Abstract
The musculoskeletal system, which comprises muscles, tendons, and bones, is an efficient tissue complex that coordinates body movement and maintains structural stability. The process of its construction into a single functional and complex organization is unclear. SRY-box containing gene 9 (Sox9) is expressed initially in pluripotent cells and subsequently in ectodermal, endodermal, and mesodermal derivatives. This study investigated how Sox9 controls the development of each component of the musculoskeletal system. Sox9 was expressed in MTJ, tendon, and bone progenitor cells at E13 and in bone at E16. We detected Sox9 expression in muscle progenitor cells using double-transgenic mice and myoblastic cell lines. However, we found no Sox9 expression in developed muscle. A decrease in Sox9 expression in muscle-associated connective tissues, tendons, and bones led to hypoplasia of the cartilage and its attachment to tendons and muscle. These results showed that switching on Sox9 expression in each component (muscle, tendon, and bone) is essential for the development of the musculoskeletal system. Sox9 is expressed in not only tendon and bone progenitor cells but also muscle progenitor cells, and it controls musculoskeletal system development.
Similar content being viewed by others
Introduction
The musculoskeletal system comprises muscles, tendons, and bones. It is an efficient tissue complex that coordinates body movement and maintains structural stability1. This requires effective linkage of muscles to bones. Many studies have reported the development of each component of the musculoskeletal system, but the process of its construction into a single functional and complex organization is unclear.
Muscles, tendons, and bones differ in their developmental origins2,3,4,5,6,7,8, yet they interact during differentiation. Chen and Galloway8 reported that artificial rupture of the myotome impedes syndetome formation. In contrast, cranial tendon progenitor cells emerge without dependence on muscles, yet muscle presence is essential for their normal differentiation8. Huang et al9. analyzed tendon development in forearms and fingers and found that tendons can be divided into a distal module showing cartilage-dependent development and a proximal module showing muscle-dependent development. In addition, the mechanical stress of muscle contraction affects the morphology of cartilage and bone during development10. Previously, we found that mutual contact between muscles and the tendon-like structure immediately promotes the development of bones11 and that there is a morphological association between muscles and bones12. In summary, studies need to focus on the analysis of muscles, tendons, and bones as a single functional organ13 rather than focusing on each as an independent tissue component.
SRY-box containing gene 9 (Sox9) is a transcription factor essential for musculoskeletal system development. Sox9 is expressed in all cartilage progenitor cells and cartilage cells except hypertrophic chondrocytes14,15, and it plays a key role in a series of processes involved in endochondral ossification16. Tendons temporally express Sox9 during the early stage of development, but Sox9 is not expressed in developed tendon cells17. In a Sox9Cre mouse cell lineage analysis, Sox9 was found to be expressed in a subset of tendon and cartilage progenitor cells18,19. Although a few studies reported high Sox9 expression in myoblastic cells in vitro20,21,22, it is unclear whether Sox9 is expressed in muscles in vivo.
In this work, we clearly identified that Sox9 is expressed in muscle progenitor cells in vivo. Since Sox9 is expressed in a subset of tendon and cartilage progenitor cells17,18, in this study, we investigated how Sox9 controls the development of each component of the musculoskeletal system.
Results
Connection between muscle progenitors and tendon-bone progenitors
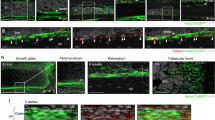
Although Sox9 is observed throughout tendon and bone progenitors17,18, little is known about the connection between muscle progenitors and Sox9+ tendon-bone progenitors. To observe this connection, we performed immunofluorescence staining with antibodies against desmin, which is a marker of the myotendinous junction (MTJ)11, and Sox9, which is a marker of tendon and bone progenitor cells17,18. In situ hybridization of Scx17,18 and alkaline phosphatase staining11 allowed us to distinguish tendon progenitors from bone progenitors. We analyzed the connection at the presumed locations in five regions: the lateral pterygoid muscle attachment to the condyle of the mandible (Fig. 1a–d,f), the triceps brachii muscle attachment to the olecranon (Fig. 1e), the intercostal muscle attachment to the ribs (Fig. 1g–i), the deltoid muscle attachment to the scapula (Fig. 1j–l), and the temporal muscle attachment to the coronoid process of the mandible (Fig. 1m–o). The progenitor cells expressing Sox9 crossed from the tendon anlage to the bone anlage, and the most forward migrating cells made contact with the desmin-accumulating MTJ (Fig. 1).
Sox9 expression in tendon and bone. (a–d,f) Sagittal plane images of the TMJ at E13.5 and (e) sagittal plane image of the triceps brachii muscle attachment to the ulna at E13.5. (a–d) Serial sections. (a) H&E staining; (b) in situ hybridization, Scx staining; (c) immunohistochemical staining of ALP and desmin; (d) immunohistochemical staining of Sox9; and (e, f) immunohistochemical staining of desmin and Sox9. (g–o) Sagittal plane images with immunohistochemical staining of (g, j, m) desmin and (h, k, n) Sox9. (i, l, o) Enlargements of (h, k, n), respectively. E13.5–E14.5 attachment regions of the (g–i) intercostal muscle to the ribs, (j–l) deltoid muscle to scapula, and (m–o) temporal muscle to coronoid process. The desmin-accumulating MTJ is in contact with Sox9+ progenitor cells. Scale bar = 50 μm (a–f, g, h, j, k, m, n) and 25 μm (i, l, o). M, muscle; T, tendon; B, condyle; SP, Sox9+ progenitor cells; Sox9, SRY-box containing gene 9; TMJ, temporomandibular joint; H&E, hematoxylin and eosin; ALP, alkaline phosphatase; MTJ, myotendinous junction.
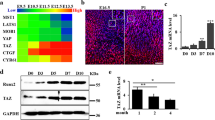
Sox9 is essential for chondrocyte differentiation and cartilage formation2. It is temporally expressed in tendons during the early stage of development but not in developed tendon cells17. To clarify the role of Sox9 expression during tendon and bone development, we analyzed the fluorescence intensity of immunohistochemical staining. The fluorescence intensity versus distance plot showed switching of Sox9 expression. At E13, the fluorescence intensity was>100 in the tendon and bone regions (Fig. 2b). At E16, the fluorescence intensity was>100 in the bone region but <100 in the tendon (Fig. 2d). During detailed observation of the connection between muscle progenitors and tendon-bone progenitors, we noticed Sox9 expression in a part of the muscle. The fluorescence intensity of Sox9 expression was>50 in the MTJ region at E13 but <50 in the MTJ region at E16 (Fig. 2b,d). The occupancy rate of Sox9 expression in the MTJ at E13 was high compared to that in the MTJ at E16 (E13: 37.56 ± 6.02%, E16: 0.40 ± 0.45%, P < 0.05) (Fig. 2e–g).
Switching of Sox9 expression during the formation of the musculoskeletal system. Sagittal plane images of the TMJ at (a) E13 and (c) E16. (b, d) Graph of the measured Sox9+ progenitor cell luminance. (e) High-magnification view of the MTJ in (a) and (f) high-magnification view of the MTJ in (c). (g) Occupancy rate of Sox9 expression. The fluorescence intensity vs. distance plot shows that Sox9 expression was identified in (a, b) future tendons, bone, and MTJ regions at E13.5 and in (c, d) cartilage cells at E16. The comparison of fluorescence intensity indicated that the intensity was>50 in the region of the MTJ at E13 but was <100 in the region of the MTJ at E16 (b, d). (e–g) The occupancy rate of Sox9 expression in the MTJ at E13 was high compared to that in the MTJ at E16 (*P < 0.05). Sox9, SRY-box containing gene 9; B, bone; M, muscle; T, tendon; MTJ, myotendinous junction.
These results demonstrated that Sox9 was expressed not only in the tendon and the bone but also in a part of the muscle: the MTJ (Fig. 2).
Sox9 expression in muscle progenitor cells in vivo
Some researchers have found Sox9 expression in myoblastic cells in vitro20,21,22. To determine whether Sox9 is expressed in muscle progenitor cells in vivo, we of double immunofluorescence staining with antibodies against desmin, which is a marker for muscle progenitors11, and Sox917,18. In previous studies of head development23,24, we defined the cells in the desmin+ area as the cranial paraxial mesoderm (CPM), from which the head muscle originates (Fig. 3a–c). Cranial neural crest cells (CNCs), from which the head tendon and bone originate, were located in the superficial layer of each pharyngeal arch and were wrapped around muscle progenitor cells23 (Fig. 3a–c). At E10, we found two masses comprising desmin+ muscle progenitor cells in the first pharyngeal pouch (Fig. 3a). A mass of muscle progenitor cells in the CPM had few Sox9+ progenitor cells compared to the number of CNCs (CNC: 88.92%±2.24%; CPM: 25.77%±0.21%; P < 0.001) (Fig. 3d). On the other hand, Sox9 was also expressed in some muscle progenitor cells in the limb (Fig. 3e–h). At E10, we could not identify desmin+ muscle progenitor cells in the limbs, which showed Sox9 expression. At E12, we detected desmin+ muscle in the limbs, and some muscle cells showed Sox9 expression (Fig. 3f,g). The limb muscle anlage had few Sox9+ progenitor cells compared to the tendon and bone anlagen (muscle: 24.21%±7.18%; tendon and bone: 96.70%±0.75%; P < 0.01) (Fig. 3h). Therefore, Sox9 was expressed in some muscle progenitor cells in vivo (Fig. 3).
Sox9 expression in muscle. (a–d) Head at E10 and (e-h) limb at E10 and E12. All panels show immunohistochemical staining of desmin (green) and Sox9 (red). (b, c) High-magnification view of a square in (a) and (g) high-magnification view of a square in (f). (d, h) Comparison of Sox9+ progenitor of CNCs with those of the CPM. (d) The mass composed of muscle progenitor cells has few Sox9+ progenitor cells compared to CNCs (P < 0.001). (h) The limb muscle has few Sox9+ progenitor cells compared to the future tendon and bone (P < 0.01). Sox9, SRY-box containing gene 9; CNC, neural crest cell; CPM, cranial paraxial mesoderm; MTJ, myotendinous junction.
To determine whether Sox9 is expressed in the muscle, we generated double-transgenic Sox9creERT2/Rosa26-loxP-stop-loxP-tdTomato reporter mice19. We detected the enrichment of tdTomato+ cells in the masseter and intercostal muscles of mice induced at E9 (Fig. 4b,f,d,h) and few tdTomato+ cells in the masseter muscle in mice induced at E15 (Fig. 4c,g). We could not identify tdTomato+ cells in the intercostal muscle in mice induced at E15 (Fig. 4e,i). The number of tdTomato+ cells in the masseter and intercostal muscles in mice induced at E9 was high compared to that in mice induced at E15 (masseter: P < 0.01, Fig. 4k; intercostal: P < 0.05, Fig. 4i). The tdTomato+ area was located between the nuclei of the muscle (Fig. 4f,h). In mice induced at E9, we could clearly identify tdTomato+ cells in the pancreas (Fig. 4j). The lumber vertebrae showed the enrichment of tdTomato+ cells in mice induced at E15 (Fig. 4m). These results clearly showed that Sox9 was expressed in muscle progenitor cells.
Lineage tracing of Sox9-expressing cells in developing muscle. (a) Schematic showing the generation of double-transgenic Sox9creERT2/Rosa26-loxP-stop-loxP-tdTomato reporter mice. We analyzed the (b, c, f, g) masseter muscle, (d, e, h, i) intercostal muscle, (j) pancreas, and (m) lumber vertebra. Mice were induced at (b, d, f, h, j) E9 and (c, e, g, i, m) E15 and analyzed at E18. The number of tdTomato+ cells in muscles induced at E9 and analyzed at E18 was much larger than that induced at E15 and analyzed at E18 (k, i; masseter: P < 0.01; intercostal: P < 0.05). Sox9, SRY-box containing gene 9.
Sox9 expression in muscle progenitor cells in vitro
We noticed that Sox9 was expressed in the cytoplasm of muscle progenitor cells in the myotome (Fig. 5a–d). Sox9 expression in sex cells translocates from the cytoplasm to the nucleus at the onset of male sexual differentiation25. To identify whether Sox9 expression during myogenesis translocates from the cytoplasm to the nucleus, we investigated the mouse myoblast C2C12 cell line. In the control group (undifferentiated C2C12 cells), Sox9 was clearly expressed in the cytoplasm (Fig. 5e–g,l,m). Reverse transcription-polymerase chain reaction (RT-PCR) showed Sox9 messenger RNA (mRNA) expression in the control group (Fig. 5h). In myogenic induction, Sox9 was expressed in the nucleus (Fig. 5i-k,o,p). In addition, in the control group, the immunofluorescence intensity of Sox9 in the cytoplasm was high compared to that in the nucleus (Fig. 5l–n). In contrast, myogenic induction resulted in Sox9 expression in the nucleus of C2C12 cells (Fig. 5o–q).
Sox9 expression in vivo and in vitro. (a–d) Desmin and Sox9 were expressed in the myotome at E10. (e–g) Sox9 expression in the control group (undifferentiated C2C12 cells). (i–k) Sox9+ progenitor cells under muscle induction (experimental group). (l–q) Single-cell analysis of C2C12 cells. (l, m, o, p) Immunohistochemical staining of Sox9 and (n, q) fluorescence intensity of Sox9 expression. (a–d) Sox9 was expressed in the cytoplasm of muscle progenitor cells. (e-h) In the control group, Sox9 was clearly expressed in the cytoplasm of C2C12 cells, and we identified Sox9 mRNA expression. (l-n) Immunofluorescence intensity of Sox9 in the cytoplasm was high compared to that in the nucleus. (i-k) In the experimental group, Sox9 was expressed in the nucleus of muscle progenitor cells. (o-q) Myogenic induction indicated Sox9 expression in the nucleus. Sox9, SRY-box containing gene 9; mRNA, messenger RNA.
Function of Sox9 in the development of the musculoskeletal system
The Wnt1 gene plays important developmental roles in the CNC4. To identify Wnt1 expression in the musculoskeletal system of the head, we used Wnt1Cre;tdTomato mice. Because muscle fibers exhibit a high level of green autofluorescence26, we could detect the muscle areas (Fig. 6a–d). tdTomato+ cells were observed throughout the muscle-associated connective tissue, tendon, and bone in the head (Fig. 6e) (Fig. 6a,b: the regions of lateral pterygoid muscle attachment to the condylar head; Fig. 6c and d: the regions of masseter muscle attachment to the mandible)27,28. Therefore, Wnt1 was expressed throughout the musculoskeletal system in the head (Fig. 6e).
Wnt1 expression in each component of the musculoskeletal system. (a,b) The region of the lateral pterygoid muscle attachment to the mandibular condyle. (c) The fibers of the masseter muscle. (d) The region of the masseter muscle attachment to the mandible. (b) High-magnification view of panel (a). (a-d) Wnt1 was expressed in the muscle-associated connective tissue, tendon, and bone. (e) Wnt1-expressing region: bone, tendon, and muscle-associated connective tissue. White arrows, tendon; yellow arrows, intramuscular tendon; CH, condylar head; LP, lateral pterygoid muscle; Ma, masseter; Mand, mandibular bone.
To determine the functional role of Sox9 in the development of the musculoskeletal system, we examined the TMJ in Wnt1Cre;Sox9flox/+ mice4. Wnt1Cre;Sox9flox/+ mice at E15.5 had a small lower jaw, a cleft secondary palate, and deformed Meckel’s cartilage and mandibular bone (Fig. 7a–h). Wnt1Cre;Sox9flox/+ mice had muscle, tendon, and bone deformation in the TMJ (Fig. 7i–p). The formation of tendon and bone was significantly decreased in Wnt1Cre;Sox9flox/+ mice at E15.5 (Fig. 7I,m). In addition, the masticatory muscles of Wnt1Cre;Sox9flox/+ mice showed hypoplasia (Fig. 7j,n). Muscle fibers of the lateral pterygoid (one of the masticatory muscles) in Wnt1Cre;Sox9flox/+ mice were narrow compared with those in control mice (Fig. 7j,n). Immature muscle cells were located in the lateral pterygoid of Wnt1Cre;Sox9flox/+ mice (Fig. 7n, arrow). The muscle fiber ends of the MTJ in control mice were sharp, while they were comparatively rounded in Wnt1Cre;Sox9flox/+ mice (Fig. 7k,l,o,p).
Function of Sox9 in the development of the musculoskeletal system. (a–p) Comparison of WntCre;Sox9floxed/+ mice with control mice. Lateral view of the head in (a, b) control and (e, f) WntCre;Sox9floxed/+ mice. (c, g) H&E staining and (d, h) 3D reconstruction of the mandibular bone and its surrounding structures. (i–p) Immunohistochemical staining of desmin (green) and Sox9 (red). Region of lateral pterygoid muscle attachment to the mandibular condyle in (i) control and (m) WntCre;Sox9floxed/+ mice. Muscle fibers in the lateral pterygoid muscle in (j) control and (n) WntCre;Sox9floxed/+ mice. MTJ in the lateral pterygoid muscle in (k) control and (o) WntCre;Sox9floxed/+ mice. (l, p) 3D reconstruction of the MTJ. Wnt1-Cre;Sox9flox/+ mice at E15.5 have a small lower jaw (b, f), a cleft secondary palate (g), and deformed Meckel’s cartilage (d, h, white arrowhead) and mandibular bone (d, h, blue arrowhead). (i, m) Wnt1-Cre;Sox9flox/+ mice have tendon and bone deformation in the TMJ. (n) Muscle fibers in Wnt1-Cre;Sox9flox/+ mice are narrow compared to those in control mice (j). The lateral pterygoid muscle in Wnt1-Cre;Sox9flox/+ mice has immature muscle cells (n, arrowheads). The muscle fiber ends of the MTJ in control mice are sharp, while the muscle fibers of the MTJ in Wnt1-Cre;Sox9flox/+ mice have comparatively rounded ends (k, l, o, p). Sox9, SRY-box containing gene 9; H&E, hematoxylin and eosin; B, bone; CH, condylar head; LP, lateral pterygoid muscle; M, muscle; MK, Meckel’s cartilage; Mn, mandibular bone; MTJ, myotendinous junction; T, tendon (i,m) or tongue (c,g); TMJ, temporomandibular joint.
Discussion
Several studies have revealed the function of Sox9 in tendon and bone development2,3,4,12,13,14,15,16,17,18,19. Nevertheless, the fundamental question of whether Sox9 is expressed in developing muscles has been largely neglected. To reveal whether Sox9 controls the development of three components (muscles, tendons, and bones) of the musculoskeletal system, we studied the expression of Sox9 in developing muscle. This study demonstrated that muscle progenitors show Sox9 expression. Therefore, we revealed that Sox9 controls all the main components of the musculoskeletal system.
Our finding that the development of muscles, tendons, and bones is controlled by the switching of Sox9 expression provides new perspectives on the development of the musculoskeletal system. In the early stage, Sox9 was expressed in progenitor cells of all components of the musculoskeletal system. Subsequently, it was detected in the MTJ, tendon, and bone. In the late embryonic stage, bones showed Sox9 expression. Therefore, it is necessary to switch on Sox9 expression in each component (muscle, tendon, and bone) for the development of the musculoskeletal system (Fig. 8). We considered that the simplification of the functioning of transcription factors allows muscles, tendons, and bones to easily establish the complex structure of the musculoskeletal system.
Schematic of switching of Sox9 expression. (a) Sox9 is expressed in progenitor cells of all components of the musculoskeletal system. (b) In the middle embryonic stage (E13), Sox9 was detected in the MTJ, tendon, and bone. (c) In the late embryonic stage (E16), bones show Sox9 expression. Therefore, it is essential to switch on Sox9 expression in each component (muscle, tendon, and bone) during the development of the musculoskeletal system. MTJ, myotendinous junction.
It is well known that tendons and bones have the same origin. Tendons and bones are derived from the sclerotome, the lateral plate mesoderm, and the neural crest2,3,4,5. Tendon progenitor cells in the trunk develop from the syndetome, which is in the rear of the sclerotome7,8. In addition, in the sclerotome, Scx and Sox5 are coexpressed in the multipotent cell group, which can differentiate into cartilage and tendons, and are then expressed specifically in tendon and cartilage regions7. Sox9 and Scx were also detected in the subpopulations of tendon/ligament progenitor cells and chondroprogenitor cells7,13. In this study, muscle progenitor cells showed Sox9 expression both in vivo and in vitro. Therefore, the components of the musculoskeletal system (muscle, tendon, and bone) originate from Sox9+ progenitor cells. However, little is known about whether muscles, tendons, and bones have the same origin. This hypothesis is supported by the differentiation of somites into muscles, tendons, and bones23.
Previous studies on myoblastic cell lines in vitro have suggested that muscles, tendons, and bones have the same origin because they retain the capacity to differentiate into a chondrogenic, osteoblastic lineage or tenogenic lineage. According to Bettex-Galland and Wiesmann29, L6 myoblasts differentiate into chondrocytes under the influence of demineralized bone. Katagiri et al20. showed that bone morphogenetic protein-2 (BMP2) converts C2C12 cells into an osteoblast lineage. L6 myoblasts show Sox9 expression, which plays a role in chondrogenesis21. According to Uemura et al30., myostatin promotes tenogenic differentiation of C2C12 myoblastic cells. C2C12 and L6 appear to be stem cells that can differentiate into each component of the musculoskeletal system.
A few researchers have reported that muscle shows Sox9 expression. Rat L6 myoblastic cells show Sox9 expression, but after several days of culture, there is a decline in the level of Sox921. We demonstrated that muscle progenitors show Sox9 expression in vivo and in vitro and that Sox9 expression in C2C12 cells translocate from the cytoplasm to the nucleus during muscle induction. Since Sox9 represses muscle gene expression22, myoblasts may retain their undifferentiated state. To retain an undifferentiated state, muscle progenitor cells expressed Sox9 in the cytoplasm (Fig. 8).
The Wnt1 gene plays an important role in the development of the neural crest and its derivatives, and the Wnt1Cre transgenic mouse line is widely used to investigate neural crest development. To clarify the role of Sox9 in the development of neural crest derivatives, Mori-Akiyama et al4. performed gross and histological observations using Wnt1Cre;Sox9flox/flox mice. The analysis showed that all cartilage and endochondral bones, such as the anterior part of the cranial base, Meckel’s cartilage, the malleus, the incus, and the nasal capsule, were missing. Although the bone features of Wnt1Cre;Sox9 flox/flox mice have been described4, little is known about the muscles and tendons of these mice. We found that Wnt1Cre;Sox9Flox/+ mice have a cleft palate, deficient Meckel’s cartilage, a small mandibular body, and hypoplastic condylar cartilage (Fig. 7). Moreover, these mice show hypoplasia of masticatory muscles and their tendons (Fig. 7). Because previous studies showed that Sox9 plays an important role in tendon and bone development18,19, it is easy to understand why tendon and bone hypoplasia is observed in Wnt1Cre;Sox9Flox/+ mice. The reason why muscle hypoplasia occurs in Wnt1Cre;Sox9Flox/+ mice is that defects in Sox9 expression in muscle connective tissues cause hypoplasia of muscle fibers (Figs. 6 and 7)27,28.
Conclusion
Sox9 is expressed in progenitor cells of all components of the musculoskeletal system. However, the muscles and tendons do not express Sox9 during the late embryonic period. Therefore, it is essential to switch on Sox9 expression in each component (muscle, tendon, and bone) during the development of the musculoskeletal system (Fig. 8). In addition, a decrease in Sox9 expression in muscle-associated connective tissues, tendons, and bones leads to hypoplasia of the muscle, tendon, and bone. Therefore, Sox9 controls the development of each component of the musculoskeletal system.
Methods
Experimental animals
All experiments in mice were performed in accordance with the National Institutes of Health (NIH) guidelines for the care and use of animals. The experiments were also approved by the Tokyo Dental College Institutional Animal Care and Use Committee (protocol #240106). Wnt1Cre (129S4. Cg-E2f1Tg(Wnt1-cre)2Sor/J), Sox9flox/flox (B6.129S7-Sox9tm2Crm/J), Sox9creER (STOCK Tg(Sox9cre/ERT2)1Msan/J), and R26tdTomato (B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). All mice were bred under specific-pathogen-free conditions. Wnt1Cre and Sox9creER mice were mated with R26tdTomato mice to generate Wnt1Cre; R26tdTomato (Sox9creER; R26tdTomato) mice. Wnt1Cre transgenic mice were mated with Sox9flox/flox mice to generate Wnt1Cre; Sox9flox/+ mice. We performed PCR to genotype each strain according to the instructions of the Jackson Laboratory. A female mouse was housed with a male mouse overnight, and noon of the day when we observed the vaginal plug was designated as E0.5.
To trace the lineages of Sox9-expressing cells in developing muscle, we generated double-transgenic Sox9creERT2/Rosa26-loxP-stop-loxP-tdTomato reporter mice in whom Cre expression in Sox9+ progenitor cells could be induced at different developmental stages by administration of tamoxifen (Tam).
Histological analysis
We fixed fetal tissues in 4% phosphate-buffered paraformaldehyde (PFA). When we made the paraffin blocks, we decalcified the specimens using 10% ethylenediaminetetraacetic acid (EDTA) for 7 days at room temperature. We prepared the paraffin blocks using standard methods and cut a series of 5-μm-thick tissue sections using a sliding microtome (Leica Biosystems, Wetzlar, Germany). When we made the frozen blocks, we incubated the tissue samples overnight in 30% sucrose in phosphate-buffered saline (PBS). Then, we embedded the tissue samples in Tissue-Tek OCT compound (Sakura Finetek, Japan). Next, we cut 15-μm-thick sections using a CM1950 cryomicrotome (Leica Biosystems, Wetzlar, Germany) and stained the sections with Hoechst 33342 (1:1000 dilution; Thermo Fisher Scientific, Waltham, MA, USA) to visualize the nuclei using an Axio Imager wide-field fluorescence microscope (Zeiss, Oberkochen, Germany). We analyzed the images using ImageJ (NIH, Bethesda, MD, USA). For 3D reconstruction, we loaded the digital images of the hematoxylin and eosin (H&E)-stained serial sections into Amira (Visage Imaging, Inc., Richmond, Australia) by using a voxel size appropriate for the section thickness.
Immunohistochemical analysis
We incubated the sections overnight at 4 °C with the following primary antibodies: mouse anti-desmin antibody (1:1000 dilution; Merck Millipore, Burlington, MA, USA) and rabbit anti-sox9 antibody (1:1000 dilution; Merck Millipore). Then, we stained them for 1.5 h at room temperature using the ABC staining kit (Funakoshi, Tokyo, Japan) and the following secondary antibodies: donkey anti-mouse immunoglobulin G (IgG) Alexa Fluor 488 (1:1000 dilution; Thermo Fisher Scientific) and donkey anti-goat IgG Alexa Fluor 555 (1:1000 dilution; Thermo Fisher Scientific). We treated a few sections with ImmPACT 3,3-diaminobenzidine (DAB) (Funakoshi, Tokyo, Japan) to detect any reactions and then inspected the sections after counterstaining with hematoxylin.
Double-staining of ALP and desmin
We stained the sections using an ALP staining kit (Primary Cell, Hokkaido, Japan) according to the manufacturer’s instructions. Briefly, we rinsed the sections in running distilled water for 1 min, after which we added 50 mL of staining solution dropwise to each section. Then, we incubated the sections for 3 h at room temperature until the ALP staining was a bright intense blue, and then we washed the sections with PBS. Next, we incubated them in 3% hydrogen peroxide with methanol for 30 min, subjected them to several additional washings with PBS, and then incubated them in 3% bovine serum albumin for 1 h to block nonspecific binding. Subsequently, we treated the sections with a primary antibody against desmin (1:1000 dilution; Abcam, Cambridge, UK) and incubated them overnight in a moisture chamber at 37 °C. Then, we applied a secondary antibody using EnVisionTM + Dual Link System-horseradish peroxidase (HRP; Dako, Tokyo, Japan) at room temperature. Finally, after several more washes with PBS, we treated the sections with ImmPACT DAB (Funakoshi) to detect any reaction and then inspected them after counterstaining with hematoxylin.
RNA in situ hybridization
The anti-sense probe for Scx has been described previously31. We labeled the probe with digoxigenin (DIG RNA labeling mix; Roche, Rotkreuz, Switzerland) and performed hybridization by following a standard protocol. Briefly, we fixed the sections for 10 min using 4% PFA, digested them using 1 μg/mL proteinase K (Roche) for 5 min, and then fixed them again for 5 min. Next, we performed acetylation for 10 min in a solution containing triethanolamine, hydrochloric acid, and acetic anhydride. We preblocked the sections using hybridization buffer (50% formamide, 5x saline sodium citrate [SSC], 50 μg/mL yeast tRNA, 1% sodium dodecyl sulfate [SDS], and 50 μg/ml heparin) and subsequently incubated them with an Scx anti-sense probe diluted to 1 ng/μL in hybridization buffer. After washing away the unbound probes using SSC buffer, we detected the probes in the sections using antidigoxigenin antibody conjugated to ALP (Roche) and BM purple (Roche).
Tamoxifen treatment
We dissolved tamoxifen (T5648; Sigma-Aldrich, St. Louis, MO, USA) in ethanol and then diluted it in corn oil (C8267; Sigma-Aldrich) at a concentration of 10 mg/mL, as described previously32. Then, we injected 1.5 mg or 3 mg of tamoxifen into the peritoneal cavity of pregnant mice at E9 or E15, respectively, and coinjected 1 mg/40 g of progesterone (P8783; Sigma-Aldrich).
C2C12 cell differentiation
We maintained C2C12 cells in Dulbecco’s Modified Eagle Medium (DMEM; Mediatech) containing 10% (vol/vol) fetal bovine serum (Tissue Culture Biologicals, Tulare, CA, USA) and incubated them in a 5% CO2 atmosphere at 37 °C. Then, we differentiated 95%–100% confluent C2C12 myoblasts into myotubes using 2% (vol/vol) horse serum (J. R. Scientific, Woodland, CA, USA) in DMEM. We maintained the C2C12 cells in 2% horse serum–containing DMEM and changed the medium every second day until the C2C12 cells fully differentiated into myotubes, which required approximately 4–6 days (muscle induction group). In the control group, 95-100% confluent C2C12 myoblasts were maintained in DMEM containing 10% (vol/vol) FBS and incubated at 37 °C with 5% CO2 for 4-6 days.
Cells were washed with warm PBS (37 °C), fixed in 4% paraformaldehyde at room temperature for 30 min and then subjected to immunofluorescence staining for myotubes and 4,6-diamidino-2-phenylindole (DAPI) staining for nuclei. The primary antibody was rabbit anti-sox9 antibody (1:1000, Merck Millipore), and the secondary antibody was donkey anti-goat IgG Alexa Fluor 555 (1:1000, Thermo Fisher Scientific). To verify the validity of the immunohistostaining results, we performed RT-PCR on the control group (undifferentiated C2C12 cells).
Statistical analysis
All statistical analyses were performed by using SPSS Statistics 21.0 (IBM, Armonk, NY, USA). P-values were calculated using Student’s t-test. The between-group differences for which P < 0.05 were considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001 are used throughout the report). Error bars show the standard deviation of the mean.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Benjamin, M. & Ralphs, J. R. The cell and developmental biology of tendons and ligaments. Int. Rev. Cytol 196, 85–130 (2000).
Akiyama, H. et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. U. S. A. 102, 14665–14670 (2005).
Christ, B., Huang, R. & Scaal, M. Formation and differentiation of the avian sclerotome. Anat. Embryol. (Berl) 208, 333–350 (2004).
Mori-Akiyama, Y., Akiyama, H., Rowitch, D. H. & de Crombrugghe, B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Natl. Acad. Sci. U. S. A. 100, 9360–9365 (2003).
Smith, T. G., Sweetman, D., Patterson, M., Keyse, S. M. & Münsterberg, A. Feedback interactions between MKP3 and ERK MAP kinase control scleraxis expression and the specification of rib progenitors in the developing chick somite. Development 132, 1305–1314 (2005).
Brent, A. E. & Tabin, C. J. Developmental regulation of somite derivatives: muscle, cartilage and tendon. Curr. Opin. Genet. Dev 12, 548–557 (2002).
Brent, A. E., Braun, T. & Tabin, C. J. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development 132, 515–528 (2005).
Chen, J. W. & Galloway, J. L. The development of zebrafish tendon and ligament progenitors. Development 141, 2035–2045 (2014).
Huang, A. H. et al. Musculoskeletal integration at the wrist underlies the modular development of limb tendons. Development 142, 2431–2441 (2015).
Rot-Nikcevic, I., Downing, K. J., Hall, B. K. & Kablar, B. Development of the mouse mandibles and clavicles in the absence of skeletal myogenesis. Histol. Histopathol. 22, 51–60 (2007).
Nara, M. et al. Developmental mechanism of muscle-tendon-bone complex in the fetal soft palate. Arch. Oral. Biol. 82, 71–78 (2017).
Yamamoto, M. et al. Morphological association between the muscles and bones in the craniofacial region. PLoS One 15, e0227301 (2020).
Subramanian, A. & Schilling, T. F. Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development 142, 4191–4204 (2015).
Ng, L. J. et al. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 183, 108–121 (1997).
Zhao, Q., Eberspaecher, H., Lefebvre, V. & De Crombrugghe, B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev. Dyn. 209, 377–386 (1997).
Akiyama, H., Chaboissier, M. C., Martin, J. F., Schedl, A. & de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16, 2813–2828 (2002).
Asou, Y. et al. Coordinated expression of scleraxis and Sox9 genes during embryonic development of tendons and cartilage. J. Orthop. Res. 20, 827–833 (2002).
Sugimoto, Y. et al. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 140, 2280–2288 (2013).
Soeda, T. et al. Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis 48, 635–644 (2010).
Katagiri, T. et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 127, 1755–1766 (1994).
Matsushita, T. et al. Expression of transcription factor Sox9 in rat L6 myoblastic cells. Connect. Tissue Res. 45, 164–173 (2004).
Hernández-Hernández, J. M. et al. Sox9 represses alpha-sarcoglycan gene expression in early myogenic differentiation. J. Mol. Biol. 394, 1–14 (2009).
Yamamoto, M. & Abe, S. Mechanism of muscle-tendon-bone complex development in the head. Anat. Sci. Int. 95, 165–173 (2020).
Yamamoto, M. et al. Desmin and nerve terminal expression during embryonic development of the lateral pterygoid muscle in mice. Arch. Oral. Biol. 59, 871–879 (2014).
Malki, S., Berta, P., Poulat, F. & Boizet-Bonhoure, B. Cytoplasmic retention of the sex-determining factor SOX9 via the microtubule network. Exp. Cell Res. 309, 468–475 (2005).
Jackson, K. A., Snyder, D. S. & Goodell, M. A. Skeletal muscle fiber-specific green autofluorescence: potential for stem cell engraftment artifacts. Stem Cells 22, 180–187 (2004).
Heude, E. et al. Unique morphogenetic signatures define mammalian neck muscles and associated connective tissues. Elife 7, e40179 (2018).
Grenier, J., Teillet, M. A., Grifone, R., Kelly, R. G. & Duprez, D. Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS One 4, e4381 (2009).
Bettex-Galland, M. & Wiesmann, U. Differentiation of L6 myoblastic cells into chondrocytes. Experientia 43, 610–611 (1987).
Uemura, K. et al. Myostatin promotes tenogenic differentiation of C2C12 myoblast cells through Smad3. FEBS Open Bio 7, 522–532 (2017).
Cesario, J. M., Almaidhan, A. A. & Jeong, J. Expression of forkhead box transcription factor genes Foxp1 and Foxp2 during jaw development. Gene. Expr. Patterns 20, 111–119 (2016).
Mizoguchi, T. et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 29, 340–349 (2014).
Acknowledgements
This study was supported by a Grant-in Aid for Scientific Research (nos. 40256300: Shinichi Abe, nos. 90733767: Masahito Yamamoto) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We sincerely thank all the staff of the Department of Anatomy, Tokyo Dental College, for their wholehearted cooperation.
Author information
Authors and Affiliations
Contributions
M.Y., S.A. and W.C. designed the study. R.N., J.J., N.H. and Y.K. performed the experiments. R.N. and M.Y. analyzed the results. M.Y. and J. J prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagakura, R., Yamamoto, M., Jeong, J. et al. Switching of Sox9 expression during musculoskeletal system development. Sci Rep 10, 8425 (2020). https://doi.org/10.1038/s41598-020-65339-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65339-9
This article is cited by
-
Multi-omics analysis of sarcospan overexpression in mdx skeletal muscle reveals compensatory remodeling of cytoskeleton-matrix interactions that promote mechanotransduction pathways
Skeletal Muscle (2023)
-
Reprogramming of human peripheral blood mononuclear cells into induced mesenchymal stromal cells using non-integrating vectors
Communications Biology (2023)
-
Skeletal muscle gene expression dysregulation in long-term spaceflights and aging is clock-dependent
npj Microgravity (2023)
-
Temporal chromatin accessibility changes define transcriptional states essential for osteosarcoma metastasis
Nature Communications (2023)
-
Tendinous annulus of Zinn for a common origin of the extraocular rectus muscles: a histological study of the orbital apex from donated elderly cadavers
Anatomical Science International (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.