet. Sci. Lett. 235, 356–360 (2005).
Nakazawa, H. Darwinian Evolution of Molecules. (Springer Singapore, 2018).
Shimamura, K., Shimojo, F., Nakano, A. & Tanaka, S. Meteorite impact-induced rapid NH3 production on early Earth: Ab Initio molecular dynamics simulation. Sci. Rep. 6 (2016).
Ferus, M. et al. On the road from formamide ices to nucleobases: IR-spectroscopic observation of a direct reaction between cyano radicals and formamide in a high-energy impact event. J. Am. Chem. Soc 134, 20788–20796 (2012).
Norton, O. R. in The Cambridge Encycropedia of Meteorites (ed O. R. Noton) 331–340 (Cambridge University Press, 2001).
Brearley, A. J., Jones, R. H. & Papike, J. J. In Planetary Materials Vol. 36 Reviews in Mineralogy (eds A. J. Brearley, R. H. Jones, & J. J. Papike) C1 (1998).
Benner, S. A. et al. When did life likely emerge on earth in an RNA-first process? ChemSystemsChem (2020).
Furukawa, Y., Sekine, T., Kakegawa, T. & Nakazawa, H. Impact-induced phyllosilicate formation from olivine and water. Geochim. Cosmochim. Acta 75, 6461–6472 (2011).
Shimamura, K., Shimojo, F., Nakano, A. & Tanaka, S. Ab initio molecular dynamics study of prebiotic production processes of organic compounds at meteorite impacts on ocean. J. Comput. Chem 40, 349–359 (2019).
Breslow, R. On the mechanism of the formose reaction. Tetrahedron Lett. 1, 22–26 (1959).
Yanagawa, H., Kobayashi, Y. & Egami, F. Genesis of amino acids in the primeval sea: formation of amino acids from sugars and ammonia in a modified sea medium. J. Biochem. 87, 359–362 (1980).
Kebukawa, Y., Chan, Q. H. S., Tachibana, S., Kobayashi, K. & Zolensky, M. E. One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity. Sci. Adv. 3, e1602093 (2017).
Hills, J. G. & Goda, M. P. The fragmentation of small asteroids in the atmosphere. Astrophys. J. 105, 1114–1144 (1993).
Pierazzo, E. & Chyba, C. F. Amino acid survival in large cometary impacts. Meteorit. Planet. Sci. 34, 909–918 (1999).
Pasek, M. & Lauretta, D. Extraterrestrial flux of potentially prebiotic C, N, and P to the early Earth. Orig. Life Evol. Biosph. 38, 5–21 (2008).
Anders, E. Pre-biotic organic-matter from comets and asteroids. Nature 342, 255–257 (1989).
Som, S. M. et al. Earth’s air pressure 2.7 billion years ago constrained to less than half of modern levels. Nat. Geosci. 9, 448–451 (2016).
Cronin, J. R. & Moore, C. B. Amino acid analyses of Murchison, Murray, and Allende carbonaceous chondrites. Science 172, 1327–1329 (1971).
Martins, Z. et al. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. Lett. 270, 130–136 (2008).
Furukawa, Y. et al. Extraterrestrial ribose and other sugars in primitive meteorites. Proc. Natl. Acad. Sci. USA 116, 24440–24445 (2019).
Glavin, D. P., Callahan, M. P., Dworkin, J. P. & Elsila, J. E. The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 45, 1948–1972 (2011).
Burton, A. S., Stern, J. C., Elsila, J. E., Glavin, D. P. & Dworkin, J. P. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 41, 5459–5472 (2012).
Chyba, C. & Sagan, C. Endgenous production, exogenous delivery and impact-shock synthesis of organic-molecules: an inventory for the origins of life. Nature 355, 125–132 (1992).
Glavin, D. P., Matrajt, G. & Bada, J. L. Re-examination of amino acids in Antarctic micrometeorites. Adv. Space Res. 33, 106–113 (2004).
Cleaves, H. J., Aubrey, A. D. & Bada, J. L. An evaluation of the critical parameters for abiotic peptide synthesis in submarine hydrothermal systems. Orig. Life Evol. Biosph. 39, 109–126 (2009).
Hartmann, W. K. & Neukum, G. Cratering chronology and the evolution of Mars. Space Sci. Rev. 96, 165–194 (2001).
Carr, M. H. & Head, J. W. Geologic history of Mars. Earth Planet. Sci. Lett. 294, 185–203 (2010).
Head, J. W. et al. Possible ancient oceans on mars: evidence from Mars orbiter laser altimeter data. Science 286, 2134–2137 (1999).
Eigenbrode, J. L. et al. Organic matter preserved in 3-billion-year-old mudstones at Gale crater, Mars. Science 360, 1096–1101 (2018).
Acknowledgements
The authors appreciate the comments received from H. Nakazawa and A. Ishida as well as discussions with them. This work was supported by KAKENHI from Japan Society for the Promotion of Science to T. K. (15H02144 and 18H03729), NINS Astrobiology Center satellite research for Y. F., and the Tohoku University FRIS research program for T. K. and Y. F.
Author information
Authors and Affiliations
Contributions
Y.F. and T.S. designed the experiments. Y.T., T. Kobayashi, and Y.F. conducted the experiments. Y.T. analyzed the samples. Y.T. and Y.F. analyzed the data. Y.F. wrote the initial manuscript. T. Kakegawa and N.T. contributed to the discussion on early Earth and early Mars.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takeuchi, Y., Furukawa, Y., Kobayashi, T. et al. Impact-induced amino acid formation on Hadean Earth and Noachian Mars. Sci Rep 10, 9220 (2020). https://doi.org/10.1038/s41598-020-66112-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66112-8
This article is cited by
-
Boron-assisted abiotic polypeptide synthesis
Communications Chemistry (2023)
-
Synthesis of prebiotic organics from CO2 by catalysis with meteoritic and volcanic particles
Scientific Reports (2023)
-
The First Nucleic Acid Strands May Have Grown on Peptides via Primeval Reverse Translation
Acta Biotheoretica (2023)
-
Extraterrestrial Life Signature Detection Microscopy: Search and Analysis of Cells and Organics on Mars and Other Solar System Bodies
Space Science Reviews (2022)
-
Frontiers in Prebiotic Chemistry and Early Earth Environments
Origins of Life and Evolution of Biospheres (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.