Abstract
Hyperuricemia has been recognized as a worldwide public health concern. This study was conducted to examine the association between serum copper (Cu) concentration and the prevalence of hyperuricemia in a middle-aged and elderly population. Serum Cu concentration was measured by Roche modular P800 using the PAESA method. Serum uric acid (UA) concentration was detected by a Beckman Coulter AU 5800. Presence of hyperuricemia was defined as serum UA ≥ 416 μmol/L for men and ≥360 μmol/L for women. The association between serum Cu concentration and the prevalence of hyperuricemia was evaluated by logistic regression. The prevalence of hyperuricemia was 17.6% (n = 6,212) in the present study. Relative to the lowest quintile, the age- and sex-adjusted odds ratios for hyperuricemia were 1.38 (95% CI: 1.12 to 1.70), 1.34 (95% CI: 1.07 to 1.66), and 1.53 (95% CI: 1.23 to 1.91) in the third, fourth, and fifth serum Cu concentration quintiles (P for trend < 0.001). Similar results were found both in men and women subgroups. None of the findings were materially altered after adjustment for additional potential confounders. In conclusion, in this population-based cross-sectional study, serum Cu concentration was positively associated with the prevalence of hyperuricemia.
Similar content being viewed by others
Introduction
Hyperuricemia is a condition characterized by abnormally elevated levels of serum uric acid (UA). The reported prevalence of hyperuricemia has been showing a rising trend in recent years1,2, which ranged from 17.0% to 22.9% in men and 5.9% to 21.6% in women1,3,4,5,6. Previous studies have suggested that hyperuricemia is associated with hypertension, metabolic syndrome, cardiovascular diseases, chronic kidney diseases, diabetes mellitus, and an increased risk of all-cause mortality7,8,9,10,11,12. However, uncertainties still exist with respect to the specific pathogenesis of hyperuricemia.
Copper (Cu) is one of the essential trace elements involved in many important biological processes of living organisms13. Cu in vivo has demonstrated both its pro- and anti-oxidant effects14. UA is directly oxidized by Cu2+15, and the Cu-reduction ability of UA was presumed to influence both the pro- and anti-oxidant behaviors of UA16. To the best of our knowledge, only one study has attempted to associate serum Cu with serum UA. Bo et al.17 detected the serum Cu and serum UA concentration in a randomly identified subgroup of men (n = 231) from a study of adults (45- to 64-year-old) in Italy, in which no significant association between serum Cu concentration and UA was concluded. However, the sample size of the study was relatively small and women subjects was not included. Up to now, there has been no research directly related serum Cu to prevalence of hyperuricemia.
To fill in this knowledge gap, we used data collected from a large population-based cross-sectional study to investigate whether there is an association between serum Cu levels and the prevalence of hyperuricemia.
Materials and methods
Study population
The present cross-sectional study was performed in Xiangya Hospital, Central South University in Changsha, Hunan Province, China3,18. All procedures were conducted in accordance with the relevant guidelines and regulations. In order to acquire data on health-related habits and demographic characteristics, registered nurses performed face-to-face interview on all the subjects during physical examination with a standard questionnaire. The included subjects were chosen based on the criteria as follows: 1) ≥40 years old; 2) receiving serum UA and serum Cu measurements; 3) providing required personal features, including age, sex and body mass index (BMI); 4) providing health-related habits, including activity level, smoking and alcohol drinking status. From October 2013 to December 2015, there were 6212 subjects in all received routine measurements like serum UA and serum Cu, and also provided detailed demographic features and required health-related habits.
Assessment of hyperuricemia
The blood samples of all the subjects were collected after a 12-hour overnight fast and then preserved at 4 °C until assayed. Serum UA concentration was detected by a Beckman Coulter AU 5800 (Beckman Coulter Inc., Brea, CA, USA), and the results were used to diagnose hyperuricemia (UA ≥ 416 μmol/L for men and ≥360 μmol/L for women).
Assessment of exposures
The serum Cu concentration was measured by Roche modular P800 using the PAESA method. The inter-assay coefficients of variation were 6.15% (9.8 μmol/L) and 4.325% (16.5 μmol/L), and the intra-assay coefficients of variation were 5.17% (16.72 μmol/L) and 5.37% (8.86 μmol/L) for serum Cu respectively. Fast blood glucose was tested using glucose oxidase enzyme method. Blood pressure was checked on an electronic sphygmomanometer. Diabetes was diagnosed as fasting glucose level ≥7.0 mmol/L or if the subject was receiving drug treatment to control blood glucose. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, or if the subject was taking antihypertensive treatment. The BMI of each subject was calculated as weight in kilograms divided by height in meters squared. The information of sports activities, smoking and drinking status was extracted from questionnaires answered by subjects. Sports activities included average frequency and average duration, and current status of smoking and drinking was evaluated in binary (yes or no).
Statistical analysis
The quantitative data were presented in form of mean ± standard deviation, and the qualitative data were presented in form of percentage. The serum Cu concentration was divided into 5 categories in terms of the quintile distribution of the sample: ≤13.60, 13.61–15.40, 15.41–17.00, 17.01–19.00 and ≥19.01 μmol/L. The one-way classification ANOVA and the Kruskal-Wallis H test was performed to evaluate continuous data for normal distribution and abnormal distribution, and the χ2 test was employed to assess the differences between qualitative data. The quintile with the lowest value of serum Cu concentration was regarded as reference, age- and sex- adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were reported to indicate the association between serum Cu and the prevalence of hyperuricemia in each quintile, respectively (Model 1). Moreover, two multivariable models (Model 2 and 3) were also used for logistic regression analyses in the whole, male and female population. The covariate adjusted model 2 included age, sex, BMI, smoking, drinking status, and we additionally adjusted for education, activity level, hypertension, diabetes in subsequent model 3. Median variables of serum Cu concentration in first to the highest quintile were used to test the linear trends by logistic regression. To increase statistical power, we additionally modelled continuous serum Cu concentrations, estimating the effect on hyperuricemia prevalence per 10 μmol/L increase. A scatter plot was employed to test linear relationship between serum Cu and UA after multivariable analyses.
The collected data were conducted by SPSS 17.0 and STATA 12.0; a P value of ≤0.05 was considered as the criterion for statistical significance.
Ethical approval and informed consent
The study protocol had been approved by the Ethics Committee of Xiangya Hospital, Central South University (reference number: 201312459). Written informed consents had been acquired from all included participants prior to the performance of research.
Results
A total of 6,212 subjects (3,700 male and 2,512 female) were recruited in the current analysis. The overall prevalence of hyperuricemia in this study was 17.6%. Table 1 shows basic characteristics of the whole population and subjects according to their hyperuricemia status. Significant differences were observed between the hyperuricemia and non-hyperuricemia population in terms of sex, smoking, drinking status, education, BMI, occupation, hypertension and diabetes.
The results of demographic characteristics and confounding factors are shown in Table 2, in accordance with the quintiles of serum Cu concentration. There were significant differences across all quartiles of serum Cu in terms of age, sex, smoking status, drinking status, education, activity level, BMI, occupation, hypertension and diabetes.
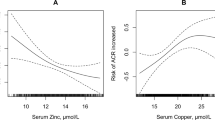
The outcomes of multivariable adjusted connections between serum Cu concentration and the prevalence of hyperuricemia are listed in Table 3. Comparing with the lowest quintile, age- and gender- adjusted ORs (Model 1) of the total population demonstrated significant increased prevalence of hyperuricemia in the third [OR 1.38 (95% CI: 1.12 to 1.70)], fourth [OR 1.37(95% CI: 1.07 to 1.66)] and highest [OR 1.53 (95% CI: 1.23 to 1.91)] quintile of serum Cu, with P for trend <0.001; significant increased prevalence of hyperuricemia was also observed in the third [OR 1.36 (95% CI: 1.10 to 1.68)], fourth [OR 1.34 (95% CI: 1.07 to 1.67)] and highest [OR 1.55, 95% CI: 1.24 to 1.94)] quintiles after adjustment for multiple confounders (Model 2) including age, sex, BMI, smoking, drinking status, with P for trend <0.001; by adding education, activity level, occupation, hypertension and diabetes, multivariable adjusted ORs (Model 3) exhibited similar results, significant higher risk of hyperuricemia was found in the third [OR 1.31 (95% CI: 1.06 to 1.62)], fourth [OR 1.30 (95% CI: 1.04 to 1.62)] and highest [OR 1.52 (95% CI: 1.21 to 1.90)] quintiles, with P for trend <0.001. Similar results were found in the male population, all three regression models revealed that men in the third, fourth and highest quintiles presented a higher risk of hyperuricemia than those in the lowest quartile. Compared with men in the lowest quartile of serum Cu, those in highest quartile had higher odds of prevalence of hyperuricemia [Model 1: OR 1.33 (95% CI: 1.03 to 1.72); Model 2: OR 1.35(95% CI: 1.04 to 1.76); Model 3: OR 1.35(95% CI: 1.04 to 1.77)], the P values for trend in Model 1 to 3 were 0.005, 0.004, 0.005, respectively. For the female population, compared with the lowest quintile, only the highest quintile showed significant increased prevalence of hyperuricemia [Model 1: OR 2.00 (95% CI: 1.16 to 3.43); Model 2: OR 1.94 (95% CI: 1.12 to 3.35); Model 3: OR 1.87 (95% CI: 1.08 to 3.23)], the P values for trend in Model 1 to 3 were 0.002, 0.003, 0.006, respectively. Results from multivariable models confirmed the positive association when serum Cu was considered as a continuous exposure variable (per 10 μmol/L increment, Table 3). In addition, serum Cu was significantly associated with UA (P < 0.001) after adjustment for age, sex, BMI, smoking, drinking, education, activity, occupation, hypertension and diabetes (Fig. 1).
Discussion
The present cross-sectional study showed a positive association between serum Cu concentrations and the prevalence of hyperuricemia, and the findings remained consistent after adjustment for confounders.
Few studies have examined the association between Cu and serum UA, and the findings are still inconclusive. By conducted a population-based study with 1197 subjects aged between 45 to 64 years, Bo et al.17 noted that UA decreased significantly from the lowest to the highest tertile of Cu intake; however, in a randomly identified subgroup of male subjects (n = 231) from this study, Bo et al. did not find any significant association between serum Cu and serum UA. Unlike Bo et al., the present study indicated the prevalence of hyperuricemia was positively associated with serum Cu concentration, which may be attribute to our large sample size. Fields et al.19 found that rats with Cu-deficient diet exhibited high levels of plasma UA; while Derouiche et al.20 reported that Cu supplementation caused an augmentation of UA in rats with or without diabetes. However, the above animal studies only provided evidence for the relationship between dietary Cu and serum UA.
Under certain conditions, Cu plays its role as an activator of Xanthine oxidase (XO). XO is a key enzyme in purine metabolism, which can oxidize hypoxanthine from nucleic acid metabolites into xanthine, and xanthine into UA. Though several studies have documented the inhibition effect of Cu on XO21,22,23, in the later study conducted by Hadizadeh et al.24, Cu was proved to be both a reversible inhibitor and an activator of XO, a progressive reduction of catalytic efficiency was observed when the Cu2+ concentration raised from 5 to 700 μM.
The biological mechanisms linking serum Cu to hyperuricemia are unclear. It has been reported that UA may function either as an anti-oxidant (mainly in plasma) or pro-oxidant (mainly in cells)25. A high UA concentration may contribute to the antioxidant behavior of UA16,26, while both UA and hyperuricemia related pathologies, such as cardiovascular disease, hypertension and obesity, are associated with oxidative stress8,9,12,27,28,29. Therefore, UA may play a very complex role through the anti-oxidant and pro-oxidant effects in hyperuricemia. Cu2+ is able to oxidize UA directly15, Cu2+/LDL ratio may be an influence factor on pro- and anti-oxidant behaviors of UA30. The Cu-reduction ability of UA explains not only its pro-oxidant behavior but also, in part, its anti-oxidant activity16. Moreover, UA is a potent mediator of inflammation. A high concentration of UA has been demonstrated to mediate inflammation by stimulating inflammatory cytokines31,32 and the nuclear factor kappa B (NF-κB) pathway33,34. On the other hand, serum Cu level was identified to be positively correlated with inflammation activity markers17,35,36, and Cu was proved to activate NF-κB37 and enhance the production of pro-inflammatory cytokines38,39,40. Based on the analysis above, the serum Cu may promote the effect of UA through the inflammatory mechanism.
The present study is characterized with several strengths. First, it is the first research work performed on a large sample (6,212 subjects) that directly relates serum Cu to hyperuricemia. The findings of this study may provide new insights into the mechanism and treatment of hyperuricemia. Second, adjustments for some potential confounding factors, such as education, activity level, occupation, hypertension and diabetes, increased reliability of the results. It should be noted that our study had several potential limitations. First, based on the cross-sectional data provided in this study, we cannot draw a conclusion that reflect the causal correlations. Further intervention trials and prospective longitudinal studies are therefore expected to establish a causal relationship between serum Cu and hyperuricemia. Second, the total serum Cu concentration was detected in this study. While approximately 90–95% of the total amount of Cu in blood serum is strongly protein-bound, mostly with α2–globulin (ceruloplasmin)13, the rest of Cu remains non-bounded (non-ceruloplasmin Cu). Ogihara et al.41 observed an elevation of non-ceruloplasmin as well as a reduction of UA in three of four untreated patients with Wilson’s disease, but the sample size was too small (only 4 patients) and all subjects were in a Wilson’s disease condition. A further study focusing on the relationship between ceruloplasmin and non-ceruloplasmin Cu with serum UA is therefore suggested.
Conclusion
In this population-based cross-sectional study, serum Cu concentration was positively associated with the prevalence of hyperuricemia. Further studies are required to estimate the exact mechanisms of the association between Cu and hyperuricemia pathogenesis.
Data availability
The data that support the findings of this study are available from the corresponding authors, TY and YW, upon reasonable request.
References
Zhu, Y., Pandya, B. J. & Choi, H. K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 63, 3136–3141, https://doi.org/10.1002/art.30520 (2011).
Benn, C. L. et al. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front. Med. 5, 160, https://doi.org/10.3389/fmed.2018.00160 (2018).
Xie, D. X. et al. Association between low dietary zinc and hyperuricaemia in middle-aged and older males in China: a cross-sectional study. BMJ Open. 5, e008637, https://doi.org/10.1136/bmjopen-2015-008637 (2015).
Chuang, S. Y., Lee, S. C., Hsieh, Y. T. & Pan, W. H. Trends in hyperuricemia and gout prevalence: Nutrition and Health Survey in Taiwan from 1993–1996 to 2005–2008. Asia Pac. J. Clin. Nutr. 20, 301–308 (2011).
Kim, Y., Kang, J. & Kim, G. T. Prevalence of hyperuricemia and its associated factors in the general Korean population: an analysis of a population-based nationally representative sample. Clin. Rheumatol. 37, 2529–2538, https://doi.org/10.1007/s10067-018-4130-2 (2018).
Liu, Z. et al. Association between Eating Away from Home and Hyperuricemia: A Population-Based Nationwide Cross-Sectional Study in China. Biomed. Res. Int. 2019, 2792681, https://doi.org/10.1155/2019/2792681 (2019).
Tamariz, L. et al. Uric acid as a predictor of all-cause mortality in heart failure: a meta-analysis. Congest. Heart Fail. 17, 25–30, https://doi.org/10.1111/j.1751-7133.2011.00200.x (2011).
Li, M. et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci. Rep. 6, 19520, https://doi.org/10.1038/srep19520 (2016).
Wei, F. et al. Associations between serum uric acid and the incidence of hypertension: a Chinese senior dynamic cohort study. J. Transl. Med. 14, 110, https://doi.org/10.1186/s12967-016-0866-0 (2016).
Kang, D. H. Hyperuricemia and Progression of Chronic Kidney Disease: Role of Phenotype Transition of Renal Tubular and Endothelial Cells. Contrib. Nephrol. 192, 48–55, https://doi.org/10.1159/000484278 (2018).
Puig, J. G. & Martinez, M. A. Hyperuricemia, gout and the metabolic syndrome. Curr. Opin. Rheumatol. 20, 187–191, https://doi.org/10.1097/BOR.0b013e3282f4b1ed (2008).
Sluijs, I. et al. Plasma uric acid is associated with increased risk of type 2 diabetes independent of diet and metabolic risk factors. J. Nutr. 143, 80–85, https://doi.org/10.3945/jn.112.167221 (2013).
Tapiero, H., Townsend, D. M. & Tew, K. D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 57, 386–398 (2003).
Ferns, G. A., Lamb, D. J. & Taylor, A. The possible role of copper ions in atherogenesis: the Blue Janus. Atherosclerosis 133, 139–152 (1997).
Becker, B. F. Towards the physiological function of uric acid. Free. Radic. Biol. Med. 14, 615–631 (1993).
Sanguinetti, S. M. et al. Nitric oxide inhibits prooxidant actions of uric acid during copper-mediated LDL oxidation. Arch. Biochem. Biophys. 423, 302–308, https://doi.org/10.1016/j.abb.2003.12.034 (2004).
Bo, S. et al. Associations of dietary and serum copper with inflammation, oxidative stress, and metabolic variables in adults. J. Nutr. 138, 305–310, https://doi.org/10.1093/jn/138.2.305 (2008).
Zeng, C. et al. Association between low serum magnesium concentration and hyperuricemia. Magnes. Res. 28, 56–63, https://doi.org/10.1684/mrh.2015.0384 (2015).
Fields, M. et al. Blood risk factor metabolites associated with heart disease and myocardial fatty acids in copper-deficient male and female rats. Proc. Soc. Exp. Biol. Med. 191, 293–298 (1989).
Derouiche, S. & Kechrid, Z. Zinc Supplementation Overcomes Effects of Copper on Zinc Status, Carbohydrate Metabolism and Some Enzyme Activities in Diabetic and Nondiabetic Rats. Can. J. Diabetes 40, 342–347, https://doi.org/10.1016/j.jcjd.2016.02.005 (2016).
Mondal, M. S., Sau, A. K. & Mitra, S. Mechanism of the inhibition of milk xanthine oxidase activity by metal ions: a transient kinetic study. Biochim. Biophys. Acta 1480, 302–310 (2000).
Kela, U. & Vijayvargiya, R. Studies on the mechanism of action of 6-mercaptopurine. Interaction with copper and xanthine oxidase. Biochem. J. 193, 799–803 (1981).
Sau, A. K., Mondal, M. S. & Mitra, S. Interaction of Cu2+ ion with milk xanthine oxidase. Biochim. Biophys. Acta 1544, 89–95 (2001).
Hadizadeh, M., Keyhani, E., Keyhani, J. & Khodadadi, C. Functional and structural alterations induced by copper in xanthine oxidase. Acta Biochim. Biophys. Sin. 41, 603–617 (2009).
Sautin, Y. Y. & Johnson, R. J. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 27, 608–619, https://doi.org/10.1080/15257770802138558 (2008).
Filipe, P. et al. Anti- and pro-oxidant effects of urate in copper-induced low-density lipoprotein oxidation. Eur. J. Biochem. 269, 5474–5483 (2002).
Lee, S. W. et al. Age-differential association between serum uric acid and incident hypertension. Hypertens. Res. 42, 428–437, https://doi.org/10.1038/s41440-018-0168-4 (2019).
Sharaf El Din, U. A. A., Salem, M. M. & Abdulazim, D. O. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J. Adv. Res. 8, 537–548, https://doi.org/10.1016/j.jare.2016.11.004 (2017).
Ali, N. et al. Prevalence of hyperuricemia and the relationship between serum uric acid and obesity: A study on Bangladeshi adults. PLoS One 13, e0206850, https://doi.org/10.1371/journal.pone.0206850 (2018).
Bagnati, M. et al. When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: a study using uric acid. Biochem. J. 340(Pt 1), 143–152 (1999).
Kanellis, J. et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 41, 1287–1293, https://doi.org/10.1161/01.HYP.0000072820.07472.3B (2003).
Lobo, J. C., Stockler-Pinto, M. B., da Nobrega, A. C., Carraro-Eduardo, J. C. & Mafra, D. Is there association between uric acid and inflammation in hemodialysis patients? Ren. Fail. 35, 361–366, https://doi.org/10.3109/0886022X.2013.764274 (2013).
Liang, W. Y. et al. Uric acid promotes chemokine and adhesion molecule production in vascular endothelium via nuclear factor-kappa B signaling. Nutr. Metab. Cardiovasc. Dis. 25, 187–194, https://doi.org/10.1016/j.numecd.2014.08.006 (2015).
Liu, S. et al. Phloretin attenuates hyperuricemia-induced endothelial dysfunction through co-inhibiting inflammation and GLUT9-mediated uric acid uptake. J. Cell Mol. Med. 21, 2553–2562, https://doi.org/10.1111/jcmm.13176 (2017).
Strecker, D., Mierzecki, A. & Radomska, K. Copper levels in patients with rheumatoid arthritis. Ann. Agric. Env. Med. 20, 312–316 (2013).
Yang, T. H. et al. Increased inflammation in rheumatoid arthritis patients living where farm soils contain high levels of copper. J. Formos. Med. Assoc. 115, 991–996, https://doi.org/10.1016/j.jfma.2015.10.001 (2016).
Wei, H., Zhang, W. J., Leboeuf, R. & Frei, B. Copper induces–and copper chelation by tetrathiomolybdate inhibits–endothelial activation in vitro. Redox Rep. 19, 40–48, https://doi.org/10.1179/1351000213Y.0000000070 (2014).
Lu, J. et al. Trace amounts of copper exacerbate beta amyloid-induced neurotoxicity in the cholesterol-fed mice through TNF-mediated inflammatory pathway. Brain Behav. Immun. 23, 193–203, https://doi.org/10.1016/j.bbi.2008.09.003 (2009).
Ansteinsson, V. et al. Zinc- and copper-induced interleukin-6 release in primary cell cultures from rat heart. Cardiovasc. Toxicol. 9, 86–94, https://doi.org/10.1007/s12012-009-9043-5 (2009).
Teles, M., Mackenzie, S., Boltana, S., Callol, A. & Tort, L. Gene expression and TNF-alpha secretion profile in rainbow trout macrophages following exposures to copper and bacterial lipopolysaccharide. Fish. Shellfish. Immunol. 30, 340–346, https://doi.org/10.1016/j.fsi.2010.11.006 (2011).
Ogihara, H., Ogihara, T., Miki, M., Yasuda, H. & Mino, M. Plasma copper and antioxidant status in Wilson’s disease. Pediatr. Res. 37, 219–226, https://doi.org/10.1203/00006450-199502000-00016 (1995).
Acknowledgements
The authors appreciate the support of Orthopedics Research Institute of Xiangya Hospital. This work was supported by the National Natural Science Foundation of China (81772413, 81601941, 81702207, 81702206), the National Key Research and Development Program of China (2018YFB1105705), the Scientific Research Project of Science and Technology Office of Hunan Province (2017TP1005), the Key Research and Development Program of Hunan Province (2018SK2070, 2018SK2071), the Young Investigator Grant of Xiangya Hospital, Central South University (2016Q03, 2016Q06, 2017Q10), the Xiangya Clinical Big Data System Construction Project of Central South University (45), the Clinical Scientific Research Foundation of Xiangya Hospital, Central South University (2015L03), the Postdoctoral Science Foundation of Central South University (182130), and the Natural Science Foundation of Hunan Province (2017JJ3491, 2017JJ3492, 2018JJ3825, 2019JJ50965).
Author information
Authors and Affiliations
Contributions
This work was completed by cooperation of all the authors. T.J., T.Y., Y.W. conceived the study, and were responsible for conception and design of the study. T.J., D.X., H.W., N.W., Z.Z. contributed to methodology and data collection. J.W. and H.H. performed the data analysis. T.J. drafted the manuscript, T.Y. and Y.W. revised and edited the manuscript. All the authors contributed to the interpretation of the data and critically reviewed the manuscript for publication. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, T., Xie, D., Wu, J. et al. Association between serum copper levels and prevalence of hyperuricemia: a cross-sectional study. Sci Rep 10, 8687 (2020). https://doi.org/10.1038/s41598-020-65639-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65639-0
This article is cited by
-
A systematic review and meta-analysis of the hyperuricemia risk from certain metals
Clinical Rheumatology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.