Abstract
At present, focal laser ablation (FLA) as a new PCa local treatment has attracted attention. We aim at comparing the survival outcomes between radiotherapy (RT) and FLA to reveal whether FLA can be used as an alternative to RT for patients with low and intermediate-risk localized PCa.We conducted analyses with data from the SEER database (2004–2015). Propensity score matching and instrumental variate (IV) were used to reduce the influence of bias and unmeasured confounders maximally.In the adjusted multivariate regression, FLA had lower overall survival (OS) benefits (HR = 1.49; 95%CI: 1.18–1.87; p < 0.001). After propensity score matching, RT still had better OS (HR = 1.50; 95%CI: 1.17–1.93; p = 0.001). The outcomes of IV-adjusted analysis showed FLA was significantly inferior to RT in OS (HR = 1.49; 95%CI: 1.18–1.87). In the subgroup analyses, for those with PSA < 4 ng/mL, FLA showed markedly worse OS and cancer-specific mortality (CSM) outcomes (OS HR = 1.89; 95%CI: 1.01–3.53; p = 0.0466 and CSM HR = 4.25; 95%CI: 1.04–17.43; p = 0.044).FLA is a promising focal therapy of PCa. But our research demonstrated RT still had an obvious advantage in survival benefits over FLA. Using FLA as an alternative treatment for RT requires careful consideration by clinicians.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is the second most frequent cancer among males, which caused roughly 358,989 deaths in 20181. Meanwhile, the popularization of prostate specific antigen (PSA) screening has increased the diagnosis of low-risk and intermediate-risk localized PCa globally2. According to the statistics from the United States in 2014, patients diagnosed as low and intermediate risk PCa accounted for 74.0% of all PCa patients3. Therefore, reasonable treatments of such patients are currently very important.
Other than Active surveillance (AS), Radiotherapy (RT) and Radical prostatectomy (RP) are the standard active treatments for patients with low-risk or intermediate-risk localized prostate cancer recommended by current clinical guidelines4. However, several important follow-up studies found that whether the patients were treated with RP or RT, many patients had problems with urinary incontinence, erectile dysfunction, or intestinal complications throughout early, intermediate, and long-term follow-up5,6.
In order to improve the quality of life of patients, a new focal therapy, focal laser ablation (FLA), has been developed to ablate tumors selectively while sparing the neurovascular bundles, sphincter, and urethra for better functional outcomes7. A recent phase II clinical trial reported that FLA was associated with favorable short-term oncologic outcomes with no major urinary, sexual, or bowel side effects8. Another small-scale longitudinal outcome study for patients with localized PCa also showed that FLA could achieve early oncologic control of localized PCa with few complications or adverse effects on quality of life9. A larger retrospective study involving 120 patients reported that the 1-year retreatment free rate of patients who had received FLA was 83%, and the sexual and urinary function did not significantly change after FLA10.
Although the short-term oncologic and functional outcomes of FLA are encouraging, current trials of FLA for PCa are all short-term single-arm studies without long-term oncologic outcomes, overall survival (OS) data or cancer-specific mortality (CSM) data. And the number of patients included in the trials is also small. Whether FLA can bring long-term survival benefits equivalent to conventional treatments like RT for patients is still unclear.
To circumvent these defects, we evaluated the overall survival and prostate cancer-specific mortality at long-term follow-up in the comparison of patients treated with FLA versus patients treated with RT.
Results
Patient characteristics
A total of 93,469 patients were included in the analysis, including 93,041 patients treated with radiation therapy and 428 patients treated with laser ablation. Patients’ characteristics were shown in Table 1. Comparing patients who were treated with laser ablation versus those undergone radiotherapy, patients treated with laser ablation were more likely to have older age (p < 0.001), lower PSA (p < 0.05), and lower GS (p < 0.05). There were no differences between both groups on T stage (p = 0.277) and race (p = 0.754). In the radiation therapy cohort, 81,015 patients died, and 1,303 of whom died of prostate cancer-specific reasons. In the focal laser ablation cohort, 356 patients died, and 7 of whom died of prostate cancer-specific reasons. Inclusion and exclusion criteria were shown in the flowchart in detail (Fig. 1).
Survival outcomes according to different treatments
From the multivariate regression analysis, FLA was associated with worse OS benefits compared with RT (Hazard ratio [HR] = 1.91; 95% confidence interval [CI]: 1.51–2.40; p < 0.001). After adjusting for the covariates including age, T stage, PSA level, GS, the results did not change significantly (HR = 1.49; 95%CI: 1.18–1.87; p < 0.001). These two treatments were evaluated in multivariate regression analysis of CSM as well. The results showed that there was no significant difference in the extent of which FLA and RT reduced CSM (HR = 1.73; 95%CI: 0.82–3.64; p = 0.147). After adjusting confounding factors, FLA and RT still maintained the same effects in reducing CSM (HR: 1.57; 95% Cl: 0.74–3.29; p = 0.237). According to the Kaplan-Meier curves, same as previous analyses, FLA performed significantly worse than RT in increasing OS, while there was no obvious difference in reducing CSM compared with RT (Fig. 2).
Kaplan-Meier survival curves of primary cohorts (RT VS FLA). A, Kaplan-Meier survival curve of OS in the comparison of RT and FLA. B, Kaplan-Meier survival curve of CSS in the comparison of RT and FLA. Abbreviation: OS = Overall survival, CSS = Cancer-specific survival, RT = Radiation therapy, FLA = Focal laser ablation. Abbreviation: RT = radiotherapy, FLA = focal laser ablation.
Survival analysis after propensity score matching
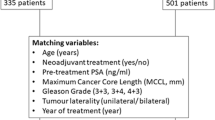
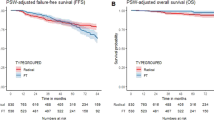
Following the propensity score matching (PSM), 2,568 RT-treated patients matched 428 FLA-treated patients. (Supplementary Table 1) The statistical differences in baseline characteristics between the two groups were eliminated after PSM (Table 2). In the matched radiation therapy cohort, 2,133 patients died, and 41 of whom died of prostate cancer-specific reasons. In the focal laser ablation cohort, 356 patients died, and 7 of whom died of prostate cancer-specific reasons. We performed another multivariate regression analysis in the matched cohort for OS and CSM, patients treated with RT still had better overall survival benefits (HR = 1.50; 95%CI: 1.17–1.93; p = 0.001), but RT and FLA remained consistent in reducing CSM performance with no significant difference (HR = 1.48; 95%CI: 0.66–3.32; p = 0.336). Kaplan-Meier analysis was performed in the matched cohort, and the results also showed that RT was better than FLA in OS, but there was no significant difference in CSS (Fig. 3).
Kaplan-Meier survival curves of matched cohorts (RT VS FLA). A, Kaplan-Meier survival curve of OS in the comparison of RT and FLA. B, Kaplan-Meier survival curve of CSS in the comparison of RT and FLA. Abbreviation: OS = Overall survival, CSS = Cancer-specific survival, RT = Radiation therapy, FLA = Focal laser ablation. Abbreviation: RT = radiotherapy, FLA = focal laser ablation.
Survival outcomes from further statistical analysis
The outcomes of instrument variate (IVA) adjusted analysis showed that the ability of FLA to improve OS was still significantly inferior to RT (HR = 1.49; 95%CI: 1.18–1.87). And same as previous analysis, there was no obvious difference of CSM in the comparison of RT and FLA (HR = 1.57; 95%CI: 0.74–3.29) (Table 3).
Our subgroup analyses showed patients with different T stage, PSA levels and Gleason scores had a different degree of reactions to FLA and RT. For those with T stage of T1, the OS of FLA was obviously worse than RT, showing that RT can bring significant OS benefits (HR = 1.51; 95%CI: 1.19–1.92; p < 0.001). The CSM outcomes had no significant difference between the two treatments (HR = 1.50; 95%CI: 0.67–3.34; p < 0.001). Similarly, FLA showed markedly worse OS and CSM outcomes compared with RT for those with PSA < 4 ng/mL (OS HR = 1.89; 95%CI: 1.01–3.53; p = 0.047 and CSM HR = 4.25; 95%CI: 1.04–17.43; p = 0.044). Notably, for patients with GS 4 + 3, RT performed significantly better than FLA in both OS and CSM (OS HR = 2.08; 95%CI: 1.15–3.76; p = 0.016 and CSM HR = 5.23; 95%CI: 1.95–14.05; p = 0.001) (Fig. 4).
Subgroup analyses of OM and CSM (RT VS FLA). A, Subgroup analysis of OM in the comparison of RT and FLA. B, Subgroup analysis of CSM in the comparison of RT and FLA. Abbreviation: OM = Overall Mortality, CSM = Cancer Specific Mortality, RT = Radiotherapy, FLA = Focal laser ablation, GS = Gleason Score, PSA = Prostate Specific Antigen, Q1-Q4 = Quartile 1 - Quartile 4.
As for sensitivity analysis, the inverse probability of treatment weighting (IPTW) adjusted model showed that FLA may be both related to a higher risk of overall death and CSM. (OS HR = 1.40; 95%CI: 1.36–1.44; p < 0.001 and CSM HR = 1.21; 95%CI: 1.12–1.31; p < 0.001). Meanwhile, the results of the standardized mortality ratio weighting (SMRW) adjusted model also indicated that RT had advantages over FLA in improving OS of patients (HR = 1.44; 95%CI: 1.03–2.02; p = 0.032) (Supplementary Table 2).
Discussion
FLA is a new organ-preserving treatment of PCa that aims to reduce complications and improve patients’ quality of life without affecting oncological control. The theory of FLA is to induce coagulation necrosis of tumor cells by heating of targeted tissue, using the high- energy delivered by laser fibers inserted through needles9,11.
Our study included long-term survival data from 428 FLA-treated patients and 93041 RT-treated patients and compared OS and CSM outcomes between RT and FLA. Our results showed that patients received RT treatment can achieve significantly better long-term OS than those received FLA. Although these two methods performed not much differently in CSM.
Several studies published in recent years showed that FLA performed well on short-term oncologic outcomes and had the ability to reduce complications that could affect the quality of life of patients9,12,13.
A Phase II evaluation of FLA including 27 FLA-treated patients reported a favorable oncologic outcome within 1 year. The targeted biopsy of the ablation zone found persistent cancer in only 1/27 men 3 months after treatment and the systematic biopsy found cancer in 10/27 men 12 months after treatment8. A larger cohort study of 120 FLA-treated patients reported a significant reduction in PSA levels after a one-year follow-up (pre-FLA mean PSA 6.05 ng/ml and post-FLA mean PSA 3.25 ng/ml, respectively), with only 17% of patients receiving tumor therapy again because of positive biopsy after MR imaging abnormalities10. This demonstrated the important role of FLA in limiting disease progression.
At the same time, the two studies above both used International Prostate Symptom Score (IPSS) and Sexual Health Inventory for Men (SHIM) to measure erectile function and urinary function before and after FLA, and the results showed that there was no significant difference. Compared with the decline in quality of life that often occurred in RT or RP, FLA showed its advantages in functional protection14. However, these studies still lacked long-term survival outcomes and comparisons of survival benefits between RT or RP.
Through our research on low risk and intermediate risk PCa patients, we could find that although the short-term oncologic and functional outcomes of FLA were excellent, RT was still significantly better than FLA in long-term survival benefits, especially for patients with T stage T1, PSA < 4 ng/mL, and GS 4 + 3. In Fig. 4, we could see that GS 4 + 3 group was the most obvious group that FLA performed inferior to RT. The latest AUA clinical guidelines also indicated that several studies had demonstrated similar results that the prognosis of Gleason Score = 4 + 3 was significantly worse than Gleason Score = 3 + 4. According to this, the intermediate-risk prostate cancer was divided into intermediate prostate cancer with good prognosis (favorable) and intermediate risk prostate cancer with poor prognosis (unfavorable)4,15,16,17. For such patients, focal therapy might no longer be effective in controlling tumor progression, compared with other whole-gland treatments.
There are some reasons why the FLA is significantly worse than RT on long-term OS benefits in the case of a small difference in CSM. First, as a kind focal therapy, FLA has more potential risk for incomplete tumor tissue clearance than whole gland therapy18. Interim results of a study of 98 patients and 138 tumor foci treated with FLA reported 23% rate of cancer residual or recurrent cancer19. Similarly, in a 2-year follow-up, there were 17/34 men with positive biopsy at 2 years after FLA13. Focal therapy is often susceptible to poor navigation, inadequacy of imaging to delineate tumor boundaries and variable precision of tissue destruction19. These risk factors of FLA may not be manifested until a long time, and ultimately reflected in the differences in OS. Second, for patients with low risk and intermediate risk PCa, the risk of cancer-specific death is inherently low20,21. Therefore, the number of samples of CSM is relatively small compared to OS (Alive or death from other diseases: 98.4% and cancer-specific deaths: 1.6% relatively). At the same time, FLA had obvious higher HR values in CSM, which also indicated that the low difference in CSM should be caused by a small sample size, not the treatments themselves. The low difference in CSM does not indicate that FLA can give patients the same level of survival benefit as RT.
The main advantages of our research are as follows. First, our research samples were of a large number, having an advantage in quantity compared with the reported studies about FLA. Second, as far as we know, our study was the first one that compared the retrospective data of FLA and RT. Third, current researches focused on short-term oncologic control, while our research mainly focused on long-term survival outcomes. We used a series of statistical analyses to reduce bias and confounding factors, confirming that RT could provide more survival benefits than FLA for patients with low and intermediate risk PCa. Our results could provide a reference for future treatment options. Clinicians should consider that RT is a more effective treatment because of its advantages over FLA in survival benefits.
There are still several limitations to our study. First, although we have used varies statistical methods to reduce the bias, it was essentially a retrospective study with a lower level of evidence than randomized controlled trial (RCT). No RCTs on FLA have been reported yet, thus more high-quality RCTs are still needed to better evaluate the effectiveness and safety of FLA, especially RCTs comparing FLA with RT, RP or AS. In addition, clear clinically relevant objectives such as negative biopsy, toxicity, and optimal follow-up schedules also require more RCTs to define22. Until these studies are completed, FLA can only be used as an experimental treatment. Second, due to the database’s limitations, patients’ baseline data were not comprehensive and there may be potential confounding factors. We selected IVA to overcome this problem. Third, functional data such as urinary function and erectile function are also absent. There were several clinical trials on FLA mentioned above suggesting that FLA has little effect on patients’ quality of life, while Prostate Testing for Cancer and Treatment (ProtecT) trial reported the negative effects of radiotherapy in erectile dysfunction, decreased bowel function, and urinary voiding and nocturia23.
In our study, we selected patients diagnosed during 2004 to 2015. The main reason for this is that the Surveillance, Epidemiology, and End Results (SEER) database began collecting TNM staging information of American Joint Committee on Cancer [AJCC] Cancer Staging Manual in 200424. Using this widely used staging system, we could enable us to more accurately include patients. However, due to the limitation of the time period, we could not ensure that all patients in the RT cohort had received the most modern radiotherapy technology currently available, such as image guided intensity modulated-dose escalated radiation therapy (IMRT). IMRT is the current mainstream radiotherapy technology, the usage of IMRT has increased from 3.1% in 2001 to 64.7% in 2013 in North America25. Compared with other radiotherapy technologies like three-dimensional conformal radiotherapy (3D-CRT), some clinical trials have revealed that IMRT has advantages in reducing side effects and biochemical recurrence rate26,27. Our study, which included some patients with outdated radiotherapy techniques, still yielded results that RT currently performed better than FLA in survival outcomes. Therefore, we think that the differences in radiotherapy technologies will not significantly affect our findings, and the advantages of RT over FLA may be more obvious in a radiation therapy cohort that includes IMRT only.
Conclusion
Although several researches have confirmed the excellent performance of FLA in controlling the progression of PCa and functional protection in the short term, for patients with low risk and intermediate risk PCa, RT still provided better long-term survival benefits. In the future, if FLA can solve its current technical shortcomings such as navigation, imaging, and precision, its therapeutic effect may be better with favorable survival benefits and functional protection at the same time. But at present, RT should have a priority over FLA in the management of low risk and intermediate risk PCa.
Materials and Methods
Patient selection
From the SEER database, patients with a diagnosis of adenocarcinoma of the prostate (International Classification of diseases-O-3 code: C61.9) between 2004 and 2015 were selected. The TNM stages were assessed by the 7th edition of AJCC Cancer Staging Manual28. Inclusion and exclusion criteria were available in Fig. 1.
Outcomes
The primary endpoint was OS. The secondary endpoint was CSM. CSM included all deaths caused by prostate cancer, complications of treatments, or unknown process in patients with active prostate cancer. Follow-up time was defined as the time between the first treatment of RT or FLA and patient death or last follow-up.
Statistical analysis
Firstly, the baseline characteristics were compared between the two groups (RT and FLA). A 2-tail t-test was used to reveal the difference in continuous variables, presenting the results as mean ± standard deviation. Likewise, a X2 test was used to expose the difference in categorical variables, and the results were presented as the frequency with its proportion.
Secondly, between the two treatment groups, multivariate Cox proportional hazard models were performed to evaluate CSM and OS rates before and after adjusting confounders.
To overcome the selection bias, we performed propensity score matching, of which the propensity scores were reckoned by logistic regression, with both two treatments (RT and FLA) as the outcome and age, T stage, PSA level, Gleason score as the pretreatment and prognostic covariates. When P > 0.05, the baseline characteristics of the matched cohort were considered to be balanced. Meanwhile, we used the Kaplan-Meier method to plot the cumulative incidence survival curve of the original cohort.
To further reduce the impact of selection bias and calculate unmeasured confounders on the outcomes, we also used the regional utilization rate as an instrument variate in the two-stage residual inclusion analysis29,30. All covariates and residual were included together to establish a new multivariate Cox proportional hazard model to demonstrate more accurate results.
Several different sensitivity analyses were used to verify the robustness of the results. (1) The analyses of OS and CSM after adjusting propensity scores; (2) Among the whole cohort, IPTW and SMRW methods were used to estimate the relationship between treatment types and outcomes. (3) Analyses of OS and CSM in different groups stratified by propensity scores. (4) Analyses of OS and CSM after the adjustment of unbalanced covariates among the matched cohort.
The statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) were used in the above statistical analyses. A p-value <0.05 was considered statistically significant.
Data availability
The data that support the findings of this study are available in SEER dataset at https://seer.cancer.gov/. These data were derived from the following resources available in the public domain: SEER Incidence Data, 1975–2016 https://seer.cancer.gov/data/.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Huland, H. & Graefen, M. Changing Trends in Surgical Management of Prostate Cancer: The End of Overtreatment? Eur. Urol. 68, 175–178 (2015).
Fletcher, S. A., et al. Contemporary national trends in prostate cancer risk profile at diagnosis. Prostate Cancer Prostatic Dis, https://doi.org/10.1038/s41391-019-0157-y
Sanda, M. G. et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J. Urol. 199, 990–997 (2018).
Resnick, M. J. et al. Long-term functional outcomes after treatment for localized prostate cancer. N. Engl. J. Med. 368, 436–445 (2013).
Wallis, C. et al. Survival and Complications Following Surgery and Radiation for Localized Prostate Cancer: An International Collaborative Review. EUR. UROL. 73, 11–20 (2018).
Mottet, N. et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. EUR. UROL. 71, 618–629 (2017).
Eggener, S. E., Yousuf, A., Watson, S., Wang, S. & Oto, A. Phase II Evaluation of Magnetic Resonance Imaging Guided Focal Laser Ablation of Prostate Cancer. J. UROL. 196, 1670–1675 (2016).
Lepor, H., Llukani, E., Sperling, D. & Fütterer, J. J. Complications, Recovery, and Early Functional Outcomes and Oncologic Control Following In-bore Focal Laser Ablation of Prostate Cancer. EUR. UROL. 68, 924–926 (2015).
Walser, E. et al. Focal Laser Ablation of Prostate Cancer: Results in 120 Patients with Low- to Intermediate-Risk Disease. J. Vasc. Interv. Radiol. 30, 401–409. e2 (2019).
Oto, A. et al. MR Imaging–guided Focal Laser Ablation for Prostate Cancer: Phase I Trial. Radiology 267, 932–940 (2013).
Natarajan, S. et al. Focal Laser Ablation of Prostate Cancer: Feasibility of Magnetic Resonance Imaging-Ultrasound Fusion for Guidance. J. UROL. 198, 839–847 (2017).
Chao, B., Llukani, E. & Lepor, H. Two-year Outcomes Following Focal Laser Ablation of Localized Prostate Cancer. Eur. Urol. Oncol. 1, 129–133 (2018).
Chen, R. C. et al. Association Between Choice of Radical Prostatectomy, External Beam Radiotherapy, Brachytherapy, or Active Surveillance and Patient-Reported Quality of Life Among Men With Localized Prostate Cancer. JAMA 317, 1141 (2017).
Mathieu, R. et al. Prognostic value of the new Grade Groups in Prostate Cancer: a multi-institutional European validation study. Prostate Cancer Prostatic Dis. 20, 197–202 (2017).
Zumsteg, Z. S. et al. Number of Unfavorable Intermediate-Risk Factors Predicts Pathologic Upstaging and Prostate Cancer-Specific Mortality Following Radical Prostatectomy: Results From the SEARCH Database. Prostate 77, 154–163 (2017).
Zumsteg, Z. S. et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur. Urol. 64, 895–902 (2013).
Ahdoot, M., Lebastchi, A. H., Turkbey, B., Wood, B. & Pinto, P. A. Contemporary treatments in prostate cancer focal therapy. Curr. Opin. Oncol. 31, 200–206 (2019).
Feller, J., Greenwood, B., Jones, W. & Toth, R. MP30-02 Transrectally Delivered, Outpatient Mri-Guided Laser Focal Therapy Of Prostate Cancer: Seven Year Interim Results Of NCT #02243033. J. UROL. 199, e374–e375 (2018).
Godtman, R. A. et al. Long-term Results of Active Surveillance in the Goteborg Randomized, Population-based Prostate Cancer Screening Trial. EUR. UROL. 70, 760–766 (2016).
Meelan, B. et al. Outcomes of initially expectantly managed patients with low or intermediate risk screen-detected localized prostate cancer. BJU INT. 110, 1672–1677 (2012).
van der Poel, H. G. et al. Focal Therapy in Primary Localised Prostate Cancer: The European Association of Urology Position in 2018. Eur. Urol. 74, 84–91 (2018).
Donovan, J. L. et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. ENGL. J. MED. 375, 1425–1437 (2016).
Schymura, M. J., Sun, L. & Percy-Laurry, A. Prostate cancer collaborative stage data items–their definitions, quality, usage, and clinical implications: a review of SEER data for 2004–2010. Cancer 120(Suppl 23), 3758–3770 (2014).
Hatano, K. et al. Current status of intensity-modulated radiation therapy for prostate cancer: History, clinical results and future directions. Int. J. Urol. 26, 775–784 (2019).
Wortel, R. C. et al. Late Side Effects After Image Guided Intensity Modulated Radiation Therapy Compared to 3D-Conformal Radiation Therapy for Prostate Cancer: Results From 2 Prospective Cohorts. Int. J. Radiat. Oncol. Biol. Phys. 95, 680–689 (2016).
Vora, S. A., Wong, W. W., Schild, S. E., Ezzell, G. A. & Halyard, M. Y. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 68, 1053–1058 (2007).
Cuccurullo, V. & Mansi, L. AJCC Cancer Staging Handbook: from the AJCC Cancer Staging Manual (7th edition). EUR. J. NUCL. MED. MOL. I 38, 408 (2011).
Terza, J. V. Two-Stage Residual Inclusion Estimation in Health Services Research and Health Economics. HEALTH SERV. RES. 53, 1890–1899 (2018).
Terza, J. V., Basu, A. & Rathouz, P. J. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J. HEALTH ECON. 27, 531–543 (2008).
Acknowledgements
This study was supported by The National Key Research and Development Program of China (Grant No.2017YFC0908003). Post-Doctor Research Project, West China Hospital, Sichuan University (Grant No. 2018HXBH085). The National Natural Science Foundation of China (Grant Nos. 81300627, 81200551, 81270841, 81460148 and 81370855). Fundings from Science and Technology Department of Sichuan Province (Grant No. 2014JY0219 and 2017HH0063). The Prostate Cancer Foundation Young Investigator Award 2013.
Author information
Authors and Affiliations
Contributions
X.Z., K.J. and S.Q. contributed equally to this study. X.Z., K.J., L.Y. and Q.W. designed the study. X.Z., K.J. and S.Q. drafted the manuscript. X.Z., D.J., Q.S. and X.L. performed the statistical analysis. X.T., X. Zheng., J.L. and K.J. performed the data collection. All authors reviewed and final approval of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementray information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, X., Jin, K., Qiu, S. et al. Comparative Effectiveness of Radiotherapy versus Focal Laser Ablation in Patients with Low and Intermediate Risk Localized Prostate Cancer. Sci Rep 10, 9112 (2020). https://doi.org/10.1038/s41598-020-65863-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65863-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.