Abstract
Low temperature affects a broad spectrum of cellular components in plants, such as chloroplasts, as well as plant metabolism. On the other hand, pseudouridine (Ψ) synthases are required for the most abundant post-transcriptional modification of RNA in Escherichia coli. However, the role of rice Ψ synthases in regulating chloroplast development at low temperature remains elusive. In this study, we identified the rice thermo-sensitive chlorophyll-deficient (tcd3) mutant, which displays an albino phenotype before the 4-leaf stage and ultimately dies when grown at 20 °C, but can grow normally at 32 °C. Genetic analysis showed that the mutant trait is controlled by a single recessive nuclear gene (tcd3). Map-based cloning, complementation and knockout tests revealed that TCD3 encodes a chloroplast-localized Ψ synthase. TCD3 is a cold-induced gene that is mainly expressed in leaves. The disruption of TCD3 severely affected the transcript levels of various chloroplast-associated genes, as well as ribosomal genes involved in chloroplast rRNA assembly at low temperature (20 °C), whereas the transcript levels of these genes were normal at high temperature (32 °C). These results provide a first glimpse into the importance of rice Ψ synthase gene in chloroplast development at low temperatures.
Similar content being viewed by others
Introduction
Rice is one of the most important food crops worldwide. Its yield potential is limited by the photosynthetic capacity of leaves that, as carbohy-drate factories, are unable to fill the larger number of florets of modern rice plants1. The development of intact chloroplasts, a prerequisite for photosynthesis, is affected by environmental factors such as temperature and light. Chloroplast development, a complex process that is coordinately regulated by plastid and nuclear genes, can be divided into three stages2,3,4: (i) the activation of plastid replication and plastid DNA synthesis; (ii) chloroplast “build-up”, characterized by the establishment of the chloroplast genetic system; and (iii) the expression of plastid and nuclear genes for the photosynthetic apparatus. In higher plants, chloroplast genes are transcribed by two types of RNA polymerase: nucleus-encoded polymerases (NEP) and plastid-encoded polymerases (PEP). NEPs are mainly responsible for transcribing the components of the transcriptional/translational machinery, such as rpoA and rpoB, while PEPs are required for the transcription of photosynthetic genes such as psbA, psbD, and rbcL. Mutations of those genes directly or indirectly affect chlorophyll biosynthesis or degradation pathways as well as photosynthesis, ultimately resulting in differences in leaf color or even plant death5,6,7,8,9. However, the roles of the many genes in the regulation of chloroplast development in higher plants remain largely unknown10.
Pseudouridine (5-ribosyluracil; Ψ) synthases, responsible for the most abundant post-transcriptional modification of cellular RNAs (pseudouridine), share a common core fold and active site structure. This core structure is modified by peripheral domains comprising accessory proteins with different amino acid sequences (depending on the family) and guide RNAs, giving rise to remarkable substrate versatility. These synthases catalyze the site-specific isomerization of uridine residues in the RNA chain and appear to employ both sequence and structural information to achieve site specificity11. All Ψ synthases identified to date from Archaea, Bacteria, and Eukarya can be classified into five families (RluA, RsuA, TruA, TruB, and TruD)12. The most well studied are from Escherichia coli, which contains 11 Ψ synthases. Of these, RsuA, RluE, RluB, and RluF belong to the RsuA family; RluA, RluC, RluD, and TruC belong to the RluA family; and TruA, TruB, and TruD are the sole members of their respective families. All of these synthases are site specific, and no overlapping functions have been detected12,13. For instance, RsuA isomerizes U516 in ribosomal RNA (rRNA), which is responsible for the universally conserved Ψ55 in the TΨC loops of all elongator tRNAs in a cell. RluA modifies two structurally distinct types of RNA at positions that share local sequence and structural similarity. TruA and RluD modify several nearby sites on a specific RNA11. Nevertheless, the modes of action of Ψ synthases remain largely unclear, especially during plant growth and development. Chlamydomonas reinhardtii Maa2, encoding a Ψ synthases, is involved in chloroplast group II intron trans-splicing of psaA RNA; its mutant (maa2) cannot grow phototrophically and is highly photosensitive14. The Arabidopsis thaliana Ψ synthase SVR1 (At2g39140) is required for normal chloroplast translation15. However, to our knowledge, no rice mutants for Ψ synthase genes have been reported, and the roles of these genes in rice are unknown.
Here, we identified tcd3, a mutant of a pseudouridine (Ψ) synthase gene in rice that displays thermo-sensitive changes in leaf color under cold stress. The chloroplast-localized Ψ synthase TCD3 appears to play an essential role in chloroplast development at low temperature in rice.
Results
Phenotypic characterization of the tcd3 mutant
We observed the leaf color of wild-type (WT) and tcd3 seedlings grown at two different temperatures, 20 °C and 32 °C (Fig. 1A,B). The tcd3 seedlings displayed yellowish white leaves and died after the 5-leaf stage when grown at 20°. However, when grown at 32 °C, the seedlings remained green, as did WT seedlings at both temperatures. These results indicate that the variation in leaf color of the tcd3 mutant is controlled by temperature and that this mutant has low-temperature-sensitive characteristics. Consistent with this conclusion, the photosynthetic pigments chlorophyll (Chl) a and b and carotenoid (Car) were almost undetectable in tcd3 seedlings grown at 20 °C (Fig. 2A), whereas there was only a slight difference in pigment levels between WT and tcd3 at 32 °C (Fig. 2B). These results indicate that tcd3 exhibits low levels of photosynthetic pigment biosynthesis at 20 °C, but can quickly recover to almost WT levels at 32 °C. As expected, the structure of WT chloroplasts was complete in seedlings grown at both 32 °C and 20 °C. However, at 20 °C, almost no intact chloroplasts and numerous bubble-like structures were observed in tcd3 (Fig. 1D), whereas tcd3 at 32 °C and WT chloroplasts appeared similar (Fig. 1C,E,F). These results indicate that the tcd3 mutation blocks chloroplast development under cold stress. Interestingly, under field conditions, the leaf Chl contents in tcd3 plants were indistinguishable from those of WT plants (Fig. S1). In addition, except for a significant reduction in panicle number and grain number, no obvious differences in other traits were observed between tcd3 and WT plants (Fig. S2), implying that the tcd3 mutation has a somewhat negative affect on the later stages of plant growth.
Characters of the tcd3 mutant and WT plants; seedling leaf color in WT and tcd3 at 20° (A) and 32° (B); chloroplast structure in WT at 20° (C) and 32° (E) and chloroplast structure in tcd3 at 20° (D) and 32° (F); To complemented plants in the tcd3 mutant (G) and control (H) backgrounds at 20° of the temperature of differentiation; segregation of T1 plants obtained from transgenic T0 plants transformed with pCAMBIA1301-TCD3 (I) and by CRISPR/Cas9 genome editing (J).
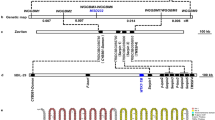
Map-based cloning of TCD3
Genetic analysis showed that the tcd3 mutant trait was caused by a recessive mutation based on the approximately 3 (green): 1 (yellowish white) ratio for phenotypic segregation in an F2 population generated from Peiai64S/tcd3 (Table S1). As a first step, we selected 236 mutant individuals in the F2 population and mapped TCD3 between markers MM0541 and RM14407 on chromosome 3 (Fig. 3A). To further fine-map TCD3, we examined 5020 F2 mutant individuals and developed five InDel molecular markers (Table S2). Ultimately, TCD3 was localized to a 179-kb region between markers ID2738 and ID2917 (Fig. 3B) spanning two BACs (AC105734, AC137635). Based on the Rice Genome Annotation Project (http://www.rice.plantbiology.msu.edu/), 32 candidate genes are predicted to be present in the target region, including one encoding a pseudouridine (Ψ) synthase (LOC_Os03g05806). We sequenced and analyzed all candidate genes and found only a G-to-A mutation at the 50th base pair (bp) and a 5-bp deletion mutation (TCTTG) at bp 51 to 55 in the fifth exon of LOC_Os03g05806 (Fig. 3C), which led to a premature translational stop and a frame-shift mutation, respectively.
Cloning of the TCD3 gene; (A), TCD3 was initially localized to a region between SSR markers MM0541 and RM14407 on chromosome 3 using 236 F2 mutant individuals; (B), TCD3 was narrowed down to a 179 kb region between ID2738 and ID2917 on BAC clones AC105734 and AC137635 using 5020 F2 mutant individuals; (C), gene model of TCD3.
Complementation and knockout of TCD3
To verify that the yellowish white phenotype of tcd3 at 20 °C is due to the mutation of LOC_Os3g05806, we performed genetic complementation of the tcd3 mutant and CRISPR/Cas9 genome editing of WT plants. In the complementation test, to quickly obtain results, we induced the differentiation of rice callus at 20 °C. The resulting To transgenic seedlings harboring pCAMBIA1301:TCD3 appeared green (Fig. 1H), whereas non-transgenic seedlings remained yellow (Fig. 1G), indicating that LOC_Os3g05806 can rescue the mutant phenotype. In addition, we obtained 19 independent transgenic (T0) plants transformed with pCAMBIA1301:TCD3. At 20 °C, we detected segregation of the mutant phenotype in the transgenic plant population (T1) and found that all green seedlings contained the transgene (Fig. 1I). As controls, we produced 14 independent lines transformed with the empty vector pCAMBIA1301, which failed to rescue the tcd3 mutant phenotype. Via CRISPR/Cas9, we obtained 13 transgenic T0 plants carrying one editing mutation of the 1-bp (G) deletion at 89 bp from the ATG start codon in TCD3 (Fig. S3). Importantly, all homozygous genome-edited T1 lines exhibited the same mutant phenotype as tcd3 mutant at 20 °C (Fig. 1J). Taken together, these results confirm that LOC_Os3g05806 is TCD3.
Expression analysis and subcellular localization of TCD3
We examined the mRNA levels of TCD3 by quantitative RT-PCR in various organs, including seedlings, roots, stems, leaves, and panicles, finding that TCD3 was mainly expressed in leaves. Lower expression levels were also detected in roots, stems, and panicles (Fig. 4A). These results support the notion that TCD3 plays an important role in leaf development, which is consistent with the rice gene expression profiling data (http://ricexpro.dna.affrc.go.jp). To further investigate the effects of temperature on TCD3 expression, we measured TCD3 transcript levels in tcd3 and WT seedlings at the 3-leaf stage grow, indicating that TCD3 is a cold-inducible gene.
Transcript levels of TCD3 in various tissues (A) and at different temperatures (B) determined by RT-PCR analysis; YR, young-seedling roots; ST, young-seedling stems; YL, young-seedling leaves; SL, the second leaf from the top; FL, flag leaf at heading; PN, young panicles. OsActin was used as a control (28 cycles for OsActin, 35 cycles for TCD3; Transcript levels (B) of TCD3 in WT and tcd3 at the 3-leaf stage at 20° and 32°, OsActin was used as a control for qPCR. Data are means ± SD (n = 3). Asterisks indicate statistically significant difference compared with WT. **P < 0.01 by Student’s t-test.
The TCD3 protein was predicted to be localized to the chloroplast according to TargetP (http://www.cbs.dtu.dk/services/TargetP/)16. To determine the actual subcellular localization of TCD3, we transiently expressed a constitutively transcribed TCD3:GFP fusion construct (35 S:TCD3:GFP) in tobacco cells. The GFP signals from TCD3:GFP were localized to chloroplasts (Fig. 5B). These findings confirm that TCD3 is localized to the chloroplast and is induced under cold stress.
Characterization of TCD3 protein by bioinformatic analysis
TCD3 consists of eight exons and nine introns (Fig. 3C) and encodes a 415 amino-acid protein with a molecular mass of approximately 45 kDa. A search of the Pfam database17 revealed that TCD3 not only contains a chloroplast transit peptide (CTP; 70 aa), but it also contains an S4 domain and a Ψ synthase domain (Fig. S4A). The tcd3 mutation site occurred in the Ψ synthase domain, which led to a change in the protein sequence and the destruction of its three-dimensional structure (Fig. S4B).
In addition, we searched various databases for sequences with similarity to each of the 11 E. coli Ψ synthases and found 22 homologous sequences in rice (Fig. S5). A phylogenetic tree showing the relationships of E. coli Ψ synthases, Arabidopsis thaliana SVR1 (At2g39140; involved in chloroplast rRNA processing)15, and Chlamydomonas Ψ synthase (Maa2)14 is shown in Fig. S6. Based on the results of phylogenetic analysis, TCD3 belongs to the RsuA family and shares the greatest sequence similarity with SVR1 (Fig. S5).
Further study showed that TCD3 is conserved in higher plants, especially in millet, maize, two ear small grass, and sorghum, with similarities of 79.3%, 76.5%, 77.5%, and 79.3%, respectively (Fig. 6B). Phylogenetic analyses showed that the TCD3 homologs could be clearly divided into two groups: monocotyledons and dicotyledons, which conforms with the taxonomy (Fig. 6A).
Phylogenic analysis of TCD3 and its homologs (A); Protein sequences are from Sorghum bicolor (8080543), Zea mays (100285657), Setaria italic (101780537, Brachypodium distachyon (100846238), Arabidopsis thaliana (At2g39140), Glycine max (100808046), Vitis vinifera (100247893), Populus trichocarpa (POPTR_0008s03540g), and Selaginella moellendorffii (SELMODRAFT_45172). Scale represents percentage substitutions per site. The rooted tree is based on multiple sequence alignment using MAFFT and was generated with MEGA 6; Amino acid sequence alignment of TCD3 with its nine homologs (B). Fully and partially conserved amino acids are shaded in black and gray, respectively. The homologous comparison is based on multiple sequence alignment using MAFFT and was generated with the program DNAMAN8.
The disruption of TCD3 alters the transcript levels of related genes
To determine the effect of the tcd3 mutation on the expression of genes related to chloroplast development and to explore the underlying pathway, we carried out RT-qPCR analysis of genes involved in Chl biosynthesis, photosynthesis, and chloroplast development in rice. At both high and low temperatures, low levels of TCD3 transcripts were detected in tcd3, confirming that the tcd3 mutation blocks the transcription of this gene (Figs. 7C and 8C). At 20 °C, Chl biosynthesis (CAO, YGL1, Cab1R) and photosynthesis (rbcS, rbcL, psaA, psbA, LHCPII)-related genes were significantly downregulated in tcd3, although PORA (Fig. 7A–C) was not, which is consistent with the chlorosis symptoms observed at low temperatures. Among chloroplast development-associated genes, except for Ftsz (encoding a component of the plastid division machinery)18,19, V3 (RNRL, encoding the large subunit of ribonucleotide reductase)18, and rps7 (encoding the small subunit ribosomal protein S7), which were upregulated in tcd3 at 20 °C, all remaining genes were strongly downregulated in the mutant. In particular, several genes were severely downregulated, including atpA, encoding an ATP synthase subunit20; OsPOLP, encoding a plastidial DNA polymerase22; rpoC1, encoding RNA polymerase β‘ subunit; S4, encoding ribosomal protein subunit S4; and 16SrRNA and 23SrRNA, encoding the large (16S) and small (23S) subunits of chloroplast ribosomes, respectively. Notably, however, the transcript levels of all up- and downregulated genes in tcd3 at 20 °C were restored to WT levels at 32 °C, and other genes were also expressed at WT levels when grown at 32 °C (Fig. 8A–C) (within a twofold range), which corresponds with the thermo-sensitivity of the mutant phenotype. In summary, the tcd3 mutation reduces the mRNA levels of certain genes involved in Chl biosynthesis and photosynthesis, as well as chloroplast development, at low temperatures.
qRT-PCR analysis of genes associated with Chl biosynthesis (A), photosynthesis (B), and chloroplast development in wild type (WT) and tcd3 at the 3-leaf stage at 20°; the relative expression level of each gene in WT and tcd3 was analyzed by qRT-PCR and normalized using OsActin as an internal control. Data are means ± SD (n = 3).
qRT-PCR analysis of genes associated with Chl biosynthesis (A), photosynthesis (B), and chloroplast development in wild type (WT) and tcd3 at the 3-leaf stage at 32°; the relative expression level of each gene in WT and tcd3 was analyzed by qRT-PCR and normalized using OsActin as an internal control. Data are means ± SD (n = 3).
Discussion
In the current study, we identified and characterized TCD3, a pseudouridine (Ψ) synthase required for chloroplast development at low temperatures in rice. Plants with a loss of function of TCD3 produced imperfect chloroplasts and exhibited a Chl-deficient phenotype at low temperatures, resulting from the abnormal expression of genes associated with Chl biosynthesis, photosynthesis, and chloroplast development. Our results provide evidence that cold-induced TCD3 plays an important role in chloroplast development in rice at low temperatures.
TCD3 regulates the first stage of chloroplast development in rice at low temperatures
Chloroplast development in rice, which is coordinately regulated by plastid and nuclear genes, can be divided into three stages2,3,4. The first stage involves OsPOLP1 (encoding a plastidial DNA polymerase) and FtsZ (encoding a component of the plastid division machinery)18,20. The second stage involves OsRpoTp (encoding a NEP), V2 (encoding a plastidial guanylate kinase), and rpoA (encoding a PEP subunit)19,20,21,22. The third stage involves PEP-transcribed plastid genes (e.g., psbA, rbcL)3. Other mutants with phenotypes similar to the cold-sensitive phenotype of tcd3 have also been reported, including the v1, v2, v3, tcd9, osv4, tcd5, tcd10, and tcd11, tsv3 and tcm1 mutants19,23,24,25,26,27,28,29,30. In detail, V1 encodes the chloroplast-localized protein NUS1, which is involved in regulating chloroplast RNA metabolism at the second stage of chloroplast development3. V2, encoding plastid or mitochondrial guanylate kinase (pt/GK), is required for the second stage of chloroplast development19. V3, encoding the large subunit of ribonucleotide reductase, is required for the first stage of chloroplast development31. More recently, we identified five additional genes that are essential for chloroplast development at low temperature: TCD9, encoding the α subunit of chaperonin protein 6025; OsV4 and TCD10, both encoding PPR proteins26,28; TCD5, encoding a monooxygenase32; TCD11, encoding plastid ribosomal protein S627; and TSV3, encoding Spo0B-associated GTP-binding (Obg) protein29. Among these, OsV4, TCD5, TCD10, and TCD11 participate in regulating the second stage of chloroplast development, while TCD9 and TSV3 participate in the first stage. In this study, the high levels of TCD3 transcript (Fig. 4B) detected at 20 °C indicate that TCD3 is a cold-inducible gene that is required for chloroplast development under cold stress. Notably, in tcd3 plants at 20 °C, we detected severely reduced transcript levels (Fig. 8C) of OsPOLP1, which is involved in the first step of chloroplast development20,22, indicating that TCD3 regulates the first stage of chloroplast development in rice. Similarly, the transcript levels of OsRpoTp (encoding a NEP), as well as NEP-dependent genes (16S rRNA, 23S rRNA, rps20, rpoB, rpoC1, V1, V2), PEP- and NEP-dependent atpA (which functions in the second stage of chloroplast development), and PEP-regulated plastid genes (Cab1R, LHCPII, psaA, psbA, rbcL, and rbcS, which function in the third stage of chloroplast development) were also severely reduced in the mutant under cold stress (Fig. 7). Perhaps the increased mRNA levels of Ftsz and V3 during the first stage of chloroplast development and of rps7 during the second stage are due to feedback effects. In addition, the recovery of the expression levels of all affected genes at 20 °C to WT levels in tcd3 at 32 °C likely accounts for the normal phenotype of the mutant at 32 °C (Fig. 8A–C). The low expression level of TCD3 at 32 °C and the high expression level at 20 °C suggest that TCD3 is indispensable for chloroplast development at low temperatures but not at high temperatures. Therefore, we conclude that TCD3 functions in the first stage of chloroplast development at low temperatures.
Possible role of TCD3 in chloroplast rRNA and tRNA metabolism at low temperatures
In this study, we identified the first Ψ synthase, TCD3, in rice. As mentioned above, Ψ synthases can be divided into five families. Based on the presence of an S4 domain and a Ψ synthase domain (Fig. S5A) and the results of homology analysis, TCD3 belongs to the RsuA family, members of which isomerize U (uridine) 516 in rRNA11. TCD3 proteins also share a conserved nine-amino acid motif (GRLDVATSG; Fig. 6B) at the active site that includes a universally conserved Asp (D) residue33. In addition, the most similar protein to TCD3 is Arabidopsis thaliana SVR1 (60.6% sequence similarity; Fig. S6), which shares the same domains. In addition to playing a role in uridine isomerization, SVR1 is also required for proper chloroplast rRNA processing and tRNA metabolism15. Surprisingly, like 16 S rRNA and 23 S rRNA (both related to chloroplast rRNA processing), the levels of nuclear (rbcS, LhcpII) and plastid (rbcL, psaA, atpA) gene expression were severely impaired in both svr115 and tcd3 (Fig. 7C). In tcd3 plants, the transcript levels of two other ribosomal protein genes (rps20 and S4) were also severely reduced. Thus, TCD3, like SVR1, might play a role in chloroplast rRNA processing and tRNA metabolism at low temperatures. Therefore, under cold stress, the tcd3 mutation affects chloroplast rRNA processing and tRNA metabolism, which in turn leads to the production of yellowish white leaves Accordingly, the loss of TCD3-mediated OsPOLP1-16S-23S rRNA mRNA expression might lead to the thermo-sensitive phenotype observed under cold stress. Why does TCD3 exhibit thermo-sensitivity? Perhaps different Ψ synthases utilize different mechanisms during environmental responses. For example, Chlamydomonas reinhardtii Maa2, which is homologous to LOC_Os3g26440 (Fig. S5), is highly photosensitive14, in contrast to the thermo-sensitivity of TCD3. The findings highlight the notion that even highly conserved genes within the same species or across species might play more diverse, complex roles than previously recognized. Under high temperature conditions, perhaps TCD3 is not required for chloroplast development because the function of TCD3 is replaced by that of other homologous genes (Fig. S5). Finally, our observations provide evidence for the versatile roles of plant Ψ synthases in development. Taken together, our results indicate that TCD3 is required for chloroplast development during the early stages of leaf development in rice under cold stress.
Materials and methods
Plant materials and growth conditions
The thermo-sensitive chlorophyll-deficient tcd3 mutant was derived from japonica rice variety Jiahua 1 (WT) treated with 60Co gamma-radiation. This thermo-sensitive leaf color mutant exhibits leaf yellowing at low temperatures. The F2 mapping population was generated from a cross between Pei’ai 64S (indica) and tcd3. All rice seedlings were grown in growth chambers under controlled conditions with a 12-h-dark/12-h-light cycle at 20 °C or 32 °C.
Phenotypic characterization and measurement of photosynthetic pigments
Germinated seeds of tcd3 and Jiahua 1 (WT) were sown on soils under controlled conditions as described above at 20 °C or 32 °C. The 3rd leaves (200 mg) from plants at the three-leaf stage were homogenized in 10 mL of 100% acetone. The absorbance value of the supernatant at 470, 645, and 663 nm was determined by spectrophotometry (Beckman Coulter, Danvers, MA, USA). The chlorophyll (Chl) and carotenoid (Car) contents were determined by spectrophotometry as described by Arnon34 and Wellburn35. In addition, WT and tcd3 plants were grown in a paddy field at Shanghai Normal University, China, in 2010. Leaf Chl SPAD values (Fig. S1) were measured using a chlorophyll meter (SPAD-502, Minolta Co. Ltd., Japan), a nondestructive, rapid method for estimating photosynthetic pigment levels36,37,38. The SPAD values were measured every week from transplantation (summer) to the heading (autumn) stage. Various yield-associated traits (Fig. S2) were investigated at maturity.
Observation of chloroplast structure by transmission electron microscopy (TEM)
Chloroplast development was observed in the third leaves of WT and tcd3 plants using TEM as described elsewhere39 with minor modifications. Briefly, the third leaves were sampled from seedlings at the 3-leaf-stage grown at 20 °C and 32 °C. Transverse leaf sections were fixed in 2.5% glutaraldehyde solution at 4° for 4 h, rinsed and incubated overnight in 1% w/v OsO4 at 4°, and embedded in Epon 812 resin. The samples were stained again and examined under a Hitachi-7650 transmission electron microscope.
Positional cloning of TCD3
Genomic DNA was extracted from rice leaves using an improved CTAB method40. Initially, 236 F2 recessive seedlings from the cross Pei’ai 64 S/tcd3 were used to identify the molecular markers linked to the mutant gene. Subsequently, 5020 F2 plants showing the mutant phenotype were used for fine mapping. New molecular markers were designed by comparing the sequences of 93–1141 and Nipponbare42 from NCBI (http://www.ncbi.nlm.nih.gov); the markers are listed in Table S2.
Complementation of the tcd3 mutant
For the complementation test, genomic DNA was extracted from WT plants and used as a PCR template with the primer pair TCD3F: 5′-GGGGTACCGCCCATACAGATCCTCG-3′ (KpnI) and TCD3R: 5′-GCGTCGACTTTCAGCAAACCCCATG-3′ (SalI), amplifying a fragment (6.9 kb) containing the target gene (TCD3) and the 1.5 kb upstream and 0.35 kb downstream sequences. The PCR products were cloned into the pMD18-T vector (TaKaRa, Dalian, China). The pMD18T-TCD3 construct was digested with KpnI and SalI and ligated into the KpnI and SalI site of binary vector pCAMBIA1301 (CAMBIA, http://www.cambia.org.au). The resulting pCAMBIA1301:TCD3 vector was transferred into Agrobacterium tumefaciens strain EHA105 and introduced into the tcd3 mutant by Agrobacterium-mediated transformation according to previously published methods43, except that the temperature used for in vitro plant differentiation was 20 °C. The genotypes of the transgenic seedlings were determined by PCR amplification of the hygromycin phosphotransferase gene (hpt) with primers HPTF (5′-GGAGCATATACGCCCGGAGT-3′) and HPTR (5′-GTTTATCGGCACTTTGCATCG-3′) and primers GUSF (5′-GGGATCCATCGCAGCGTAATG-3′) and GUSR (5′-GCCGACAGCAGCAGTTTCATC-3′) as selection markers. The resulting T0 transgenic seedlings were grown in a paddy field after screening for hygromycin-tolerant plants and confirmation by DNA sequencing. All T1 seedlings were grown at 20° and used to examine the segregation of the mutant phenotype.
Knockout of TCD3
To experimentally affirm that TCD3 is responsible for the phenotypic changes observed in tcd3, CRISPR/Cas9 genome editing was performed on WT plants. To generate the Cas9 targeting construct for TCD3 using CRISPR Primer Designer software(http://www.crispr.dbcls.jp/)44, annealed gRNA oligonucleotide pairs with recognition sequences were designed (F1: 5′-GCCGGACTTCAAC ATCACCTTCG-3′, R1: 5′-AAACCGAAGGTGATGTTGAAGTC-3′; F2: GCCGGACTTCAACATCACCTTCG, R2: AAACCGAAGGTGATGTTGAAGTC; F3: GCCGCCTGCGTGCTCCTCCGCTG, R3: AAACCAGCGG AGGAGCACGCAGG; F4: GCCGGCGGAGGCGGAGGCCAAGA). The recognition sequences were inserted into the region between the OsU6 promoter and the gRNA scaffolds (from the pYLgRNA-OsU6 vector) of the Cas9 expression backbone vector (pYL-CRISPR/Cas9-MH) at the BsaI sites as previously described45. The resulting plasmid (CRISPR/Cas9 expression) and the empty vector were introduced into Agrobacterium strain EHA105 and used to infect calli from WT plants43. The resulting T1 seedlings were grown and used to examine the segregation of the mutant phenotype at 20 °C.
Subcellular localization
To investigate the subcellular localization of TCD3, cDNA fragments encoding the 144 amino-acid N-terminal region of TCD3 were amplified from total RNA extracts using following primers (5′-GAAGATCTATGGCCCTCGCCGCCGCCGCCGCCGCCGCG-3′ and 5′-GGGGTACCCCCCTCTCCCTCACCTTGGCAT-3′) and introduced into vector pMON530-GFP at the BglII and KpnI sites (the underlined sequences represent cleavage sites). The resulting pMON530:CaMV35S:TCD3-GFP plasmid was verified by sequencing and introduced into Agrobacterium strain EHA105. The localization of TCD3 was investigated by transient expression analysis of the GFP fusion protein in tobacco (Nicotiana tabacum) cells via confocal microscopy as described previously25. GFP fluorescence in the transformed protoplasts was imaged by confocal laser-scanning microscopy (LSM5 PASCAL; ZEISS, http://www.zeiss.com).
RT-PCR and quantitative RT-PCR (qRT-PCR) analysis
Total RNA from WT plants was extracted from seedling roots (ST), young stems (YS), the third leaves of plants at the 3-leaf stage, the second leaves (SL) from the tops of plants, and flag leaves (FL) and young panicles (PN) from plants at the heading stage using an RNA Prep Pure Plant kit (Tiangen Co., Beijing, China) to investigate the tissue-specific expression patterns of TCD3. The RNA was reversely transcribed into cDNA and used for RT-PCR as described previously25. In addition, RNA from the third leaves of WT and tcd3 seedlings at the 3-leaf stage grown at 20 °C and 32 °C was extracted as described above and used to measure the transcript levels of TCD3 as well as Chl biosynthesis, chloroplast development, and photosynthesis-associated genes (atpA, Cab1R, CAO, FtsZ, HEMA, LHCPII, OsPOLP, OsRpoTp, OsV4, PORA, psaA, psbA, rbcL, rbcS, rpoB, rpoC, rps7, rps20, RNRL, S4, V1, V2, YGL-1, 16SrRNA, 23SrRNA) in rice. The qPCR was performed using a SYBR Premix Ex TaqTM kit (TaKaRa) on an ABI7500 Real-Time PCR System (Applied Biosystems; http://www.appliedbiosystems.com), and the relative quantification of gene expression data was performed as described in Livak and Schmittgen46. The specific primers for qPCR were partially referred to Wu et al.47 based on sequences from NCBI; the primers are listed in Table S3. The rice OsActin gene was used as a reference gene.
Sequence and phylogenetic analyses
Gene prediction and structure analysis were carried out using the GRAMENE database (http://www.gramene.org). Homologs of TCD3 were identified by BLASTP analysis against the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov) and subjected to multiple sequence alignment using DNAMAN version 6.0 (Lynnon Biosoft, USA). The signal peptide was predicted with SignalP version 2.047. The phylogenetic tree was constructed using MEGA version 6 software (http://www.megasoftware.net).
References
Von, C. S., Quick, W. P. & Furbank, R. T. The development of C4 rice: current progress and future challenges. Science 336, 1671–1672 (2012).
Kusumi, K., Chono, Y., Gotoh, E., Tsuyama, M. & Iba, K. Chloroplast biogenesis during the early stage of leaf development in rice. Plant Biotechnol. J. 27, 85–90 (2010).
Kusumi, K., Sakata, C., Nakamura, T. & Kawasaki, S. A plastid protein NUS1 is essential for build-up of the genetic system for early chloroplast development under cold stress conditions. Plant J. 68, 1039–1050 (2011).
Kusumi, K. & Iba, K. Establishment of the chloroplast genetic system in rice during early leaf development and at low temperatures. Front Plant Sci. 5, 1–6 (2014).
Beale, S. I. Green genes gleaned. Trends in Plant Science 10, 309–312 (2005).
Morita, R., Sato, Y., Masuda, Y., Nishimura, M. & Kusaba, M. Defect in non-yellow coloring 3, an alpha/beta hydrolase fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J. 59, 940–952 (2009).
Terry, M. J. & Kendrick, R. E. Feedback inhibition of chlorophyll synthesis in the phytochrome chromophore-deficient aurea and yellow-green-2 mutants of tomato. Plant Physiol. 119, 143–152 (1999).
Chen, G., Bi, Y. R. & Li, N. EGY1 encodes a membrane-associated and ATP-independent metalloprotease that is required for chloroplast development. Plant J. 41, 364–375 (2005).
Kushnir, S. et al. A mutation of the mitochondrial ABC transporter Sta1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell 13, 89–100 (2001).
Pfalz, J. & Pfannschmidt, T. Essential nucleoid proteins in early chloroplast development. Trends Plant Sci. 18, 186–194 (2013).
Hamma, T. & Ferré-D’Amaré, A. R. Pseudouridine synthases. Chem. Biol. 13, 1125–1135 (2006).
Kaya, Y. & Ofengand, J. A novel unanticipated type of pseudourdine synthase with homologs in bacteria archaea and eukarya. RNA 9, 711–721 (2003).
Gutgsell, N. et al. Deletion of the Escherichia coli pseudouridine synthase gene truB blocks formation of pseudouridine 55 in tRNA in vivo, does not affect exponential growth, but confers a strong selective disadvantage in competition with wild-type cells. RNA 6, 1870–1881 (2000).
Perron, K., Goldschmidt‐Clermont, M. & Rochaix, J. A factor related to pseudouridine synthases is required for chloroplast group II intron trans-splicing in Chlamydomonas reinhardtii. Embo. J. 18, 6481–6490 (1999).
Yu, F., Liu, X., Alsheikh, M., Park, S. & Rodermel, S. 2008 Mutations in suppressor of variegation1, a factor required for normal chloroplast translation, suppress var2-mediated leaf variegation in Arabidopsis. Plant Cell 20, 1786–804 (2008).
Emanuelsson, O., Nielsen, H. & Brunakb, S. 2000 Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 300, 1005–1016 (2000).
Finn, R. D. et al. The Pfam protein families database. Nucleic Acids Res. 38, 211–222 (2010).
Yoo, Y. et al. Rice Virescent3 and Stripe1 encoding the large and small subunits of ribonucleotide reductase are required for chloroplast biogenesis during early leaf development. Plant Physiol. 150, 388–401 (2009).
Vitha, S., McAndrew, R. S. & Osteryoung, K. W. FtsZ ring formation at the chloroplast division site in plants. J. Cell Biol. 153, 111–120 (2001).
Hiratsuka, J., Shimada, H., Whittier, R., Ishibashi, T. & Sakamoto, M. The complete sequence of the rice (Oryza sativa L.) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol. Gen. Genet. 217, 185–194 (1989).
Kusumi, K., Yara, A., Mitsui, N., Tozawa, Y. & Iba, K. Characterization of a rice nuclear-encoded plastid RNA polymerase gene OsRpoTp. Plant Cell Physiol. 45, 1194–1201 (2004).
Iba, K., Takamiya, K., Toh, Y., Satoh, H. & Nishimura, M. Formation of functionally active chloroplast is determined at a limited stage of leaf development in virescent mutants of rice. Devel. Genet. 12, 342–348 (1991).
Kusumi, K., Mizutani, A., Nishimura, M. & Iba, K. A Virescent gene v1 determines the expression timing of plastid genes for transcription translation apparatus during early leaf development in rice. Plant J. 12, 1241–1250 (1997).
Jiang, Q. et al. Importance of the rice TCD9 encoding a subunit of chaperonin protein60 (Cpn60α) for the chloroplast development during the early leaf stage. Plant Sci. 215–216, 172–179 (2014).
Gong, X. et al. The rice OsV4 encoding a novel pentatricopeptide repeat protein is required for chloroplast development during the early leaf stage under cold stress. J. Integr. Plant Biol. 56, 400–410 (2014).
Wang, W. et al. The rice TCD11 encoding plastid ribosomal protein S6 is essential for chloroplast development at low temperature. Plant Science 259, 1–11 (2017).
Wu, L. et al. The rice pentatricopeptide repeat gene TCD10 is needed for chloroplast development under cold stress. Rice 9, 67 (2016).
Lin, D. et al. Rice TSV3 encoding Obg-like GTPase protein is essential for chloroplast development during the early leaf stage under Cold Stress. G3 (Bethesda) 8, 253–263 (2018).
Lin, D. et al. TCM1 encoding a component of TAC complex is required for chloroplast development under cold stress. The Plant Genome 11(1), 1–13 (2018).
Yoo, S. C. et al. Rice Virescent3 and Stripe1 encoding the large and small subunits of ribonucleotide reductase are required for chloroplast biogenesis during early leaf development. Plant Physiol. 150, 388–401 (2009).
Wang, F. et al. Temperature-sensitive albino gene TCD5, encoding a monooxygenase, affects chloroplast development at low temperatures. Journal of Experimental Botany 67(17), 5187–5202 (2016).
Ofengand, J. Ribosomal RNA pseudouridines and pseudouridine synthases. Febs Lett. 514, 17–25 (2002).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Plant Physiol. 24, 1–15 (1949).
Wellburn, A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144, 307–313 (1994).
Peng, S., Garcia, F. V., Lasa, R. C. & Classman, K. G. Adjustment for specific leaf weight improves chlorophyll meter’s estimate of rice leaf nitrogen concentration. Agron J. 85, 987–990 (1993).
Turner, F. T. & Jund, M. F. Chlorophyll meter to predict nitrogen topdress requirement for semidwarf rice. Agron J. 83, 926–928 (1991).
Dwyer, L. M., Tollenaar, M. & Houwing, L. A nondestructive method to monitor leaf greenness in corn. Can J. Plant Sci. 71, 505–509 (1991).
Inada, N., Sakai, A., Kuroiwa, H. & Kuroiwa, T. Three-dimensional analysis of the senescence program in rice (Oryza sativa L.) coleoptiles. Planta 206, 585–597 (1998).
Murray, M. G. & Thompson, W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8, 4321–4326 (1980).
Yu, J. et al. A draft sequence of the rice genome (Oryza sativa L. Ssp. indica). Science 296, 79–92 (2002).
Goff, S. A. et al. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296, 92–100 (2002).
Hiei, Y., Ohta, S., Komari, T. & Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6, 271–282 (1994).
Naito, Y., Hino, K., Bono, H. & Ui-Tei, K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123 (2015).
Ma, X. et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284 (2015).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Wu, Z. M. et al. A chlorophylldeficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol. 145, 29–40 (2007).
Nielsen, H. & Krogh, A. Prediction of signal peptides and signal anchors by a hidden Markov model. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6, 122–130 (1998).
Acknowledgements
We sincerely thank Dr. Nancy Hofmann for her critical reading of and suggestions for our manuscript. We are grateful to Prof. Zhongnan Yang for kindly providing pMON530-GFP vector and for his constructive comments as well. The project was financially supported by Minister of Science and Technology of China (MOST) (2016YFD0100902), the Shanghai Municipal Science and Technology Commission (19391900200 and 16391900700), Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-02-E00039), the Natural Science Foundation of China (No. 30971552), and International Cooperation Project of South Korea and China (PJ013647).
Author information
Authors and Affiliations
Contributions
D.L. and Y.D. provided the mutant rice seeds. R.K., D.L., L.W., J.X. and Y.D. generated F2 and F3 seeds for genotyping and phenotyping. R.K., L.W., L.C., Y.W., D.L. and Y.D. performed the experiments of phenotype assays and molecular analysis. R.K., D.L., Y.D., Z.P. and G.L. designed and discussed the research plan. R.K., D.L. and Y.D. wrote the manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, D., Kong, R., Chen, L. et al. Chloroplast development at low temperature requires the pseudouridine synthase gene TCD3 in rice. Sci Rep 10, 8518 (2020). https://doi.org/10.1038/s41598-020-65467-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65467-2
This article is cited by
-
Transcriptomic analysis of hub genes regulating albinism in light- and temperature-sensitive albino tea cultivars ‘Zhonghuang 1’ and ‘Zhonghuang 2’
Plant Molecular Biology (2024)
-
CRISPR-Cas System, a Possible “Savior” of Rice Threatened by Climate Change: An Updated Review
Rice (2023)
-
Genome-wide identification, characterization, and expression analysis unveil the roles of pseudouridine synthase (PUS) family proteins in rice development and stress response
Physiology and Molecular Biology of Plants (2023)
-
The emerging role of epitranscriptome in shaping stress responses in plants
Plant Cell Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.