Abstract
Oceans are vast, dynamic, and complex ecosystems characterized by fluctuations in environmental parameters like sea surface temperature (SST), salinity, oxygen availability, and productivity. Environmental variability acts as the driver of organismal evolution and speciation as organisms strive to cope with the challenges. We investigated the evolutionary consequences of heterogeneous environmental conditions on the mitogenome of a widely distributed small pelagic fish of Indian ocean, Indian oil sardine, Sardinella longiceps. Sardines were collected from different eco-regions of the Indian Ocean and selection patterns analyzed in coding and non-coding regions. Signals of diversifying selection were observed in key functional regions involved in OXPHOS indicating OXPHOS gene regulation as the critical factor to meet enhanced energetic demands. A characteristic control region with 38–40 bp tandem repeat units under strong selective pressure as evidenced by sequence conservation and low free energy values was also observed. These changes were prevalent in fishes from the South Eastern Arabian Sea (SEAS) followed by the Northern Arabian Sea (NAS) and rare in Bay of Bengal (BoB) populations. Fishes belonging to SEAS exhibited accelerated substitution rate mainly due to the selective pressures to survive in a highly variable oceanic environment characterized by seasonal hypoxia, variable SST, and food availability.
Similar content being viewed by others
Introduction
Adaptive mechanisms are essential in marine fishes to survive in rapidly changing oceans and consequently understanding signals of adaptation will pave the way for sustainable utilization of marine resources. Ecophysiological adaptations at the cellular level provide resilience to the organism and the role of the mitochondrion and mitochondrial genome in these processes has been regarded as pivotal in recent investigations1,2,3. Mitochondria play important roles in the bioenergetics of tissues by producing 95% of eukaryotic cell energy (ATP) through the process of oxidative phosphorylation (OXPHOS) and the five major protein complexes involved in OXPHOS are crucial in the electron transport system and ATP synthesis4. Mitogenomic investigations on Pacific salmon revealed that key adaptations in OXPHOS proteins are important in lineage sorting5 whilst positively selected sites in cytochrome b region of a widely distributed European anchovy were correlated with thermal clines6. Mutations of genes involved in OXPHOS have been correlated with a wide range of environmental factors like hypoxia7, heat stress2, cold stress8, nutrient availability9 and the difference in expression of genes10. Such mutations have been indicated as providing adaptation to different thermal regimes in Drosophila11 and differential aerobic capacity in Killifish12. These investigations emphasize the importance of identifying the loci involved in selection as these loci could be used as markers to study environmental adaptation.
Tropical Indian ocean has been warming for over a century at a rate that is faster than any other region of the tropical oceans which influences the sea surface temperature (SST) patterns globally13. Seasonality in the Arabian sea was well profound in the horizontal section plots of SST, DO and Chlorophyll-a, while its counterpart Bay of Bengal (BoB) showed minimum variation in the above properties. Seasonal dynamics in the Northern Arabian Sea (NAS) has mainly been driven by both monsoonal forcing through intensive mixing and upwelling processes. During the summer monsoon, generally from June-September, Findlater jet14 (strong winds that blow from the southwest forming an intense low-level jet) create open ocean upwelling and downwelling in its left and right side of the path, while intense upwelling can be seen along the west coast15 and southeastern Arabian Sea16. In winter monsoon, evaporative cooling results in high dense water mass in the NAS that sinks to deeper depths and uplift subsurface waters to surface layers17 while the southern Arabian Sea becomes stratified due to the intrusion of the BoB waters18. Positive water balance in the BoB creates strong and stable stratified surface layers. Weak monsoonal winds19 may not be able to break these layers most of the time except, cyclones, strong cold-core eddies, strong winds, etc. Transitional periods such as inter-monsoon spring and intermonsoon fall are associated with primary and secondary heating periods that warm the surface layers and create stratification in both the seas.
The inhabitants of this ecosystem will be under an intense selection pressure to meet the enhanced energetic demands due to the increased SST as well as the changes in salinity, dissolved oxygen, food availability, and hydrological factors20. Mitochondrial genome adaptations may provide resilience to these climatic factors by changes in the efficiency of the OXPHOS complex21,22, which could be monitored over time to understand spatial and temporal patterns in the distribution of some sentinel species like Indian oil sardine. Further, conservation and management strategies can be devised to protect or conserve the adapted populations ensuring the sustainability of sardine populations, which constitute a cheap source of protein for the burgeoning human population, especially in developing countries like India23,24.
Control region, the non-coding content present in the mtDNA is responsible for the regulation of replication and transcription of mitogenome25 with many conserved sequence elements/domains, binding sites for nuclear-encoded factors, replication initiation sites, transcription initiation sites, and termination associated sites26. Many control region segments can form stable intra-sequence secondary structures25,27 which are identified as recognition sites/binding sites for many regulatory proteins like transcriptional factors25,27,28.
The Indian oil sardine Sardinella longiceps is distributed across wide environmental clines in the Indian Ocean, mainly in the northeast, southeast, southwest and northwest Indian coast, Gulf of Oman and Gulf of Aden29. Temperature followed by salinity and dissolved oxygen availability are the vital factors determining the seasonal fluctuations in distribution and abundance of small pelagic fishes like sardines and anchovies30,31. The availability of nitrogen from upwelling and sinking events in addition to external sources, especially runoff from rivers32,33, also influences the distribution and abundance. Fluctuations in these parameters induce physiological stress and consequent changes in metabolic rate, survival, and persistence. Small pelagic fishes like Indian oil sardine have high-metabolic requirements with high dependence on aerobic metabolism, making them vulnerable to environmental perturbations. Being ectothermic, their body temperature and metabolic rates are highly dependent on sea temperature. Temperature and salinity clines were reported in the Indian Ocean between the Arabian Sea on the west and the Bay of Bengal on the east34. The wide distribution of these sardines across these environmental gradients indicates their excellent adaptive capacity to different ecozones and transitional zones. In the present study, we investigated the signals of adaptation in the mitogenome by sampling fishes from widely spaced ecoregions and analyzing selective constraints. Forty-five complete mitogenomes, along with 350 complete mitochondrial control regions were analyzed from Indian oil sardines collected from the Indian ocean, mainly the eastern Indian Ocean (Bay of Bengal) and western Indian ocean (South Eastern Arabian Sea and Northern Arabian Sea). Subsequently, we investigated signals of positive/purifying selection and its correlation with geographical distribution. We also analyzed the mitochondrial non-coding control region/D loop sequences for functional constraints and their geographical significance if any.
Results
Mitogenome sequencing and assembly
The size of the mitogenome ranged from 16598 to 16676 bp depending on the size variation in the control region. No identical sequences were found. The maximum likelihood tree of the whole mitogenome sequence revealed different clades with moderate bootstrap support (Supplementary Fig. A1). Descriptive statistics of nucleotide data sets are given in Table 1. The level of nucleotide diversity was low for the whole mitogenome (ranging from 0.0060 to 0.00132) (Supplementary Table T3) with only 1131 segregating sites whereas haplotype diversity was high with each genome representing a unique haplotype (45) as evident in haplotype network (Supplementary Fig. A2). The significant negative Fu’s Fs and Tajima’s D for the whole genome (−8.642 and −2.319) and concatenated protein-coding gene data set (−11.318 and −2.370) (Table 1) indicated an excess of rare nucleotide site variants and rare haplotypes respectively compared to what would be expected under neutrality35. The values Fu’s FS, Fu & Li’s F*36, and Fu’s & Li’s D are presented in Table 1.
Evidence for natural selection
The relative mutation rate varied between genes. Relative mutation rates (µ relative) calculated for different gene regions indicated ND4 (1.4) and ND5 (1.46) genes as rapidly evolving with the highest number of non-synonymous mutations (29 and 24 respectively) (Table 1). No non-synonymous substitutions were observed in ND4L and ND6 genes. Control region, ND genes and ATPase evolved faster than other regions. Cytochrome c oxidase COX (Complex IV) evolved slower than ND genes. tRNAs and 12 S rRNAs were the slowest evolving genes (Table 1).
Signals of significant selection were evident in many codons among the 3798 codons analyzed. FUBAR analysis identified purifying selection as pervasive in the data set with 680 of the codons significant (Supplementary Table T6). Signatures of positive selection (10 sites) were less prevalent than purifying selection and they were observed in Complex I (ND1, ND2, ND4, and ND5), Complex III (CYT B), Complex IV (C01, CO2, and CO3) and Complex V (ATP6). MEME analysis showed that there are 26 sites under episodic diversifying selection (P < 0.1). TreeSAAP analysis detected many significant amino acid physiochemical property changes in the positively selected regions of S. longiceps mitogenome, with conservative amino acid changes dominating over radical changes. Among this, only those sites identified as positively selected at least by two methods were selected for further analysis (Table 2).
Twelve sites were identified as positively selected in mitochondrial complex I (NADH: ubiquinone oxidoreductase) of S. longiceps and all of them were located in transmembrane helices (#29ND1, #30ND1, #302ND2, #148ND4, #9ND5, #97ND5, #98ND5, #225ND5, #226ND5, #227ND5 and #236ND5) except one which is in the intra-helix loop (#228ND5) (Fig. 2). Some of these sites occur in the functional domains of mitochondrial complex I especially on ND2 (#302 Leu-Gln located in C-terminus), ND4 (#148 Thr-Asn in proton-conducting membrane transporter (Proton_antipo_M) and ND5 (#97 Ala-Gly, #98 Leu-Val, #225 Ala-Thr, #226 Thr-Asn, #227 Gly-Cys, #228 Lys-Asn & #236 Pro-Ser clustered in Proton_antipo_M & N-terminal (Proton_antipo_N). Position 228 (ND5) plays an important role in proton translocation (as a proton donor or acceptor) and hydrogen bond formation37. This position has recorded a shift in an amino acid residue from Lysine to Asparagine in the present study. Asparagine is more polar than lysine which may be important in hydrogen bond formation.
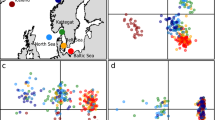
Map showing sampling locations of S. longiceps from 3 ecoregions in Indian Ocean. Sample Site: NAS (Northern Arabian Sea), SEAS (South Eastern Arabian Sea) and BoB (Bay of Bengal). The map was drawn using Adobe Photoshop CS6 (https://www.adobe.com/in/products/photoshop.html?promoid=PC1PQQ5T&mv=other).
Positive selected sites identified in NADH dehydrogenase (Complex I) of S. longiceps. Grey structures represent nuclear-encoded subunits. (a) OXPHOS Complex I with mitochondrial-encoded subunits are represented in different colors: ND2 in yellow, ND4L in blue, ND1 in orange, ND3 in magenta, ND4 in cyan, ND5 in green, ND6 in red. Individual core subunits (b) ND5, (c) ND4, (d) ND2 and (e) ND1 with amino acid site number on positively selected sites. The protein structures were generated using Geneious R788.
Twelve sites were observed to be under positive selection in mitochondrial complex IV (3 sites in CO1; 3 sites in CO2 and 2 sites in CO3). The sites in CO1 (#25Leu-Arg, #114 Gly-Ala and #262Asn-asp) are located in the transmembrane helix and two of these sites (#25 & #114) align with amino acid residues that have been reported to participate in polypeptide binding at Subunit I/VIIc interface & Subunit I/VIIa interface respectively38,39,40. Among three sites observed under positive selection in the CO2 gene, amino acid position 50 (Leu-Gln) lies in the intra-helix loop, position 63 (Glu-Gly) in the transmembrane helix and 152 (Val-ser) in the Beta strand. Among the two sites identified in CO3, position 16 (Trp-Gly) is aligned with the transmembrane helix and position 117 (Pro-Leu, Ser) with the intra-helix loop (Fig. 3).
Positive selected sites in Cytochrome C Oxidase (Complex IV) and Cytochrome bc 1 (Complex III) of S. longiceps. Grey structures represent nuclear-encoded subunits. (a) OXPHOS Complex IV (Homodimer) with mitochondrial-encoded subunits represented in different colors: CO1 in orange, CO2 in yellow, CO3 in magenta. (e) OXPHOS Complex III with mitochondrial-encoded subunit represented in magenta color. Individual core subunits (b) CO1, (c) CO2, (d) CO3 and (f) CYT B with amino acid site number at positively selected sites. The protein structures were generated using Geneious R788.
Four sites were under positive selection in mitochondrial complex III (#70 Cys-Trp, #250 Leu-Gln, #311 Lys-Gln, and #320 Leu-Ile in CYTB gene). Among these sites, one (#311) aligned with an amino acid residue reported to participate in polypeptide binding in the inter-chain domain interface and it is located in the transmembrane helix (Fig. 3). Two sites were under positive selection in mitochondrial complex V (#114 Val-Cys, ala #185 Ile-Gln in ATP6) with site #114 located in the transmembrane helix-4 and the other site #185 in the intra-helix loop connecting helix-5 and 6 (Supplementary Fig. A8).
Individuals with positively selected sites were prevalent in the South Eastern Arabian Sea (SEAS) samples. Around 26 sites are identified as positively selected in all populations. Among those sites, 81% were observed in individuals from SEAS, 39% from NAS, and 35% from BoB with some of the sites shared among regions. Seven positively selected sites (Two each in ND1 and ND5 genes and one each in ND4, CO1 and CO2 respectively) were specific to SEAS and two sites (one each in CO2 and CYTB) to NAS populations (Table 2; Fig. 4) indicating the presence of locally adapted variants.
Graphical representation of the geographical distribution of positively selected sites and Control region repeat unit types in the mitogenome of S. longiceps in the 3 eco-regions of the Indian Ocean, (a) Frequency of positively selected sites in NAS, (b) Frequency of positively selected sites in SEAS, (c) Frequency of positively selected sites in BoB, (d) Frequency of haplotype with Type 1 repeat unit in NAS, (e) Frequency of haplotype with Type 2 repeat unit in SEAS and (f) Frequency of haplotype with repeat unit Type 3 in BoB. The map was drawn using Adobe Photoshop CS6 (https://www.adobe.com/in/products/photoshop.html?promoid=PC1PQQ5T&mv=other).
Types of repeat units in the mtDNA control region
The length of the control region of S. longiceps ranged from 900−980 bp (GenBank Accession No: KJ466087–KJ466091; KJ472113–KJ472120; KJ888156–KJ888390; KP000859–KP000897), due to variation in the number of tandem repeats and poly-A in different haplotypes (Supplementary Fig. A4). The control region contained different conserved sequence regions like Termination Associated Sequence (TAS) at the 3′ end and Conserved Sequence Box (CSB D, CSB1, CSB2, and CSB3)41. (Supplementary Fig. A3). Two hundred and fifty-nine haplotypes with 3 types of repeats were found among the 304 control regions analyzed. The tandem repeat was found in between TAS and poly-A (length between 38–40 bp), the repeat units were repeated once (in the majority of haplotypes), twice and three times (in few haplotypes). Type 1 with one repeat unit (38 bp) was the most abundant (present in all the ecoregions). Type 2 (38 bp) with two repeat units and type 3 (40 bp) with three repeat units were found only in a few individuals (from SEAS and NAS). There are sub-types for Type 3 with some variation in the repeat unit (Type 3a, 3b, and 3c).
Patterns of the predicted secondary structure of the control region
Several secondary structures (size of 10 bp or more) were identified in the control region L-strand (Supplementary Fig. A5). The conserved sequences like TAS and CSBs were always associated with a secondary structure. All secondary structures predicted for the mtDNA L-strand were also observed for the L-strand mRNA transcript with some minor changes. Palindromic sequences were observed in the region with length variation and repeat units. Few large and short stem-loop structures with low free energy ∆G (−0.101 to −0.384 kcal/mol) were observed in the repeat region (Fig. 5; Supplementary Table T7, Fig. A10). Multiple stem-loop structures have been observed in haplotypes with Type 3 repeat unit sequences whilst, no complex structure observed in Type 1 and Type 2 haplotypes. The stem was formed by the 3′ end of the repeat unit and 5′ upstream sequence. In Type 3 repeat unit, several stem-loop structures with internal bulges were observed. The L strand mRNA transcript of the repeat region is also forming similar structures with greater negative folding energies (∆G) (Fig. 5; Supplementary Table T7, Fig. A10).
Graphical representation of some predicted secondary structures of repeat unit Type 1, 2 and 3 of mtDNA control region. a) Predicted secondary structure of repeat unit Type 1, b) predicted secondary structure of repeat unit Type2, c) predicted secondary structures of repeat unit Type 3. The secondary structures were generated using ‘mfold’ web server97,98.
The robustness of the predicted secondary structure of the control region was assessed by comparing its folding potential (Free energy, ∆G = kcal/mol) with that of tRNA, which is confirmed to form a functional secondary structure. The relative free energy (∆G/Length) of tRNA ranged from −0.42 to −0.08 and that of predicted repeat unit structures of control region from −0.384 to −0.101 (Supplementary Table T7). Type 1 and Type 2 repeat units have recorded lower free energy than Type 3 repeat units (Supplementary Table T7), indicating the higher folding potential of haplotypes with Type 1 and Type 2 over Type 3 repeat units. Type 1 haplotypes with one repeat unit were the most abundant in all geographic locations of the Indian Ocean. Haplotypes with two and three repeat units were less abundant and restricted to the Western Indian Ocean (both eco-regions) (Fig. 4).
The Tajima’s D value was significant (zero/negative) for tRNA and coding regions, which indicated their functional constraints (Table 1). The mitochondrial control regions involved in secondary structure formation also recorded negative and highly significant values (Supplementary Fig. A3). The repeat unit position alone indicated a value of −2.14526 (P < 0.01) (Supplementary Fig. A3). These results suggest that some regions in the control region (TAS, CSD, and repeat unit) are also under purifying selection force similar to the coding region. The number of substitutions/rates of evolution in paired sites was comparatively lower than the unpaired sites.
Environmental data
Wide variations in temperature, dissolved oxygen, salinity, and chlorophyll-a were observed between NAS, SEAS, and BoB. NAS encompasses the Persian Gulf, Gulf of Oman, Red Sea and the northeast Arabian Sea where a unimodal pattern of sea surface temperature (SST) is observed with the highest temperature (24–27 °C) during the northeast monsoon season (October-March) and lowest temperature (20–22 °C) during the southwest monsoon season (June–September). The NAS also is characterized by a very high chlorophyll-a concentration (4–10 mg/m3) during May-June, lasting up to October (during the southwest monsoon season). The average sea surface salinity (SSS) is also higher along with NAS throughout the year (36–38ppt)34 (Fig. 6, Supplementary Table T5, Fig. A12). SEAS exhibits a typical bimodal pattern of SST with the warm (29–30 °C) spring intermonsoon (April–May) and the fall intermonsoon (October–November) and the cool (26–28 °C) southwest monsoon (June–September) and the northeast monsoon seasons (December-March) (Fig. 6, Supplementary Table T5, Fig. A12). High chlorophyll-a concentration (Fig. 6, Fig. A12) observed at SEAS (Malabar upwelling zone) is due to the intense coastal upwelling from May to September, and it peaks during July and August (5–10 mg/m3). By October, it recedes to a low (1–2 mg/m3) chlorophyll-a concentration and maintains up to May. SEAS is also characterized by a very low dissolved oxygen (1–2 mg/L) during the southwest monsoon season while it is 2–4 mg/L in NAS and 3–5 mg/L in BoB. Coastal upwelling along the Somalia coast and SEAS brings not only subsurface cool, nutrient-rich waters but also less oxygenated waters to surface layer, while coastal upwelling regions in the BoB are well oxygenated (Fig. 6, Fig. A12). During this season, high chlorophyll-a concentration along the coast is well corroborated with low temperature and low dissolved oxygen. On the contrary, BoB is characterized by stable dissolved oxygen (3–5 mg/L), reduced salinity (28–33ppt), reduced temperature (28–30 °C), and low chlorophyll-a (0–3 mg/m3) environment than SEAS and NAS, throughout the year. Variations in frequencies of the positively selected amino acid substitutions of the dataset between SEAS, NAS and BoB were positively and highly correlated to fluctuations in annual Sea surface Temperature (SST) (represented as standard deviation) (parameter estimate = 2.04, SE = 0.04, P = 0.01), fluctuations in annual chlorophyll-a (represented as standard deviation) (estimate = 1.24, SE = 0.23, P = 0.03) and negatively to Fall (October, November, December) - Dissolved Oxygen (DO) (estimate = −4.68, SE = 0.27, P = 0.02). Moderate correlation was obtained for the amino acid substitutions under selection with fluctuations in annual DO (represented as standard deviation) (estimate = 0.99, SE = 0.4, P = 0.057), Winter -SST (estimate = 0.47, SE = 0.031, P = 0.057), Spring-DO and Summer -Chlorophyll-a (estimate = 2.13, SE = 0.13, P = 0.057).
Monthly Chlorophyll a (mg/m3), Sea Surface Temperature- SST (°C) and Dissolved Oxygen (µmol/kg) for the Bay of Bengal and Arabian Ocean during July to September. Chlorophyll a and Sea Surface Temperature gradients are represented as coloured shades. Dissolved Oxygen is represented as contour lines. The images were generated in ODV 5.1.7 (https://odv.awi.de/).
Discussion
The highly variable environment of the SEAS induced maximum selective pressures in the mitogenome of Indian oil sardine indicating the importance of variable environmental factors on mitogenomic evolution and subsequent adaptation. The positive and diversifying selection was detected for fixed amino acid replacements in key regions involved in oxidative phosphorylation complexes, Complex I: NADH dehydrogenase (ND1, ND2, ND4, and ND5), Complex III: Cytochrome b (CYT B), Complex IV: Cytochrome C Oxidase (CO1, CO2, and CO3) and Complex V: ATP Synthase (ATP6 gene). Seven positively selected sites were specific to SEAS and 2 sites to NAS eco-region. S. longiceps has a characteristic control region with 38–40 bp tandem repeat units (palindromic sequences within it) and they are under intense selective pressure similar to the coding region. We predicted stable intra-strand secondary structures with high folding potential/low free energy (−0.101 to −0.384 kcal/mol) in the repeat unit. Haplotypes with one repeat unit are the most abundant haplotypes in the samples probably due to the lower free energy and consequent enhanced stability (high folding potential). Haplotypes with two and three repeat units are less abundant and they are restricted to the SEAS. The observed genetic diversity and positive selection in the mtDNA of S. longiceps may be driven by the pressures of the heterogeneous environment. High selective pressures were evident in both coding and non-coding regions in samples collected from SEAS followed by NAS. Variation in frequencies of the positively selected sites between SEAS, NAS and BoB correlated significantly with fluctuations in environmental parameters mainly temperature, dissolved oxygen and chlorophyll-a. The SEAS is considered as a region with a complex interplay between many oceanographic processes that vary spatially and temporally42.
The NADH dehydrogenase complex is the first and largest multimeric enzyme of the five complexes constituting the oxidative phosphorylation pathway43. It provides electrons for the reduction of quinine to quinol which is available from oxidation of the NADH and translocates four protons (H+) across the inner membrane. The subunits ND2, ND4, and ND5 directly act as proton pumps for H+ ions and the changes in amino acids may have some adaptive value. Among the nine positively selected sites in S. longiceps, all of them were located in transmembrane helices especially in proton-conducting membrane transporter. Extensive non-synonymous mutations have been reported in ND genes in many fishes5,44,45,46,47. The high rate of mutations observed in complex I may be associated with the position of the ND genes in the mitogenome. They are found immediately upstream from the origin of L-strand replication (OriL) and downstream from the origin of H-strand (OriH) replication. During replication, these genes stay single-stranded for more time compared to other genes, thus they are prone to a high rate of mutation48.
Cytochrome b is a part of respiratory protein complex III, which is the middle component of the mitochondrial respiratory chain, coupling the transfer of electrons from ubihydroquinone to cytochrome c with the generation of an electrochemical gradient across the mitochondrial membrane. Substitution at amino acid position 311 reported to participate in polypeptide binding in inter-chain domain interface may influence the structure and function of cytochrome b. The amino acid replacements can result in regional changes to hydrophobicity and structure within the protein and can alter the coupling efficiency of complex III. In humans, mutations characterized by enhanced binding of water at Qi site have been linked to increased longevity49 whereas, in yeast, mutation at Qo binding site have been linked to reduced catalytic efficiency and increased oxygen radical production50. Thus, the substitution at polypeptide binding site of Cytochrome b in the Indian oil sardine may have some functional relevance which needs further investigation.
Cytochrome c oxidase (complex IV) catalyzes the final step in the mitochondrial electron transfer chain and is considered as one of the major regulation sites for OXPHOS40. It receives an electron from each of the four cytochrome c molecules which transfers electrons between complex III and IV and transfers them to one oxygen molecule. During this process, it converts one molecular oxygen to two molecules of water by using four protons from the inner aqueous phase to make water and also, translocates four protons across the membrane. The conserved nature of most of the key amino acid residues reported to participate in the electron transfer pathway, putative water exit pathway, ion/chemical binding and putative proton exit pathway in complex IV indicates that these regions are constrained functionally. Mutations observed outside the key functional residues could be related to relaxed purifying selection49.
Polymorphisms in regions reported to participate in polypeptide binding at mitochondrial and the nuclear-encoded subunits interface (Subunit I/VIIc interface & Subunit I/VIIa interface; complex IV)38,39,40 may change the structure and efficiency of the OXPHOS complex possibly playing a role in adaptation. Thus, the co-evolution between mitochondrial and nuclear-encoded subunits due to genome-genome interactions can affect the OXPHOS function and regulation in S. longiceps. Such co-evolution has been reported in cytochrome c oxidase (complex IV) of primates51 and NADH dehydrogenase complex of humans52. Positively selected sites that appear to interact with other COX subunits (Complex IV) were also reported from high-performance fish like Scombroidei53.
ATP synthase (complex V) is composed of a soluble catalytic F1 region and a membrane-inserted FO region. The observed amino acid replacement in the ATP 6 gene (corresponding to a subunit), located in the transmembrane helix-4 (#114) and coil connecting helix-5 and 6 (#185) may have a role in the arrangement of a-helices, which is functionally important and likely involved in proton translocation.
In a population with low effective population size (Ne), fixation of slightly deleterious mutations by drift may leave similar signals as positive selection54. But in a pelagic species like S. longiceps with large Ne (Ne mtDNA 1.1 × 106 to 1.31 × 109)55, the effect of genetic drift will be minimal in mtDNA evolution54. A previous investigation using two mitochondrial DNA markers (partial gene regions) indicated a lack of significant genetic differentiation in Indian oil sardine populations along the Indian coast55 which may be due to the low resolving power of the markers56. On the contrary, microsatellite markers indicated significant genetic differentiation between populations of NAS and other regions57. Similarly, the evidence of positive selection in regions participating at mitochondrial and the nuclear-encoded subunits interface interactions (CO1, Subunit I/VIIc interface & Subunit I/VIIa interface in complex IV) from SEAS and NAS signals possible reproductive isolation, sympatric speciation and diversification in sardines. Polymorphisms in regions involved in mito-nuclear interactions may disrupt mito-nuclear interactions resulting in reproductive isolation and speciation58. Further investigations using genome-wide markers like SNPs may provide more clarity to these findings.
The distribution of mitochondrial control region haplotypes also corroborated the conclusions made out of functional region analysis as all the three types of control region were present in SEAS. The haplotypes with repeat units having the highest folding potential and low free energy are abundant in all oceanic regions. The distribution of other haplotypes with more than one repeat unit is restricted to the SEAS. The folding potential, the number of substitutions/rates of evolution in paired sites, and Tajima’s D statistics analysis showed that control regions are under strong functional constraints. Haplotypes having one repeat unit (Type 1; Supplementary Fig. A10-A), with lower free energy ∆G (high folding potential), were the most abundant. The haplotypes with two (Type 2; Supplementary Fig. A10-B) and three repeat units (Type 3; Supplementary Fig. A10-B) have greater folding energies and they are less abundant and restricted to the SEAS. Different models such as slipped-strand mispairing59, intermolecular recombination, transposition60, and misalignment during replication have been suggested as the mechanism behind observed polymorphisms25. In mammals, these deletions are closely linked to mitochondrial diseases and proven to be associated with site-specific breakage hotspots59.
The TAS, poly-A, and secondary structure-forming repeat units were conserved within species. The loop forming regions are protected from mutations that are more likely to occur during replication as it forms a single-stranded structure (D-loop). This strongly indicates the purifying selection pressure on these regions to maintain the intra-strand loop structure and the significant negative Tajima’s D strongly corroborates this hypothesis. The position of the loop forming region between TAS and poly-A indicates its possible role in replication initiation and termination of elongation in proposed models of mitochondrial replication61. The occurrence of the secondary structure near the hot spot of polyadenylation sites (Poly A)62 strengthens its possible role in transcription termination. The secondary structure may act as a punctuation point for correct mRNA processing63,64. The link between mitochondrial structural variants/haplogroups and mtDNA copy number variation (by influencing the replication machinery) has been reported in humans contributing to the adaptation of the human populations to different climatic zones22,65,66.
Theoretical and empirical evidence has suggested the role of many environmental parameters for species persistence, adaptation, and phenotypic and genotypic diversification67,68. The temperature has been proposed as one of the main factors driving evolutionary diversification due to enhanced mutation rates in mitochondrial as well as nuclear genome in many taxa, which also is the reason for species diversity in tropics as compared to temperate waters69,70. It has been demonstrated that fluctuations in the environmental factors promote phenotypic and evolutionary diversification in organisms71,72,73. The Arabian Sea Large Marine Ecosystem is considered as one of the major upwelling systems in the world causing variations in temperature, dissolved oxygen, salinity, and chlorophyll-a. SEAS exhibit wide fluctuations in temperature values annually as compared to NAS and BoB. Mitochondria play essential roles in aerobic metabolism which is a temperature-sensitive process and consequently, mitochondria are considered as possible sites of processes influencing the thermal limits of organisms. Thus, thermal acclimation alters mitochondrial properties to maintain aerobic scope74. Thermal acclimation in ectotherms may happen by maintaining the stability of OXPHOS proteins for which a few amino acid substitutions may be necessary75,76. SEAS is also characterized by variations in chlorophyll-a values and oxygen minimum zones (hypoxia). Starvation (reduced chlorophyll-a in this case) can drive mtDNA evolution by acting as a force to generate energy more efficiently by improving the efficiency of the coupling of energy production in the OXPHOS pathway77,78,79. Hence, productivity variations of the sardine habitat may lead to the evolution of genotypes with more efficient OXPHOS pathways. Similarly, the occurrence of oxygen minimum zones and consequent hypoxia in SEAS (during southwest monsoon) also demand efficient coupling of energy production80. Thus, the abundance of selective signatures/higher rate of selected genotypes in SEAS may be a response to the uncertain environmental conditions (hypoxia, temperature, and productivity) which warrant ecotypes of high metabolic efficiency for survival and reproduction, compared to the stable environment of NAS and highly stable environment of BoB.
There are many reports regarding the correlation between genetic diversity of OXPHOS genes, and environmental pressures such as hypoxia9, heat stress81, cold stress8, and nutrient availability9. Adaptive evolution in mitogenome in response to temperature and salinity has been reported in, Atlantic salmon, Pacific salmon, Atlantic cod and Killer whale populations5,45,82,83. Thus, the prevalence of diversifying selection in the SEAS indicates the action of evolutionary forces in the mitochondrial OXPHOS complex associated with metabolic adaptation to the dynamic and highly productive environment. Two positively selected sites in ND1 (#29,#30) and ND5 (#97, #98) genes and one site each in ND4 (#148), CO1 (#25), CO2 (#152) and ATP6 (#185) respectively were specific to SEAS and one site each in CO2 (#50) and CYTB (#250) specific to NAS populations. These functional genes and regulatory elements have the potential to act as markers for inferring population genetic structure, plastic responses, adaptation, and functional gene evolution in marine fishes and thus could be valuable for the management and conservation of this important resource. Further studies could be carried out in Indian oil sardine to identify genome level adaptations that will provide holistic information concerning their adaptive capacity. The accelerated warming events in the Indian Ocean necessitate species with adaptive potential and enhanced fitness to increased SST and associated changes in hydrography. The present study assumes great relevance from this point of view as Indian oil sardines with adaptive signals can be further monitored for their spatial and temporal distribution to derive clues regarding climatic impacts in the Indian ocean. Further, information from genomic and mitogenomic investigations can be correlated with fitness consequences at each eco-region by common-garden experiments83,84,85. The locally adapted populations will exhibit higher fitness in each eco-region. These investigations are vital to conserve and understand the dynamics of small pelagic resources in space and time as they form the mainstay of food security of many coastal states of developing nations.
Materials and Methods
Sample collection, DNA extraction, mitogenome sequencing, and assembly
A total of 350 individuals of the Indian oil sardines were collected during 2015–2017, from the three eco-regions of the Indian Ocean, NAS (117nos), SEAS (117nos), and BoB (116nos) (Fig. 1; Supplementary Table T2). Muscle tissues stored in 95% ethanol were used for genomic DNA extraction using DNEASY blood and tissue kit (Qiagen). All the fishes sampled in this study were handled in strict accordance with the guidelines for the care and use of fish in research by De Tolla et al.86 and the protocols were approved by the ethical committee of the ICAR- Central Marine Fisheries Research Institute, Kochi.
Complete mitochondrial genomes of 45 individuals (15 each from the 3 eco-regions) were amplified and sequenced using 16 novel primer pairs (Supplementary Table T1) and the mitogenome of Sardinella longiceps was used as a template (GenBank Accession No: KR000002.1)41. Sequences were manually checked, aligned, and assembled in MEGA687 and Geneious R788 against the S. longiceps mitogenome41. Annotated mitogenome sequences have been submitted to NCBI, GenBank (Accession numbers MG251937–MG251981). In addition to mitogenome sequences, we also generated control region sequences of an additional 305 individuals to understand structural variations and selective constraints.
Four sets of nucleotide sequence data (1. whole mitogenome nucleotide sequence - 16598 to 16676 bp. 2. concatenated dataset of 13 protein-coding genes - 11418 bp, 3. 22 tRNAs and 4. control region - 1032 to 1108 bp) along with amino acid sequence data (amino acid sequences of 13 protein-coding genes) were prepared and analyzed using MEGA6 and Geneious R7. Descriptive statistics, the number of polymorphic sites (S), nucleotide diversity (π)89, haplotype diversity (Hd)89, the average number of pairwise nucleotide differences (K)90, Fu’s FS, Fu & Li’s F*36 and the total number of synonymous and non-synonymous mutations were generated for nucleotide data sets. The number of non-synonymous substitutions per non-synonymous site (Ka), number of synonymous substitutions per synonymous site (Ks), Ka/Ks89, and theta (θ) were calculated91 . The θ-values were used to calculate the relative mutation rate of individual genes relative to the whole mitogenome using the equation µgene = ((µmitogenome* θmitogenome)/θgene)46. All these analyses were performed using DnaSP92 and MEGA6. The maximum likelihood tree was generated using MEGA6 for nucleotide and amino acid data sets with 1000 bootstrap replicate and GTR substitution model (selected using the J Model Test93). Sardinella maderensis sequence was used as an outgroup to root both the trees.
Selection analyses
The concatenated dataset of 13 protein-coding genes and the Maximum likelihood tree generated from it was used to conduct whole mitochondrial genome scans to detect signals of natural selection. We analyzed the data with the approximate hierarchical Bayesian method (FUBAR- Fast Unconstrained Bayesian Approximation) and mixed effect method (MEME- Mixed Effect Model of Evolution) available in DATA MONKEY94. The MEME analyzes the distribution of synonymous and non-synonymous substitution rates from site to site and branch to branch at a site. But FUBAR is considered as more dependable when the strength of selection varies across sites because it uses settings that are less sensitive to model specifications. HKY 85 nucleotide substitution model was used for analysis. For each method we selected a threshold P-value; P < 0.05 for MEME and posterior probability >0.9 for FUBAR. We used TreeSAAP95 to understand changes in physicochemical properties of amino acids caused by replacements, as it compares the amino acid changes inferred from a given tree with a model having 31 predicted physicochemical amino acid property changes, under an assumption of neutrality. The Z test was used to analyze the changes in the amino acid properties, which is categorized into eight magnitude groups96. The positive and negative Z-scores indicate positive and negative selection respectively. In this analysis, we considered only 6, 7, and 8th category amino acid changes with strong statistical support (P < 0.001).
All amino acid data sets were aligned in MEGA6. The 3D homology model of protein subunits with positively selected sites observed was constructed with the SWISS-MODEL server97 using the vertebrate protein model of Bovine corresponding to subunit (available in Protein Data Base, https://www.rcsb.org/). Finally, we located positively selected sites identified in the three-dimensional structure of protein subunit and compared it with the functionally important amino acid residues. The number of positively selected sites was compared between eco-regions to correlate it with habitat characteristics and identify any signals of local adaptation.
Control region, tRNA sequence analysis, and secondary structure prediction
Repeatability of Sanger sequencing of the control region of three major types was assessed by sequencing the same individual twice so that these sequences could be treated as control samples. Based on the number of repeat units, we elucidated the types of control region sequences present in the dataset. Prediction of the secondary structure of control region sequences was carried out by the free energy minimization method following nearest neighbor thermodynamic rules (with 15 window length and 25 step size) using ‘mfold’ webserver98. RNA dataset of control region types and 22 tRNA was generated using MEGA6. The structure and free energy for the RNA datasets were calculated using RNA mfold in ‘mfold’ web server.
We calculated Tajima’s D and the relative mutation rate in control region data to understand the deviation from neutrality/functional constraints on the control region. Sequences spanning the secondary structures were tested for the extent of conservation by comparing relative mutation rates and polymorphisms. Inter-specific comparisons of control region sequences were carried out with those of Clupeoids (using mitogenome sequences deposited in NCBI, GenBank) to deduce identity, sequence conservation, and polymorphisms. All these analyses were performed using DnaSP and MEGA6.
Environmental data
Monthly climatology data of Sea surface Temperature SST (°C) SSS (ppt) and Dissolved Oxygen DO (µmol/kg) was taken from World Ocean Data 2018 available at https://www.nodc.noaa.gov/OC5/woa18/woa18data.html. While monthly average Chlorophyll-a (mg/m3) data spanning from the year 2002 to 2015 was downloaded from MODIS site (https://modis.gsfc.nasa.gov/data/dataprod/chlor_a.php) and subjected to objective analysis before generating monthly climatology. The data was analyzed by using Ferret and visualized in Ocean Data View (ODV 5.1.7). Seasonal climatology data (Winter - (January, February, March), Spring - (April, May, June), Summer - (July, August, September), Fall - (October, November, December)) for ecoregions were prepared by estimating the mean and standard deviation of annual SST, SSS, DO and Chlorophyll-a was estimated as a measure of degree variability in annual climatology. We used generalized linear models in R 3.6.2 with a binomial link to examine variations in the frequencies of amino acid substitutions under selection (as described by Consuegra et al.45) in NAS, SEAS, and BoB with SST (Winter, Spring, Summer, and Fall), SSS (Winter, Spring, Summer, and Fall), DO (Spring, Summer, and Fall), Chlorophyll-a (Spring, Summer, and Fall), the standard deviation of annual SST (fluctuations in annual SST), the standard deviation of annual SSS (fluctuations in annual SSS), the standard deviation of annual DO (fluctuations in annual DO) and standard deviation of annual Chlorophyll-a (fluctuations in Chlorophyll-a).
Data availability
Mitochondrial DNA sequence data associated with this manuscript have been deposited in GenBank under the accession numbers: MG251937 - MG251981, KJ466087–KJ466091, KJ472113–KJ472120, KJ888156–KJ888390, KP000859–KP000897.
References
Ballard, J. W. O. & Pichaud, N. Mitochondrial DNA: more than an evolutionary bystander. Funct. Ecol. 28, 218–231 (2014).
Morales, H. E. et al. Mitochondrial-nuclear interactions maintain a deep mitochondrial split in the face of nuclear gene flow. BioRxiv 1, 095596 (2016).
Wang, D. Molecular Basis for Adaptive Response to Environmental Toxicants or Stresses. In Molecular Toxicology in Caenorhabditis elegans (ed. Wang, D.) 411-428 (Springer (2019).
Letts, J. A., Fiedorczuk, K. & Sazanov, L. A. The architecture of respiratory supercomplexes. Nature 537, 644–648 (2016).
Garvin, M. R., Bielawski, J. P. & Gharrett, A. J. Positive Darwinian selection in the piston that powers proton pumps in complex I of the mitochondria of Pacific salmon. Plos One 6, e24127 (2011).
Silva, G., Lima, F. P., Martel, P. & Castilho, R. Thermal adaptation and clinal mitochondrial DNA variation of European anchovy. P. Roy. Soc. Lond. B. Bio. 281, 20141093 (2014).
Scott, G. R. et al. Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol. Biol. Evol. 28, 351–363 (2010).
Stier, A., Massemin, S. & Criscuolo, F. Chronic mitochondrial uncoupling treatment prevents acute cold-induced oxidative stress in birds. J. Comp. Physiol. B. 184, 1021–1029 (2014).
Da Fonseca, R. R., Johnson, W. E., O’Brien, S. J., Ramos, M. J. & Antunes, A. The adaptive evolution of the mammalian mitochondrial genome. BMC Genomics 9, 119 (2008).
Garvin, M. R., Thorgaard, G. H. & Narum, S. R. Differential expression of genes that control respiration contribute to thermal adaptation in redband trout Oncorhynchusmykiss gairdneri. Genome. Biol. Evol. 7, 1404–1414 (2015).
Doi, A., Suzuki, H. & Matsuura, E. T. Genetic analysis of temperature-dependent transmission of mitochondrial DNA in. Drosophila. Heredity 82, 555–560 (1999).
Brennan, R. S., Hwang, R., Tse, M., Fangue, N. A. & Whitehead, A. Local adaptation to osmotic environment in killifish, Fundulus heteroclitus, is supported by divergence in swimming performance but not by differences in excess post-exercise oxygen consumption or aerobic scope. Comp. Biochem. Phys. A. 196, 11–19 (2016).
Roxy, M. K., Ritika, K., Terray, P. & Masson, S. The curious case of Indian ocean warming. J. Climate. 27, 8501–8509 (2014).
Findlater, J. A major low level air current near the Indian Ocean during the northern summer. Q. J. Roy. Meteor. Soc. 95, 362–380 (1969).
Schott, F. A. & McCreary, J. P. Jr The monsoon circulation of the Indian Ocean. Prog. Oceanogr. 51, 1–123 (2001).
Smitha, B. R., Sanjeevan, V. N., Vimalkumar, K. G. & Ravichandran, C. On the upwelling off the southern tip and along the west coast of India. J. Coastal. Res. 24, 95–102 (2008).
Qasim, S. Z. Oceanography of the northern Arabian Sea. Deep Sea Res. Part 1 Oceanogr. Res. Pap. 29, 1041–1068 (1982).
Prasanna Kumar, S. et al. Why is the Bay of Bengal less productive during summer monsoon compared to the Arabian Sea? Geophys. Res. Lett. 29, 88-1–88-4 (2002).
Shenoi, S. S. C., Shankar, D. & Shetye, S. R. Differences in heat budgets of the near-surface Arabian Sea and Bay of Bengal: Implications for the summer monsoon. J. Geophys. Res. 107, 3052 (2002).
Teske, P. R. et al. Thermalselection as a driver of marine ecologicalspeciation. Proc. R. Soc. B 286, 20182023 (2019).
Garvin, M. R., Bielawski, J. P., Sazanov, L. A. & Gharrett, A. J. Review and meta-analysis of natural selection in mitochondrial complex I in metazoans. J. Zool. Syst. Evol. Res. 53, 1–17 (2015).
Lajbner, Z., Pnini, R., Camus, M. F., Miller, J. & Dowling, D. K. Experimental evidence that thermal selection shapes mitochondrial genome evolution. Sci. Rep. 8, 9500 (2018).
Devaraj, M. & Martosubroto, P. Small pelagic resources and their fisheries in the Asia-Pacific Region. Proceedings of APFIC working party on Marine Fisheries (RAP Publishers (1997).
CMFRI. Annual report 2017–2018 (Central Marine Fisheries Research Institute (2018).
Pereira, F. et al. Evidence for variable selective pressures at a large secondary structure of the human mitochondrial DNA control region. Mol. Biol. Evol. 25, 2759–2770 (2008).
Miya, M. & Nishida, M. The mitogenomic contributions to molecular phylogenetics and evolution of fishes: a 15-year retrospect. Ichthyol. Res. 62, 29–36 (2015).
Katz, L. & Burge, C. B. Widespread selection for local RNA secondary structure in coding regions of bacterial genes. Genome Res. 13, 2042–2051 (2003).
Walberg, M. W. & Clayton, D. A. Sequence and properties of the human KB cell and mouse L cell D-loop regions of mitochondrial DNA. Nucleic Acids Rese. 9, 5411–5421 (1981).
Munroe, T. A. & Priede, I. G. Sardinella longiceps (errata version published in 2017). The IUCN Red List of Threatened Species, 2010e, T154989A115258997 (2010).
Peck, M. A., Reglero, P., Takahashi, M. & Catalan, I. A. Life cycle ecophysiology of small pelagic fish and climate-driven changes in populations. Prog. Oceanogr. 116, 220–245 (2013).
Sato, M. et al. Coastal upwelling fronts as a boundary for planktivorous fish distributions. Mar. Ecol. Prog. Ser. 595, 171–186 (2018).
Checkley, D. M. Jr, Asch, R. G. & Rykaczewski, R. R. Climate, anchovy, and sardine. Annu. Rev. Mar. Sci. 9, 469–493 (2017).
Reiss, C. S., Checkley, D. M. Jr. & Bograd, S. J. Remotely sensed spawning habitat of Pacific sardine (Sardinops sagax) and Northern anchovy (Engraulis mordax) within the California Current. Fisheries Oceanogr. 17, 126–136 (2008).
Chatterjee, A. et al. A new atlas of temperature and salinity for the North Indian Ocean. J. Earth. Syst. Sci. 121, 559–593 (2012).
Harpending, H. C. Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum. Biol. 66, 591–600 (1994).
Fu, Y. X. & Li, W. H. Statistical tests f neutrality of mutations. Genetics 133, 693–709 (1993).
Zhu, J., Vinothkumar, K. R. & Hirst, J. Structure of mammalian respiratory complex I. Nature 536, 354–358 (2016).
Tsukihara, T. et al. Structures of Metal Sites of Oxidized Bovine Heart Cytochrome c Oxidase at 2.8\AA. Science 269, 1069–1074 (1995).
Crofts, A. R. The cytochrome bc 1 complex: function in the context of structure. Annu. Rev. Physiol. 66, 689–733 (2004).
Li, Y., Park, J. S., Deng, J. H. & Bai, Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 38, 283–291 (2006).
Sebastian, W., Sukumaran, S., Zacharia, P. U. & Gopalakrishnan, A. The complete mitochondrial genome and phylogeny of Indian oil sardine, Sardinella longiceps and Goldstripe Sardinella, Sardinella gibbosa from the Indian Ocean. Conserv. Genet. Resour. 10, 735–739 (2017).
Narvekar, J. et al. Winter‐time variability of the eastern Arabian Sea: A comparison between 2003 and 2013. Geophys. Res. Lett. 44, 6269–6277 (2017).
Sazanov, L. A. A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat. Rev. Mol. Cell. Bio. 16, 375–388 (2015).
Caballero, S., Duchene, S., Garavito, M. F., Slikas, B. & Baker, C. S. Initial evidence for adaptive selection on the NADH subunit Two of freshwater dolphins by analyses of mitochondrial genomes. Plos One 10, e0123543 (2015).
Consuegra, S., John, E., Verspoor, E. & De Leaniz, C. G. Patterns of natural selection acting on the mitochondrial genome of a locally adapted fish species. Genet. Sel. Evol. 47, 1–10 (2015).
Jacobsen, M. W., Da Fonseca., R. R., Bernatchez, L. & Hansen, M. M. Comparative analysis of complete mitochondrial genomes suggests that relaxed purifying selection is driving high nonsynonymous evolutionary rate of the NADH2 gene in whitefish Coregonus ssp. Mol. Phylogenet. Evol. 95, 161–170 (2016).
Teacher, A. G., Andre, C., Merila, J. & Wheat, C. W. Whole mitochondrial genome scan for population structure and selection in the Atlantic herring. BMC Evol. Biol. 12, 248 (2012).
Marshall, H. D., Coulson, M. W. & Carr, S. M. Near neutrality, rate heterogeneity, and linkage govern mitochondrial genome evolution in Atlantic cod (Gadus morhua) and other gadine fish. Mol. Biol. Evol. 26, 579–589 (2008).
Beckstead, W. A., Ebbert, M. T., Rowe, M. J. & McClellan, D. A. Evolutionary pressure on mitochondrial cytochrome b is consistent with a role of CytbI7T affecting longevity during caloric restriction. Plos One 4, e5836 (2009).
Wenz, T. et al. Mutational analysis of cytochrome b at the ubiquinol oxidation site of yeast complex III. J. Biol. Chem. 282, 3977–3988 (2007).
Osheroff, N. et al. The reaction of primate cytochromes c with cytochrome c oxidase. Analysis of the polarographic assay. J. Biol. Chem. 258, 5731–5738 (1983).
Gershoni, M. et al. Disrupting mitochondrial–nuclear coevolution affects OXPHOS complex I integrity and impacts human health. Genome Biol. Evol. 6, 2665–2680 (2014).
Dalziel, A. C., Moyes, C. D., Fredriksson, E. & Lougheed, S. C. Molecular evolution of cytochrome c oxidase in high-performance fish Teleostei: Scombroidei. J. Mol. Evol. 62, 319–331 (2006).
Hauser, L. & Carvalho, G. R. Paradigm shifts in marine fisheries genetics: ugly hypotheses slain by beautiful facts. Fish Fish. 9, 333–362 (2008).
Sukumaran, S., Sebastian, W. & Gopalakrishnan, A. Population genetic structure of Indian oil sardine, Sardinella longiceps along Indian coast. Gene 576, 372–378 (2016).
Hauser, L., Turan, C. & Carvalho, G. Haplotype frequency distribution and discriminatory power of two mtDNA fragments in a marine pelagic teleost (Atlantic herring, Clupea harengus). Heredity 87, 621–630 (2001).
Sebastian, W., Sukumaran, S., Zacharia, P. U. & Gopalakrishnan, A. Genetic population structure of Indian oil sardine, Sardinella longiceps assessed using microsatellite markers. Conserv. Genet. 18, 951–964 (2017).
Burton, R. S., Pereira, R. J. & Barreto, F. S. Cytonuclear genomic interactions and hybrid breakdown. Annu. Rev. Ecol. Evol. 44, 281–302 (2013).
Samuels, D. C., Schon, E. A. & Chinnery, P. F. Two direct repeats cause most human mtDNA deletions. Trends Genet. 20, 393–398 (2004).
Mita, S. et al. Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic. Acids. Res. 18, 561–567 (1990).
Yasukawa, T., Yang, M. Y., Jacobs, H. T. & Holt, I. J. A bidirectional origin of replication maps to the major noncoding region of human mitochondrial DNA. Mol. Cell. 18, 651–662 (2005).
Slomovic, S., Laufer, D., Geiger, D. & Schuster, G. Poly-adenylation and degradation of human mitochondrial RNA: the prokaryotic past leaves its mark. Mol. Cell. Biol. 25, 6427–6435 (2005).
Ojala, D., Merkel, C., Gelfand, R. & Attardi, G. The tRNA genes punctuate the reading of genetic information in human mitochondrial DNA. Cell 22, 393–403 (1980).
Mignotte, F., Gueride, M., Champagne, A. M. & Mounolou, J. C. Direct repeats in the non-coding region of rabbit mitochondrial DNA: Involvement in the generation of intra-and inter-individual heterogeneity. Eur. J. Biochem. 194, 561–571 (1990).
Melo-Ferreira, J. et al. The elusive nature of adaptive mitochondrial DNA evolution of an arctic lineage prone to frequent introgression. Genome Biol. Evol. 6, 886–896 (2014).
Suissa, S. et al. Ancient mtDNA genetic variants modulate mtDNA transcription and replication. Plos Genet. 5, e1000474 (2009).
Coyne, J. A. & Orr, H. A. Speciation (Sinauer Associates Sunderland (2004).
Thompson, J. N. Relentless Evolution (University of Chicago Press (2013).
Jablonski, D. The tropics as a source of evolutionary novelty through geological time. Nature 364, 142–144 (1993).
Gillooly, J. F., Allen, A. P., West, G. B. & Brown, J. H. The rate of DNA evolution: effects of body size and temperature on the molecular clock. Proc. Natl. Acad. Sci. USA 102, 140–145 (2005).
Dean, A. M., Lehman, C. & Yi, X. Fluctuating Selection in the Moran. Genetics 205, 1271–1283 (2017).
Melbinger, A. & Vergassola, M. The Impact of Environmental Fluctuations on Evolutionary Fitness Functions. Sci. Rep. 5, 15211 (2015).
Fuentes, M. A. & Ferrada, E. Environmental Fluctuations and Their Consequences for the Evolution of Phenotypic Diversity. Aip. Conf. Proc. 5, 16 (2017).
Eddie, K. H. Ho & Aneil F. Agrawal. Mutation accumulation in selfing populations under fluctuating selection. The Society for the Study of Evolution. Evolution 72-9, 1759–1772 (2019).
Iftikar, F. I. & Hickey, A. J. Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. Plos One 8, e64120 (2013).
Somero, G. N. Proteins and temperature. Annu. Rev. Physiol. 57, 43–68 (1995).
Baris, T. Z., Crawford, D. L. & Oleksiak, M. F. “Acclimation and acute temperature effects on population differences in oxidative phosphorylation.”. Am. J. Physiol-Reg. I. 310, R185–R196 (2015).
Rion, S. & Kawecki, T. J. Evolutionary biology of starvation resistance: what we have learned from Drosophila. J. Evolution. Biol. 20, 1655–1664 (2007).
Ballard, J. W. O. & Melvin, R. G. Linking the mitochondrial genotype to the organismal phenotype. Mol. Ecol. 19, 1523–1539 (2010).
Ruiz-Pesini, E., Mishmar, D., Brandon, M., Procaccio, V. & Wallace, D. C. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science 303, 223–226 (2004).
Solaini, G., Baracca, A., Lenaz, G. & Sgarbi, G. Hypoxia and mitochondrial oxidative metabolism. BBA-Bioenergetics 1797, 1171–1177 (2010).
Berg, P. R. et al. Adaptation to low salinity promotes genomic divergence in Atlantic cod Gadus morhua. Genome Biol. Evol. 7, 1644–1663 (2015).
Foote, A. D. et al. Positive selection on the killer whale mitogenome. Biol. Lett-UK. 7, 116–118 (2011).
de Villemereuil, P., Gaggiotti, O. E., Mouterde, M. & Till-Bottraud, I. Common garden experiments in the genomic era: new perspectives and opportunities. Heredity 3, 249–254 (2016).
Gueye, M., Kantoussan, J. & Tine, M. Common Garden Experiments Confirm the Impact of Salinity on Reproductive Traits that is Observed in Wild Populations of the Back-Chinned Tilapia Sarotherodon melanotheron. Int. J. Aquac. Fish. Sci. 2, 031–037 (2016).
DeTolla, L. J. et al. Guidelines for the care and use of fish in research. Ilar J. 1(37), 159–173 (1995).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
Kearse, M. et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 (2012).
Nei, M. Molecular Evolutionary Genetics (Columbia University Press, New York (1987).
Tajima, F. Evolutionary relationship of DNA sequences in finite populations. Genetics 105, 437–460 (1983).
Nei, M. & Gojobori, T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3, 418–426 (1986).
Librado, P. & Rozas, J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009).
Posada, D. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25, 1253–1256 (2008).
Pond, S. L. K. & Frost, S. D. Datamonkey: rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics 21, 2531–2533 (2005).
Woolley, S., Johnson, J., Smith, M. J., Crandall, K. A. & McClellan, D. A. TreeSAAP: selection on amino acid properties using phylogenetic trees. Bioinformatics 19, 671–672 (2003).
McClellan, D. A. & Ellison, D. D. Assessing and improving the accuracy of detecting protein adaptation with the TreeSAAP analytical software. Int. J. Bioinformat. Res. Appl. 6, 11–17 (2010).
Schwede, T., Kopp, J., Guex, N. & Peitsch, M. C. SWISS-MODEL: an automated protein homology-modeling server. Nucleic. Acids. Res. 31, 3381–3385 (2003).
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003).
Acknowledgements
The authors would like to thank Director, Central Marine Fisheries Research Institute (CMFRI), and Dr. P. Vijayagopal (Head, Marine Biotechnology Division, CMFRI) for providing facilities to carry out this work. WS received a Senior Research Fellowship from the ICAR-NICRA project. We also place on record our sincere thanks to Dr. Mohammed Koya, Dr. K.R. Sreenath, Dr. N.K. Sanil, Dr. P.K. Asokan, and Dr. Muktha Menon for helping with sample collection. We would like to thank Dr. S. Prasanna Kumar, Emeritus scientist and former acting director, CSIR-National Institute of Oceanography, Goa for the critical comments and suggestions on this manuscript. This work was carried out under the institute project MBT/GEN/25 receiving funding support from the Indian Council of Agricultural Research (ICAR).
Author information
Authors and Affiliations
Contributions
W.S. conceived the idea, executed the research, and wrote the initial manuscript. S.S. coordinated the research and wrote the initial manuscript. K.R.M. and P.K.D.K. analyzed the oceanographic data and contributed to writing up the manuscript. A.G. and P.U.Z. reviewed the initial manuscript and approved it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sebastian, W., Sukumaran, S., Zacharia, P.U. et al. Signals of selection in the mitogenome provide insights into adaptation mechanisms in heterogeneous habitats in a widely distributed pelagic fish. Sci Rep 10, 9081 (2020). https://doi.org/10.1038/s41598-020-65905-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65905-1
This article is cited by
-
Ancient reindeer mitogenomes reveal island-hopping colonisation of the Arctic archipelagos
Scientific Reports (2024)
-
The sequence and de novo assembly of the genome of the Indian oil sardine, Sardinella longiceps
Scientific Data (2023)
-
Genetic analyses reveal panmixia in Indian waters and population subdivision across Indian Ocean and Indo-Malay Archipelago for Decapterus russelli
Scientific Reports (2023)
-
A vignette of mitogenome and evolutionary status of the giant form of Sthenoteuthis oualaniensis (Lesson, 1830) endemic to the northwestern Arabian Sea
Marine Biology (2023)
-
Positive selection over the mitochondrial genome and its role in the diversification of gentoo penguins in response to adaptation in isolation
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.