Abstract
Neck circumference (NC) has been proposed as a simple and practical tool, independently associated with cardiometabolic risk factors. However, the association of NC with inter-muscular adipose tissue (IMAT) is still to be determined. We aimed to examine the association of NC with thigh IMAT, and visceral adipose tissue (VAT) measured with computed tomography (CT) in overweight/obese women. 142 premenopausal overweight and obese Caucasian women participated in this cross-sectional study. NC was measured with an inextensible metallic tape above the thyroid cartilage according to International Society for Advancement of Kinanthropometry protocol. Thigh IMAT and VAT volumes were measured with a single cross-sectional CT. Regarding the covariates, fat mass (FM) was assessed with dual-energy x-ray absorptiometry and physical activity was objectively measured with accelerometry. NC was positively associated with thigh IMAT and VAT volumes (standardized β coefficient: β = 0.45, P-value = ≤0.001, β = 0.60, P = ≤ 0.001; respectively), which persisted after adjusting for age, height, overall FM or moderate-to-vigorous physical activity. Our findings show that NC is associated with thigh IMAT volume in overweight and obese premenopausal Caucasian women, regardless of the amount of lower-body fatness. These results suggest underscoring the relevance of NC as a marker of adipose tissue content in thigh skeletal muscle.
Similar content being viewed by others
Introduction
Obesity is associated with significantly higher all-cause mortality in almost two thirds of the adult population in developed countries1. Although the deleterious effects of greater adiposity are well documented, obesity-related metabolic consequences are more associated with regional body fat distribution, ectopic fat deposition, and cellular infiltration, rather than absolute quantity2,3. An increased upper-body fat has been associated with adverse metabolic complications, in particular the visceral adipose tissue (VAT) located in the thoracic, abdominal and pelvic cavities4,5. In addition, a higher intermuscular adipose tissue (IMAT) located between muscle groups and beneath the fascia6 is related to greater risk of all-cause mortality. Each one-standard deviation increase in IMAT (~6.8% greater IMAT) is associated with a 40% greater mortality risk over a 10-year period7. IMAT volume is higher in individuals with obesity and type 2 diabetes8,9 and is associated with high levels of inflammation, insulin resistance, obesity, sarcopenia, and lower physical performance in adults and the elderly population10,11. Recently, Bergia et al. showed that greater IMAT in the thigh is a better predictor of cardiometabolic risk than greater IMAT in the calf in adults who are overweight and obese11. Interestingly, IMAT is a unique ectopic adipose depot involved in a range of adverse health outcomes similar to VAT12.
In the body, adipose tissue two compartments are clearly distinguished: Subcutaneous adipose tissue (SAT) (80% of all body), and VAT (intra-abdominal fat)13. IMAT have been definite as part of SAT depot located between muscle groups and beneath the muscle fascia14. Advances in imaging technology have enabled the identification of IMAT and VAT thought the use of highly sensitive imaging techniques as computed axial tomography (CT) scan and magnetic resonance imaging (MRI)15.
Recently, emerged evidence reveals that volume of neck adipose tissue is independently associated with cardiovascular risk factors, metabolic syndrome prevalence and long-term mortality16,17,18,19,20. Consequently, neck circumference (NC) has been proposed as a novel upper-body fatness anthropometric measurement for weight management in children and adults21,22, due to the associated low cost and feasibility23,24. Previous studies observed a strong association between NC, total abdominal fat, SAT and VAT using CT or MRI17,25,26. However, the association of a larger NC with a higher IMAT is still to be determined. Still, IMAT determination is limited by the radiation exposure associated with CT and by the relatively high cost of magnetic resonance imaging (MRI) analysis precluding its use at clinical settings. Therefore, simple anthropometric indicators, such as NC, waist circumference (WC), body mass index (BMI) but also fat mass (FM) or fat mas index (FMI) will be more practical to predict cardiovascular risk factors at clinical settings as alternatives to estimate IMAT. Nevertheless, the usefulness of these practical anthropometric measures and indexes of adiposity as surrogates of IMAT at the tissue level is still unclear.
Simple and feasible anthropometric measures such as BMI and WC have been explored as markers of IMAT27,28,29,30. The usefulness of simple, low cost and feasible anthropometric indicators, such as NC, over other commonly used overall or central fat mass (FM) indexes has not been explored as potential markers of IMAT. Therefore, in the present investigation we aimed to examine the association of NC and other simple measures such as WC, BMI, FM and FMI with thigh IMAT, and VAT measured with computed tomography (CT) in overweight/obese women.
Methods
Research design and participants
The baseline data of 142 premenopausal overweight and obese Caucasian women participating in a randomized controlled trial (Clinical Trials, ID: NCT00513084) were used. The inclusion criteria were being >24 years, >24.9 kg/m2 BMI, not being pregnant, without any cardio-metabolic disease, not being on any medications or not having had an intervention that affects the weight or body composition. All the assessment was performed in the Faculty of Human Movement, Technical University of Lisbon, from 2002 till 2003. The informed consent was signed by the participants. The study protocol was performed according to the principles of the Helsinki Declaration and approved by the Human Research Ethics Committee of the University of Lisbon.
Procedures
Anthropometry
With the participants in anatomical position, standing or sitting with the head in the Frankfort plane and shoulders relaxed, NC was measured with a plastic tape calibrated weekly over the thyroid cartilage, and perpendicular to the longitudinal axis of the neck, according to International Society for Advancement of Kinanthropometry protocol31. Based on test-retest in 10 participants, the coefficient of variation (CV) for the NC was 0.45%. Weight and height were measured using a scale (SECA, Hamburg, Germany) and a stadiometer (Seca, Hamburg, Germany) previously calibrated and WC was measured according to Lohman et al.’s32 procedures. All measurements were made in duplicate, using the mean for the analysis. BMI was calculated by dividing body weight by the squared height in meters (kg/m2).
Body composition
Single cross-sectional CT
Using a CT scan (Somaton Plus; Siemens, Sorheim, Germany), participants were assessed in supine position with arms extended above their head. A single cross-sectional CT at the L4-L5 intervertebral space image was acquired to measure the VAT area. The boundary between VAT and abdominal subcutaneous adipose tissue was defined using the abdominal and oblique muscles in continuity with the deep fascia of the paraspinal muscles and the anterior aspect of the vertebral body33. Cross-sectional CT thigh images were also obtained using contiguous 7-mm–thick cross-sectional images of both legs obtained between the inferior ischial tuberosity and the superior border of the patella. The IMAT region was measured between muscle groups and underneath the fascia. The IMAT volume (cubic centimeters) identified in each image was calculated by multiplying the image thickness (7 mm) by the tissue area (square centimeters), and IMAT volume (litters) was then converted to mass units (kilograms) multiplying the volume by the assumed constant of fat density (0.92 kg/L)34.
All images were obtained using 120 kVp, 480 mA, and 512 × 512 matrix with a 48-cm field of view. CT data were analysed by specific software (Slice-O-matic, Version 4.2, Tomovision,Montreal, Canada) based on image morphology. A combination of watershed techniques and edge detection filters was employed. Different tissues were identified using boundaries in Hounsfield Units (HU) set to −29 to +150 for muscle, −190 to −30 for IMAT and subcutaneous adipose tissue, and −150 to −50 for VAT35.
Dual energy X-ray absorptiometry (DXA)
Total fat mass, fat-free mass, lean soft tissue, appendicular lean soft tissue mass, leg fat and trunk fat were measured with a pencil beam mode DXA (QDR-1500 Hologic, Waltham, Mass, USA). The equipment measures the attenuation of X-rays pulsed between 70 and 140 kV synchronously with the line frequency for each pixel of the scanned image. Following the protocol of DXA described by the manufacturer, a step phantom with six fields of acrylic and aluminium of varying thickness and known absorptive properties was scanned to serve as an external standard for the analysis of different tissue components. The same technician positioning the participants performed the scans and executed the analysis according to the operator’s manual using the standard analysis protocol. Based on test–retest using 10 participants, the coefficients of variation (CV) in our laboratory of FM is 2.9%. FMI was calculated as FM in kg divided by squared height in m.
Physical activity assessment
All participants were asked to use an accelerometer (ActiGraph, GT1M model, Fort Walton Beach, Florida, USA), worn on the right hip near the iliac crest during 7 consecutive days including weekend days36. The delivery and reception of the accelerometers, as well the explanation of its use, were personally carried out with the participants (Ward et al. 2005). The devices were activated on the first day at 07:00, and data were recorded in 10-s epochs. The device activation and data download were performed using the software Actilife Lifestyle (ver. 3.2). Processing was performed using the software MAHUffe (ver. 1.9.0.3; available at www.mrc-epid.cam.ac.uk) from the original downloaded files (*.dat). For the analyses, a valid day was defined as having 600 or more minutes (10 h) of monitor wear, corresponding to the minimum daily use of the accelerometer37. Apart from accelerometer nonwear time (i.e., when it was removed for sleeping or water activities), periods of at least 60 consecutive min of zero activity intensity counts were also considered as nonwear time. The amount of activity assessed by accelerometry was expressed as the number of minutes per week spent in moderate-to-vigorous physical activity (MVPA) using the cutoff values proposed by Troiano38 for moderate intensity (2020–5998 counts/min, corresponding to 3–5.9 METs) and vigorous intensity (≥5999 counts/min, corresponding to ≥6 METs).
Statistical analyses
Descriptive statistics were performed to describe the characteristics of the study participants. Pearson correlation analyses were used to examine the relationship of NC and other anthropometric indicators with thigh IMAT and VAT.
Multivariate lineal regression was performed to examine the unadjusted associations of NC, BMI, WC, FM and FMI with thigh IMAT and VAT indicators (model 1) or to adjust for age (model 2). Statistical analyses were performed using the SPSS software version 21.0 (SPSS, Chicago, IL, USA) and statistical significance was set at P-value < 0.05.
Results
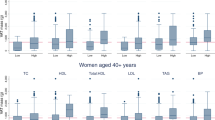
Table 1 displays the descriptive characteristics of the participants of the study. The accelerometer wear mean time was 13.8 ± 1.3 h/day and participants spend the 71% of their waking time (16.91 ± 1.5 h/day) in sedentary behavior. Figure 1 illustrates the significant correlations between NC, BMI, waist circumference, FM and FMI with thigh IMAT and VAT.
In Table 2, the unadjusted (Model 1) or adjusting for age (Model 2) show that NC, BMI, WC, FM and FMI were associated with thigh IMAT and VAT in premenopausal women (all P > 0.01). Values of NC in the unadjusted analyses (Model 1) were positively associated with thigh IMAT, and VAT (Standardized β coefficient: β = 0.45, P-value ≤ 0.001, β = 0.60, P = ≤ 0.001; respectively). When linear regression analyses were additionally adjusted for age, NC remained significantly associated with thigh IMAT (β = 0.41, P = ≤ 0.001). Similarly, NC was a significant predictor of VAT, independently of age (β = 0. 54, P = ≤ 0.001). We repeated the analyses adjusting for height, FM (kg) and MVPA and the results did not change (data not shown).
Discussion
The current investigation shows, for the first time, a positive association of NC with thigh IMAT volume in overweight and obese premenopausal Caucasian women. These findings suggest that NC could be a valid measure of ectopic fat deposition as well as abdominal body fat in overweight and obese premenopausal women.
Our results extend previous findings that underlined (the fact) that NC has as a simple and practical indicator of adiposity21,39 and is positively associated with VAT measured with MRI and CT16,17,25,40. In addition, we observed that, after adjusting for age, NC, BMI, WC, FM, and FMI remained significant predictors of VAT. Interestingly, NC was similar to other indicators as VAT predictor. As no previous investigations explored the association between NC with thigh IMAT, no comparisons can be provided. However it should be underscored the role of intermuscular depots of fat located in the thigh as a relevant predictor of cardiometabolic risk factors in adults who are normal, overweight or obese11,41. Taken together, these findings uncover the potential role of NC as a subrrogate of a patogenic neck adipose tissue due to a greater flow of systemic free fatty acids (FFA), that could partly explain the cardiometabolic risk missed by VAT17,40. In fact, previous evidence has showed that elevated FFA concentrations reproduce the metabolic abnormalities of obesity and that the upper body fat increased in women with obesity would be the most important contributor in the systemic FFA release42.
In this investigation, we observed that anthropometric indicators and FM markers were correlated with ectopic fat deposition, being this association particularly higher for WC and FM. In additon, we showed that an increase in adjusted BMI, WC, FM and FMI markers explained an increase of 0.5–0.6 units in IMAT, while for NC this assocation had a lower variation (0.45 units of IMAT). These results extend previous observations between anthropometric markers with body fat as the outcome of interest23,43. In a sample of overweight and obese adults where body-fat percentage was measured by a bioelectrical impedance analysis (BIA), Joshipura et al.43 showed a lower coefficient of correlation of NC (r = 0.45, P = <0.001) compared to WC (r = 0.62, P = <0.001) and BMI (r = 0.65, P = <0.001), even after adjusting for age, gender, smoking status, and physical activity. Similarly, Assyov et al.23 in a sample of adults that included pre and postmenopausal women with obesity found that WC (r = 0.60, P = < 0.001) showed a higher association with adiposity compared to NC (r = 0.43, P < 0.001). Nevertheless, NC presents a better association in the assessment of metabolic health compared to WC, specifically with fasting plasma glucose, fasting insulin, uric acid, HDL cholesterol and serum triglycerides26,43. Recently, similar results have been shown by Borel et al. in 305 women with severe obesity where NC was a better anthropometric marker to identify a high cardiometabolic risk compared to WC and BMI18. The interesting observation is that FMI presented a slightly lower ability to predict VAT and IMAT in the adjusted model compared to BMI. This is in line with Ortega et al.’s findings44, who showed that BMI was a stronger predictor of CVD mortality than total adiposity markers, particularly, fat-mass percentage and FMI assessed using accurate methods.
It is important to underscore that the reference techniques used in this study to assess IMAT and VAT along with DXA measures for total and regional body composition determination strengthen the findings of this study. Also, the inclusion of accelerometry data to assess the role of habitual physical activity as a potential confounder in these findings was explored. Nevertheless, some limitations should also be addressed. The cross-sectional nature of this study does not establish causality, and a possible reverse causality between anthropometric measures and adipose tissues compartments should not be disregarded. Our findings can only be generalized to Caucasian overweight/obese premenopausal women.
In conclusion, we suggest that a larger NC is associated with a higher volume of VAT but also with a higher amount of ectopic fat deposition in the thigh skeletal muscle, underscoring the relevance of NC as an indicator of adipose tissue content in thigh skeletal muscle. Additionally, when WC, BMI or adiposity are not available or are invalidated (edema, abdominal distension or increase in lean mass) in the clinical practice, NC should be used as an alternative anthropometric measure to predict thigh IMAT and VAT in overweight and obese premenopausal women due to its simplicity, feasibility and low cost.
Change history
16 July 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Flegal, K. M., Kit, B. K., Orpana, H. & Graubard, B. I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. Jama 309, 71–82, https://doi.org/10.1001/jama.2012.113905 (2013).
Krotkiewski, M., Bjorntorp, P., Sjostrom, L. & Smith, U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J. Clin. Invest. 72, 1150–1162, https://doi.org/10.1172/jci111040 (1983).
Dube, M. C. et al. The contribution of visceral adiposity and mid-thigh fat-rich muscle to the metabolic profile in postmenopausal women. Obesity 19, 953–959, https://doi.org/10.1038/oby.2010.348 (2011).
Piche, M. E. et al. Regional body fat distribution and metabolic profile in postmenopausal women. Metabolism 57, 1101–1107, https://doi.org/10.1016/j.metabol.2008.03.015 (2008).
Karpe, F. & Pinnick, K. E. Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. Nat. Rev. Endocrinol. 11, 90–100, https://doi.org/10.1038/nrendo.2014.185 (2015).
Gallagher, D. et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am. J. Clin. Nutr. 81, 903–910, https://doi.org/10.1093/ajcn/81.4.903 (2005).
Zhao, Q. et al. Greater skeletal muscle fat infiltration is associated with higher all-cause mortality among men of African ancestry. Age Ageing 45, 529–534, https://doi.org/10.1093/ageing/afw062 (2016).
Goodpaster, B. H. et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 26, 372–379 (2003).
Miljkovic-Gacic, I. et al. Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am. J. Clin. Nutr. 87, 1590–1595, https://doi.org/10.1093/ajcn/87.6.1590 (2008).
Lim, J. P. et al. Inter-muscular adipose tissue is associated with adipose tissue inflammation and poorer functional performance in central adiposity. Arch. Gerontol. Geriatr. 81, 1–7, https://doi.org/10.1016/j.archger.2018.11.006 (2018).
Bergia, R. E., III & Kim, J. E. Differential Relationship between Intermuscular Adipose Depots with Indices of Cardiometabolic Health. 2018, 2751250, https://doi.org/10.1155/2018/2751250 (2018).
Yim, J. E. et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int. J. Obes. 31, 1400–1405, https://doi.org/10.1038/sj.ijo.0803621 (2007).
Ibrahim, M. M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes. reviews: an. Off. J. Int. Assoc. Study Obes. 11, 11–18, https://doi.org/10.1111/j.1467-789X.2009.00623.x (2010).
Vettor, R. et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am. J. Physiol. Endocrinol. Metab. 297, E987–998, https://doi.org/10.1152/ajpendo.00229.2009 (2009).
Lemos, T. & Gallagher, D. Current body composition measurement techniques. Curr. Opin. endocrinology, diabetes, Obes. 24, 310–314, https://doi.org/10.1097/med.0000000000000360 (2017).
Rosenquist, K., Therkelsen, K., Massaro, J., Hoffmann, U. & Fox, C. Development and reproducibility of a computed tomography-based measurement for upper body subcutaneous neck fat. J. Am. Heart Assoc. 3, e000979, https://doi.org/10.1161/jaha.114.000979 (2014).
Torriani, M. et al. Compartmental neck fat accumulation and its relation to cardiovascular risk and metabolic syndrome. Am. J. Clin. Nutr. 100, 1244–1251, https://doi.org/10.3945/ajcn.114.088450 (2014).
Borel, A. L. et al. Waist, neck circumferences, waist-to-hip ratio: Which is the best cardiometabolic risk marker in women with severe obesity? The SOON cohort. PLoS One 13, e0206617, https://doi.org/10.1371/journal.pone.0206617 (2018).
Tal, S. & Litovchik, I. The association between neck adiposity and long-term outcome. 14, e0215538, https://doi.org/10.1371/journal.pone.0215538 (2019).
Alzeidan, R. & Fayed, A. Performance of neck circumference to predict obesity and metabolic syndrome among adult Saudis: a cross-sectional study. 6, 13, https://doi.org/10.1186/s40608-019-0235-7 (2019).
Arias Tellez, M. J., Martinez-Tellez, B., Soto, J. & Sanchez-Delgado, G. Validity of neck circumference as a marker of adiposity in children and adolescents, and in adults: a systematic review. Nutr. Hosp. 35, 707–721, https://doi.org/10.20960/nh.1582 (2018).
Kroll, C. & Mastroeni, S. The accuracy of neck circumference for assessing overweight and obesity: a systematic review and meta-analysis. 44, 667–677, https://doi.org/10.1080/03014460.2017.1390153 (2017).
Assyov, Y., Gateva, A., Tsakova, A. & Kamenov, Z. A comparison of the clinical usefulness of neck circumference and waist circumference in individuals with severe obesity. Endocr. Res. 42, 6–14, https://doi.org/10.3109/07435800.2016.1155598 (2017).
Ben-Noun, L., Sohar, E. & Laor, A. Neck circumference as a simple screening measure for identifying overweight and obese patients. Obes. Res. 9, 470–477, https://doi.org/10.1038/oby.2001.61 (2001).
Li, H. et al. Neck circumference as a measure of neck fat and abdominal visceral fat in Chinese adults. BMC Public. Health 14, 311, https://doi.org/10.1186/1471-2458-14-311 (2014).
Luo, Y. et al. Neck circumference as an effective measure for identifying cardio-metabolic syndrome: a comparison with waist circumference. Endocrine 55, 822–830, https://doi.org/10.1007/s12020-016-1151-y (2017).
Ruan, X. Y. et al. Estimating whole body intermuscular adipose tissue from single cross-sectional magnetic resonance images. J. Appl. Physiol. 102, 748–754, https://doi.org/10.1152/japplphysiol.00304.2006 (2007).
Koster, A. et al. Fat distribution and mortality: the AGES-Reykjavik Study. Obesity 23, 893–897, https://doi.org/10.1002/oby.21028 (2015).
Yaskolka Meir, A. et al. Intermuscular adipose tissue and thigh muscle area dynamics during an 18-month randomized weight loss trial. J. Appl. Physiol. 121, 518–527, https://doi.org/10.1152/japplphysiol.00309.2016 (2016).
Santanasto, A. J. et al. Body Composition Remodeling and Mortality: The Health Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci 72, 513–519, https://doi.org/10.1093/gerona/glw163 (2017).
Stewart, A., Marfell-Jones, M., Olds, T. & Ridder, D. H. International Society for Advancement of Kinanthropometry. International standards for anthropometric assessment. Lower Hutt, New Zealand: International Society for the Advancement of Kinanthropometry, 50–53 (2011).
T. G. Lohman, A. F. R., and R. Martorell. Eds. Anthropometric Standardization Reference Manual, Human Kinetics Publishers. Vol. Champaign, Ill (USA, 1988).
Ferland, M. et al. Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. Br. J. Nutr. 61, 139–148, https://doi.org/10.1079/bjn19890104 (1989).
Snyder, W. S. et al. Report on the Task Group on Reference Man., (Paergamon Press, Oxford, UK, 1984).
Mourtzakis, M. et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. physiology, Nutr., Metab. = Physiologie appliquee, nutrition et. metabolisme 33, 997–1006, https://doi.org/10.1139/h08-075 (2008).
Trost, S. G., McIver, K. L. & Pate, R. R. Conducting accelerometer-based activity assessments in field-based research. Med. Sci. Sports Exerc. 37, S531–543 (2005).
Ward, D. S., Evenson, K. R., Vaughn, A., Rodgers, A. B. & Troiano, R. P. Accelerometer use in physical activity: best practices and research recommendations. Med. Sci. Sports Exerc. 37, S582–588 (2005).
Troiano, R. P. A timely meeting: objective measurement of physical activity. Med. Sci. Sports Exerc. 37, S487–489 (2005).
Preis, S. et al. Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart study. J. Clin. Endocrinol. Metab. 95, 3701–3710, https://doi.org/10.1210/jc.2009-1779 (2010).
Pandzic Jaksic, V. et al. Neck adipose tissue - tying ties in metabolic disorders. Horm Mol Biol Clin Investig 33, https://doi.org/10.1515/hmbci-2017-0075 (2018).
Bang, E., Tanabe, K., Yokoyama, N., Chijiki, S. & Kuno, S. Relationship between thigh intermuscular adipose tissue accumulation and number of metabolic syndrome risk factors in middle-aged and older Japanese adults. Exp. Gerontol. 79, 26–30, https://doi.org/10.1016/j.exger.2016.03.010 (2016).
Nielsen, S., Guo, Z., Johnson, C. M., Hensrud, D. D. & Jensen, M. D. Splanchnic lipolysis in human obesity. J. Clin. Invest. 113, 1582–1588, https://doi.org/10.1172/jci21047 (2004).
Joshipura, K., Munoz-Torres, F., Vergara, J., Palacios, C. & Perez, C. M. Neck Circumference May Be a Better Alternative to Standard Anthropometric Measures. J. Diabetes Res. 2016, 6058916, https://doi.org/10.1155/2016/6058916 (2016).
Ortega, F. B., Sui, X., Lavie, C. J. & Blair, S. N. Body Mass Index, the Most Widely Used But Also Widely Criticized Index: Would a Criterion Standard Measure of Total Body Fat Be a Better Predictor of Cardiovascular Disease Mortality? Mayo Clin. Proc. 91, 443–455, https://doi.org/10.1016/j.mayocp.2016.01.008 (2016).
Acknowledgements
Our research was supported by the Portuguese Foundation for Science, Technology grant (Sapiens 358007/99), the Oeiras City Council, Becel Portugal, Roche Pharmaceuticals Portugal, Compal Portugal and partially by the University of Granada Plan Propio de Investigación 2016 -Excellence actions: Unit of Excellence on Exercise and Health (UCEES), and the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades, and European Regional Development Funds (ref. SOMM17/6107/UGR) and the Fundación Carolina (C.2016-574961).
Author information
Authors and Affiliations
Contributions
Designed research: M.J.A.T., A.M.S., J.R.R., L.B.S.; Conducted research: M.J.A.T., A.M.S., J.R.R.; Provided essential reagents or provided essential materials: A.M.S., L.B.S.; Analysed data or performed statistical analysis: M.J.A.T., A.M.S., J.R.R.; Wrote manuscript: M.J.A.T., A.M.S., J.R.R.; Critical review of the manuscript and scientific assistance: M.J.A.T., A.M.S., J.R.R., S.S.M., A.L.P., T.L.B., C.S.M., P.M.R., J.L.T.B., P.J.T., L.B.S.; Had primary responsibility for final content: L.B.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arias Tellez, M.J., Silva, A.M., Ruiz, J.R. et al. Neck circumference is associated with adipose tissue content in thigh skeletal muscle in overweight and obese premenopausal women. Sci Rep 10, 8324 (2020). https://doi.org/10.1038/s41598-020-65204-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65204-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.