Abstract
We examined the effect of L-arginine - (i) on the growth of L. rhamnosus GG (LrG) and (ii) combined LrG synbiotic on the growth of cariogenic S. mutans. Viability of LrG was assessed using MTT/XTT assays, confocal imaging with ADS activity measurement. The effect of L-arginine (0.5%/1%/2%) (2×/24 h) with LrG on S. mutans was evaluated by measuring the colony forming units, biofilm biomass, real-time qPCR and confocal imaging. The pH of the spent media was measured immediately and 24 h post-treatment with assessment of lactic acid. The LrG viability was highest with 2% L-arginine (p < 0.001). Confocal imaging showed that 2% L-arginine increased biofilm thickness of LrG. The 2% L-arginine and LrG synbiotic significantly inhibited the growth of S. mutans (p < 0.001) reducing the viable counts (p = 0.002) and biofilm biomass (p < 0.001). The pH of spent media was the highest when treated with 2% L-arginine and LrG synbiotic (p < 0.001) with no difference between post-treatment and 24 h post-treatment (p > 0.05). Conversely, the 2% L-arginine and LrG synbiotic showed the lowest lactic acid production (p < 0.001). This study demonstrated that L-arginine enhanced the growth of LrG. The 2% L-arginine and LrG synbiotic synergistically inhibits the growth of S. mutans with significant potential to develop as an anti-caries regimen.
Similar content being viewed by others
Introduction
Dental caries, a dysbiosis-regulated biofilm-mediated disease, is an important public health problem worldwide1. Despite being the gold standard for caries control, fluoride has limited action on oral biofilms2. Recently developed strategies to combat caries focus on ecological approaches, interfering with the physical and metabolic activities of the biofilm with an objective of preventing dysbiosis. Such interventions use either antimicrobial strategies or approaches to enhance the growth of health-promoting bacteria3. The latter approach can be holistically summarized as a biotic means of caries prevention, which includes the use of prebiotics and probiotics.
Prebiotics, probiotics and synbiotics are well known for their beneficial health effects on the human gastrointestinal tract4. A prebiotic is a substrate that is selectively utilized by host microorganisms, thereby conferring a health benefit. It stimulates the growth of beneficial bacteria and suppress the growth of pathogens. Arginine has been used as an oral prebiotic to enhance the growth of alkalogenic health-promoting bacteria - Streptococcus sanguinis, Streptococcus parasanguinis and Streptococcus gordonii, with subsequent inhibition of the cariogenic bacterium - Streptococcus mutans5. Furthermore, it enhances the antimicrobial and remineralization effects of fluoride6,7. However, prolonged use of arginine may facilitate plaque alkalization, promoting the overgrowth of oral anaerobes such as Porphyromonas gingivalis5.
Probiotics are live microorganisms that confer a health benefit on the host when administered in adequate amounts. It provides a local protective effect by counteracting the activities of the pathogens and a systemic indirect effect on immunological amelioration. Lactobacillus rhamnosus GG is the world’s most researched probiotics with profound health benefits systemically and antagonistic effects on cariogenic bacteria; however, its effect in the oral environment is brief8,9. Recently, it has been shown that prebiotics and probiotics can potentially be developed into novel synbiotics against oral pathogens10 for future dental caries management11.
Based on such a premise, the use of synbiotics might represent a novel ecological-based caries- preventive strategy to promote the growth of healthy arginolytic bacteria (S. sanguinis and S. gordonii) by prebiotics and simultaneously enrich the growth of probiotics L. rhamnosus GG for enduring oral colonization. Accordingly, the objectives of the study were to examine the effect of (i) arginine on the growth of L. rhamnosus GG and (ii) the combined L-arginine and L. rhamnosus GG synbiotic on the growth of cariogenic S. mutans. The null hypotheses tested in the present study were: (i) L-arginine has no effect on the growth of L. rhamnosus GG and (ii) the combination of L-arginine with L. rhamnosus GG does not affect the growth of S. mutans.
Results
Effect of L-arginine on growth of probiotic L. rhamnosus GG
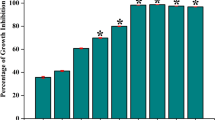
The biofilm viability results are shown in Fig. 1a. Both MTT and XTT assays showed that the percentage survival of L. rhamnosus GG increased with arginine concentration. The cell viability of the L. rhamnosus GG with 2% L-arginine group was significantly higher than 0.5% L-arginine and the control groups (p < 0.05). Representative live/dead images are displayed in Fig. 1b. Live bacteria were stained green and dead bacteria/damaged bacterial cells were stained red with propidium iodide. Distinguishable dead/damaged bacterial cells (red staining) of L. rhamnosus GG could be seen in biofilms treated with 0.5% L-arginine, which were not evident in the other groups (Fig. 1b). The 2% L-arginine increased biofilm thickness of L. rhamnosus GG (Fig. 1b). The Arginine Deiminase System (ADS) activity measured by ammonia detection could not be determined for all the groups, suggesting that L. rhamnosus GG might not be metabolizing arginine through the ADS operon. The results of the first experiment demonstrated that 2% L-arginine significantly enhanced the growth of L. rhamnosus GG biofilm.
The effect of L-arginine on L. rhamnosus GG: (a) L.rhamnosus GG cell viability using MTT and XTT assays (Data was analyzed using Kruskal-Wallis test with Dunn-Bonferroni’s multiple comparison test at p < 0.05. The capital alphabets – A/B/C show significant difference among the groups with XTT assay and the small alphabets – a/b show significant differences among the groups with MTT assay); and (b) Representative confocal microscopic images (100×) of biofilms treated with 0.5%, 1%, 2% L-arginine and 0.9% NaCl.
Effect of L-arginine with L. rhamnosus GG on growth of cariogenic S. mutans
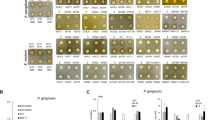
The real-time PCR results showed that the inhibitory effect of L-arginine + L. rhamnosus GG on S. mutans proportions increased with arginine concentration, with 2% L-arginine + L. rhamnosus GG group more evident than 0.5% L-arginine + L. rhamnosus GG and 1% L-arginine + L. rhamnosus GG groups (p < 0.001) (Fig. 2a). The total bacterial concentration for L. rhamnosus GG + S. mutans was further computed with respect to treatments with L-arginine at concentrations – 0.5%, 1%, and 2% and the control – 0.9% NaCl (Fig. 2b). The total bacterial concentration decreased with increasing arginine concentration, with the lowest observed in the 2% L-arginine + L. rhamnosus GG group.
The effect of combined L-arginine and L. rhamnosus GG on S. mutans: (a) Proportional bacterial quantitative data of S. mutans (Capital alphabets – A/B indicate significant differences among the treatment groups) and L. rhamnosus GG (small alphabets – a/b indicate significant differences among the treatment groups) for S. mutans biofilms treated with L-arginine (0.5%, 1%, 2%), 0.9% NaCl and L.rhamnosus GG; (b) Total bacterial concentration estimated by real-time PCR for S. mutans biofilms treated with L-arginine (0.5%, 1%, 2%), 0.9% NaCl and L.rhamnosus GG (small alphabets – a/b indicate significant differences among the groups); (c) Colony forming units for S. mutans on agar media (small alphabets – a/b indicate significant differences among the groups); (d) Relative percentage of biofilm biomass measured by CV assay (small alphabets – a/b indicate significant differences among the groups); and (e) Representative confocal microscopic images (100×) of S. mutans biofilms treated with 0.5%, 1%, 2% L-arginine and 0.9% NaCl and L rhamnosus GG. Data for real-time qPCR and CFU were analyzed by 1-way ANOVA SNK test at p < 0.05; and data for CV assay was analyzed using Kruskal-Wallis test with Dunn-Bonferroni’s multiple comparison test at p < 0.05.
Both the CFU count for S. mutans (Fig. 2c) and the quantified percentage biofilm biomass of S. mutans (Fig. 2d) decreased with increasing arginine concentration with the 2% L-arginine + L. rhamnosus GG group showing significantly lower biofilm biomass than the other groups (p < 0.001). Representative confocal microscopic image of biofilm revealed that the 2% L-arginine + L. rhamnosus GG group had noticeably reduced biofilm thickness (Fig. 2e). These results showed that the 2% L-arginine + L. rhamnosus GG significantly inhibited the growth of cariogenic S. mutans, demonstrating a synergistic synbiotic effect.
pH measurement and generated lactic acid
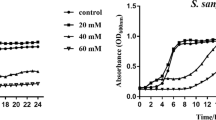
Post-treatment and 24 h post-treatment pH of the spent media of L. rhamnosus GG biofilms treated with L-arginine or 0.9% NaCl are shown in Fig. 3a. For both time points, pH of the spent media for L. rhamnosus GG biofilm increased with increasing concentration of arginine, with the highest pH observed in the 2% arginine group (p < 0.05). Lactic acid generated in the spent media for L. rhamnosus GG biofilms treated with different concentrations of L-arginine or 0.9% NaCl is shown in Fig. 3b. Lactic acid production was significantly lower in 2% L-arginine, 0.5% L-arginine and 0.9% NaCl groups.
pH of spent media and lactic acid production: (a) Post-treatment and 24 h post-treatment pH of spent media for L. rhamnosus GG biofilms treated with 0.5%, 1%, 2% L-arginine and 0.9% NaCl (small alphabets – a/b/c/d show significant difference among the groups at post-treatment phase; and capital alphabets – A/B/C/D show significant difference among the groups at 24 h post-treatment phase); (b) Lactic acid generated in spent media for L. rhamnosus GG biofilms treated with 0.5%, 1%, 2% L-arginine and 0.9% NaCl (small alphabets – a/b show significant difference among the groups); (c) Post-treatment and 24 h post-treatment pH of spent media for S. mutans biofilms treated with L-arginine (0.5%, 1%, 2%), 0.9% NaCl and L.rhamnosus GG (small alphabets – a/b/c/d show significant difference among the groups at post-treatment phase; and capital alphabets – A/B/C/D show significant difference among the groups at 24 h post-treatment phase); and (d) Lactic acid generated in spent media for S. mutans biofilms treated with L-arginine (0.5%, 1%, 2%), 0.9% NaCl and L. rhamnosus GG (small alphabets – a/b show significant difference among the groups). All data were analyzed using 1-way ANOVA with SNK test at p < 0.05.
Conversely, the post-treatment and 24 h post-treatment pH of the spent media of S. mutans biofilm treated with L-arginine and L. rhamnosus GG or 0.9% NaCl are shown in Fig. 3c. For both time points, pH of the spent media with S. mutans biofilm also increased with increasing concentration with arginine, with the highest pH in the 2% L-arginine + L. rhamnosus GG group (p < 0.05). Similarly, the lactic acid generated in the spent media for S. mutans biofilms treated with L-arginine and L. rhamnosus GG or 0.9% NaCl are shown in Fig. 3d. Lactic acid production was the least in 2% L-arginine + L. rhamnosus GG group (p < 0.05). Overall, 2% L-arginine and L. rhamnosus GG significantly antagonized S. mutans growth by creating a synbiotic synergism against S. mutans.
Discussion
The present study demonstrated the feasibility and beneficial effects of a novel synbiotic with synergistic inhibitory effect on cariogenic bacterium S. mutans. Our results demonstrated that the use of L-arginine as a prebiotic enhanced the growth of the probiotic – L. rhamnosus GG in a dose-dependent manner. Thus, the null hypotheses that L-arginine has no effect on the growth of L. rhamnosus GG and the combined L-arginine and L. rhamnosus GG synbiotic does not affect the growth of S. mutans are to be rejected.
Probiotics are beneficial microorganisms that modulate the bacterial balance in the digestive system. For probiotics to be useful in the oral cavity, it must be able to adhere, colonize oral tissue and compete with pathogenic microorganisms at the binding site10. The probiotic L. rhamnosus GG, was used in the current study due to its ability to inhibit a large variety of human pathogenic oral bacteria, including S. mutans, S. sobrinus and P. gingivalis12,13,14. However, L. rhamnosus GG are not well-adapted for long-term persistence in the oral cavity. This problem may overcome by combining L. rhamnosus GG with L arginine, forming a synbiotic to improve the survival and adherence of L. rhamnosus GG. Recently, a study had shown that L. rhamnosus LRB a probiotic strain isolated from the oral cavity, similar to L. rhamnosus GG demonstrated a strong antimicrobial activity against pathogenic S. mutans and several oral streptococci15. Future studies can be directed to evaluate the synbiotics potential of arginine and L. rhamnosus LRB, as the strain is closely genetically related to L. rhamnosus GG with an intra-oral origin.
The preferred medium for culture of L. rhamnosus GG is MRS broth; while they only survive in BHI broth for short period of time. However, in this study, the growth of L. rhamnosus GG in BHI broth was significantly enhanced by the presence of L-arginine, with the highest percentage of survival of L. rhamnosus GG observed with 2% L-arginine. Due to the presence of the prebiotic arginine, L. rhamnosus GG acquire higher tolerance to the environmental conditions.
The effect of L-arginine and L. rhamnosus GG synbiotic on S. mutans biofilms was further assessed by evaluating the colony-forming unit counts of biofilm and biofilm biomass. The S. mutans viable counts and proportional biofilm biomass were significantly reduced in the 2% L-arginine + L. rhamnosus GG group. The results were mainly attributed to the diminished virulence of S. mutans as a result of the synergistic inhibitory effect of the synbiotic on both the growth and viable counts of S. mutans14,16,17.
L-arginine is known to destabilize human oral biofilms, affecting the adhesion properties of S. mutans18,19. Conversely, a number of clinical trials have shown significantly lower levels of S. mutans and caries incidence after consumption of the probiotic L. rhamnosus GG20,21,22. The inhibitory capacity of L. rhamnosus GG is still unclear, but has been associated with coaggregation of S. mutans23 preventing their adherence to the tooth surfaces, bactericidal effects on S. mutans23, reduced production of insoluble extracellular polysaccharides in biofilm formation24 and reduction of salivary counts of S. mutans25.
The real-time PCR results identified that the combined 2% L-arginine + L. rhamnosus GG synbiotic significantly inhibited the growth of S. mutans; while no significant difference in the proportion of S. mutans and total bacterial concentration were observed between the 2% L-arginine + L. rhamnosus GG synbiotic and the control groups. The reasons could be of two-folds: either the qPCR had amplified DNA from both the live and dead bacteria or the growth of bacterial cells embedded deep within the biofilm matrix had been inhibited due to matrix pressure without affecting its virulence in the control group26.
The pH of the spent media for L. rhamnosus GG was the highest and remained unchanged immediate and 24 h post treatment with 2% L-arginine. The alkaline pH environment provided by 2% L-arginine could have modulated the metabolic activities of L. rhamnosus GG and augmented its growth27. Further, L. rhamnosus GG has limited potential to synthesize certain amino acids (including arginine) for its growth and thus, requires exogenous supplementation of the amino acids for substantial growth and metabolic activity28,29. Thus, the enhanced growth of L. rhamnosus GG in the presence of L-arginine (at 2% concentration) is due to the active utilization of amino acid biosynthesis pathway of L. rhamnosus GG which thereby improves bacterial metabolism and growth30.
The ADS activity remained undetermined for all the groups, suggesting that L. rhamnosus GG could not be recognized as an arginolytic bacteria as opposed to a recently discovered novel Streptococcus strain A12, which demonstrated ADS activity at high levels, moderating plaque pH as well as interfering with the growth and virulence of caries pathogens, S. mutans. The combination of an arginine with ADS-positive probiotics into synbiotics may constitute a nonpharmaceutical advancement in caries prevention11,31. The pH of the spent media for S. mutans was the highest for 2% L-arginine and L. rhamnosus GG group and remained constant over time, suggesting a shift in dental plaque ecology by the synbiotic. The change in microbial ecology is also supported by lactic acid measurements, which was significantly reduced in the 2% L-arginine + L.rhamnosus GG group, indicating that the virulence of S. mutans had been affected with a general reduction in the acidogenicity of the oral microbiota. Lactic acid production by bacteria might lead to extracellular acidic pH shift, but L-arginine, either due to its inherent potential or the ammonia production through arginolytic oral commensals buffer the acidic pH. Conversely, Lin et al.32 examined the effect of probiotics on the pH of a dental biofilm following a sugar rinse among high caries-risk children aged between 7-11 years reported an increase in the pH of the biofilm and a reduction in the recovery time of pH with a short-term probiotics intake.
The ab initio contemplated model on the mechanism of actions of the synbiotics and the beneficial effects associated with the synbiotic administration are presented in Fig. 4. We propose that the L-arginine molecules deliver its ecological-based caries-preventive treatment through two pathways. The first pathway is the prebiotic effect on the oral arginolytic commensals – S. sanguinis and S. gordonii, whereby arginine augments the growth of the arginolytic bacteria and affect the oral biofilm ecology33,34,35. In the prebiotic pathway, L-arginine will be metabolized by arginolytic commensals into ammonia and ATP through the ADS operon36,37. The health-associated commensals utilize the ATP for their survival, help in maintaining homeostasis and creating a non-conducive environment for cariogenic S. mutans38. Additionally, the probiotic L.rhamnosus GG utilizes L-arginine, creating a stable environment conducive for its growth. Thus, the probiotic pathway supplements the brief availability of L. rhamnosus GG in the oral cavity, thereby augmenting its sustenance8,9.
The increased sustainability of the probiotic bacteriotherapy will enhance the pathogenic colonization resistance and immune modulation potential16. The synbiotic therapy through its synergistic mechanism will further assist in reducing the pathogenic biofilm formation39. Thus, the synbiotic therapy of L-arginine combined L. rhamnosus GG potentially enhances a metabolic interactive organization in oral biofilms, that could physiologically generate microbial colonies with increased homeostasis40. Overall, the synbiotic therapy will serve as an oral biofilm modifier and can be an effective anti-caries treatment, especially for high caries-risk patients that addresses the much-needed ecological-based caries preventive approach3. Such synbiotics can be incorporated into mouth rinses or dentifrice for daily use. The twice daily use of the synbiotics might help maintain ecological homeostasis in patients after meal consumption, whereby the low plaque pH and its detrimental cariogenic effects can be prevented. Apart from anti-caries benefits, this combination might have some potential systemic benefits, as both L-arginine and the prebiotic L. rhamnosus GG have demonstrated systemic benefits individually.
This study showed that synbiotics, having both prebiotics and probiotics properties, are more effective than probiotics alone in antagonizing the cariogenic bacterium S. mutans. However, the results should be viewed with caution, as the study uses a mono-species biofilm in a closed microbial (microtiter biofilm) model as compared to the open model that undertakes a continuous culture system with pulsed treatment. Future studies should investigate the effects of synbiotics, alone and in combination with fluorides, on polymicrobial models, to provide conclusive recommendations for clinical use.
Methods
Culturelle (i-Health, Inc., Denmark) – a probiotic dietary supplement was routinely cultured at 37 °C for 72 h under anaerobic conditions (85% N2, 10% H2, 5% CO2) to obtain L. rhamnosus GG in BHI broth (pH-7). The cells were then adjusted to a concentration of 107 cells/ml in BHI broth (pH-7) with concentration estimated using UV-Vis Spectrophotometer (Beckman Coulter, CA, USA) at 660 nm. The strain was matched to an in-house L. rhamnosus GG ATCC 53103 using real-time quantitative polymerase chain reaction (qPCR) and was found to conform to the reference strain. S. mutans UA159 (ATCC 700610) was similarly cultured as L. rhamnosus GG and adjusted to a concentration of 107 cells/ml in BHI broth. The L-arginine treatments were disposed at concentrations −0.5 w/v %, 1 w/v %, and 2 w/v % in 0.9% NaCl; whereby 0.9% NaCl was treated as control. For all experiments, prior to biofilm analysis, the wells were washed with PBS twice.
For the first experiment, L. rhamnosus GG cells were cultured in a 96-well microplate to receive the 1st L-arginine treatment. The second treatment of test solutions were added after 4 h during the 24 h incubation period in an anaerobic chamber (85% N2, 10% H2, 5% CO2). After 24 h, the spent media was removed to analyze biofilm using MTT and XTT assays for probiotic cell viability, ADS activity, and confocal laser scanning microscopy (CLSM).
For the second experiment, S. mutans cells were primarily cultured in a 96-well microplate. The treatment in this section, comprised of L. rhamnosus GG and pre-determined concentrations of L-arginine combined at 2-different segments 4 h apart each. The treated wells were incubated in an anaerobic chamber (85% N2, 10% H2, 5% CO2) for 24 h. After the incubation period, the biofilms were analyzed by counting colony forming units for S. mutans on spiral plated blood agar plates, biofilm biomass determination using crystal violet (CV) assay, and real-time qPCR to determine proportional bacterial cells for S. mutans (a priori to test the hypothesis) and L. rhamnosus GG with total bacterial concentration in the biofilm.
For both sets of experiments, pH of the spent media was measured and the amount of lactic acid generated in the media was quantified.
Probiotic cell viability
The probiotic L. rhamnosus GG cell viability was assessed using MTT and XTT assays after 24 h incubation as per a previous study41. For the probiotic cell viability assay, the spent media was removed followed by biofilm wash with PBS.
MTT reagent was dissolved in PBS and dispensed on L. rhamnosus GG biofilm. The reagent with biofilm was incubated for 4 h at 37 °C. After 4 h, MTT solvent – dimethyl sulfoxide (DMSO) was added and incubated for 20 min on an orbital shaker at room temperature. The viability was analyzed with a microplate reader at 590 nm.
The reagent was prepared by combining XTT Developer Reagent and Electron Mediator Solution with a prior thawing of all components at room temperature. The prepared agent was further introduced to the biofilms and mixed for 5 min on an orbital shaker at room temperature. Further, the biofilms treated with XTT reagent were incubated at 37 °C in a CO2 incubator for 4 h. Prior to reading the plate, the reduced XTT dye was allowed to mix on an orbital shaker for 5 min for homogenization. Following which the mix was removed in an Eppendorf tube to centrifuge at 10,000 rpm for 15 min. The supernatant was dispensed in a microplate to measure absorbance at 450 nm41. All obtained OD values for MTT and XTT assay were normalized to the respective controls for further analysis.
Confocal laser scanning microscopy
Qualitative assessment of L. rhamnosus GG biofilm grown with twice-daily treated L-arginine was evaluated using CLSM. For the purpose of this experiment, the biofilms were grown on hydroxyapatite (HA) discs (5 mm × 2 mm; Clarkson Chromatography Products, Inc., USA) instead of microplate which were dip-washed in PBS prior to staining with LIVE/DEAD BacLight (SYTO 9/propidium iodide) Kit (Invitrogen detection technologies, USA) for 30 min at room temperature. The biofilms were scanned with two-photon laser scanning microscope (Olympus, FLUOVIEW FV1000, USA) at excitation/emission wavelengths for SYTO 9 – 480/500 nm and Propidium Iodide – 490/635 nm. The biofilms were scanned at 3 randomly selected view fields at 100-x magnification. Similarly, to examine the effect of combined L-arginine (at concentrations – 0.5%, 1%, and 2%, respectively) with L. rhamnosus GG on S. mutans biofilm, the bacterial cells were inoculated on HA discs and the biofilms were eventually stained to scan random 3-view fields at 100-x with CLSM.
Colony forming units
The total number of S. mutans viable cells in the second experiment was determined by counting CFU on spiral plated (Autoplate 4000, Spiral Biotech Inc., MA, USA) biofilm suspension in BHI broth on Horse Blood Agar (HBA). The HBA plates with biofilm suspensions were incubated for 72 h anaerobically prior to CFU counting. The CFU counting for each suspension was determined based on the diluted suspensions approximated and grown on the agar plates identified in the feasibility study phases of the experimental protocol.
Biofilm biomass
Biofilm biomass was determined by crystal violet (CV) staining assay as per previous studies42,43. The PBS washed biofilms from the second experiment were fixed with methanol for 5 min which was eventually allowed to dry for 15 min. The fixed biofilms were then stained with 0.1% CV stain for 15 min. After staining, the biofilms were washed with PBS thrice and dried for 2 h until complete evaporation of moisture. The dye was then extracted with 95% ethanol and absolute acetone for 5 min. The solution was allowed to homogenize prior to reading with microplate for absorbance at 570 nm. The data was normalized to the control.
Arginine deiminase system (ADS) activity
ADS activity (as µmol of ammonia) was measured by quantification of ammonia generated from the incubation of biofilm with Nessler’s reagent at 37 °C as per a previous study44. The ammonia produced was detected by Nessler’s reagent (Sigma-Aldrich, St. Louis, USA) using 8-point reference of ammonium sulfate (10 mM high-point reference) subjected to a standard curve (R2 = 0.999). Each sample was assayed in triplicate. ADS activity determined was recorded as µmol of ammonia liberated in the biofilm.
pH measurement
The pH of spent media (BHI) was determined using pH electrode (CyberScan pH 500, Thermo Scientific, USA) calibrated to standard solutions (4.01, 7.00, 10.01). For the purpose of pH evaluation, respective inoculums were introduced in a sterile container to receive the electrode for assessment. The pH was determined at two time-points – post intervention treatment and after 24 h incubation in the anaerobic chamber (85% N2, 10% H2, 5% CO2). Prior to each measurement, the electrode was first rinsed with DI water and then thoroughly disinfected with 70% v/v. ethanol. The instrument was re-calibrated at intervals preferably between group measurements to achieve a stand-alone optimum electrode standardization for pH measurements.
Lactic acid determination
Lactic acid (in g/L) generated post 24 h incubation in anaerobic chamber was determined using a spectrophotometric method as per a previous study45. The spent media was centrifuged at 10,000 g for 5 min. The supernatant was drawn as a test solution to determine lactic acid. Briefly, the supernatant was added to 0.2% FeCl3 solution and vortexed for 60 s. The absorbance of the working solution was measured at 390 nm against a series of reference solutions derived standard curve (R2 = 0.999). The lactic acid concentration was calculated based on the standard curve to obtain measurements in g/L.
Quantitative polymerase chain reaction (qPCR)
The molecular based qPCR determination of total bacterial cells was primarily used to examine the S. mutans and then proportional S. mutans and L. rhamnosus GG cells in the second experiment that highlights the effect of the proposed therapy on cariogenic S. mutans, considering it as a dual-species biofilm. The DNA quantification (in CFU/ml) was done as per our previous study7.
Biofilms were transferred to PBS in an Eppendorf tube which were further centrifuged at 14,000 rpm for 10 min. Following which the supernatant was discarded and the bacterial pellet was suspended in 20 mM Tris-HCl, pH: 8.0; 2 mM EDTA; 1.2% Triton and cells lysed with 20 mg/ml lysozyme incubated in a water bath at 37 °C overnight. The genomic isolation of DNA was performed using QIAamp DNA Isolation kit (Qiagen, Hilden, Germany) following manufacturer instructions. S. mutans ATCC 700610 and L. rhamnosus GG ATCC 53103 isolated DNA’s served as positive controls for the reaction.
The oligonucleotide primers used in the present study for S. mutans and L. rhamnosus GG were F: 5′-GCCTACAGCTCAGAGATGCTATTCT-3′; R: 5′-GCCATACACCACTCATGAATTGA-3′ and F: 5′-CAGAAATCAAAGAAGACAAACTCGTTA-3′; R: 5′-CCATGTAAACGGACAATGGGAGT-3′14; respectively. Similarly, the Taqman probes (Applied Biosystems, USA) for S. mutans and L. rhamnosus GG used in the present study were 5′-FAM-TGGAAATGACGGTCGCCGTTATGAA-TAMRA-3′ and 5′-6FAM-CGGATTTCCAAAGCAATTCTTAACGATGAAAATG-TAMRA-3′14; respectively. The probes and primers were mixed with Taqman universal PCR mix and isolated DNA for the reaction.
The cycle condition for the reaction was set as follows: 50 °C/2 mins; 95 °C/10 mins; 50 cycles of 95 °C/15 sec and 58 °C/1 min. The standard curve was obtained from the known concentrations of isolated DNA from the respective positive controls whereas the negative control was sterilized Milli-Q water instead of isolated DNA from the samples/controls. The reaction was performed using Step One Plus (Applied Biosystems, USA) subjected to description of appropriate controls and reaction initiated in a microAMP optical PCR reaction plate for the system.
Statistical analysis
All experiments were performed at least in triplicate. The statistical analysis for the parametric data was performed using 1-way ANOVA followed by SNK test; whereas for non-parametric data the analysis was done using Kruskal-Wallis test with Dunn-Bonferroni’s multiple comparison test. Post-treatment and 24 h post-treatment pH measurements were compared with paired sample t-test. For both data categories, the statistical significance was set at p < 0.05.
Conclusion
This study highlights that L-arginine enhances the growth of Lactobacillus rhamnosus GG. The combined 2% L-arginine and Lactobacillus rhamnosus GG synbiotic synergistically inhibits the growth of Streptococcus mutans and has significant potential to develop as an anti-caries regimen.
References
Kassebaum, N. J. et al. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 96, 380–387 (2017).
Dang, M. H., Jung, J. E., Lee, D. W., Song, K. Y. & Jeon, J. G. Recovery of Acid Production in Streptococcus mutans Biofilms after Short-Term Fluoride Treatment. Caries Res. 50, 363–371 (2016).
Philip, N., Suneja, B. & Walsh, L. J. Ecological Approaches to Dental Caries Prevention: Paradigm Shift or Shibboleth? Caries Res. 52, 153–165 (2018).
Markowial, P. & Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 9, 1021 (2017).
Zheng, X. et al. Combinatorial effects of arginine and fluoride on oral bacteria. J. Dent. Res. 94, 344–53 (2015).
Bijle, M. N., Ekambaram, M., Lo, E. C. & Yiu, C. K. Y. The combined enamel remineralization potential of arginine and fluoride toothpaste. J. Dent. 76, 75–82 (2018).
Bijle, M. N. A., Ekambaram, M., Lo, E. C. M. & Yiu, C. K. Y. The combined antimicrobial effect of arginine and fluoride toothpaste. Sci. Rep 9, 8405 (2019).
Seminario-Amez, M., López-López, J., Estrugo-Devesa, A., Ayuso-Montero, R. & Jané-Salas, E. Probiotics and oral health: A systematic review. Med. Oral Patol. Oral Cir. Bucal 22, e282–e288 (2017).
Twetman, S. & Keller, M. K. Probiotics for caries prevention and control. Adv. Dent. Res 24, 98–102 (2012).
Kojima, Y., Ohshima, T., Seneviratne, C. J. & Maeda, N. Combining prebiotics and probiotics to develop novel synbiotics that suppress oral pathogens. J. Oral Biosci 58, 27–32 (2015).
Zaura, E. & Twetman, S. Critical Appraisal of Oral Pre- and Probiotics for Caries Prevention and Care. Caries Res., https://doi.org/10.1159/000499037 (2019).
Kõll, P. et al. Characterization of oral lactobacilli as potential probiotics for oral health. Oral Microbiol. Immunol. 23, 139–147 (2008).
Meurman, J. H., Antila, H., Korhonen, A. & Salminen, S. Effect of Lactobacillus rhamnosus strain GG (ATCC 53103) on the growth of Streptococcus sobrinus in vitro. Eur. J. Oral Sci. 103, 253–258 (1995).
Pham, L. C. et al. Effects of Lactobacillus rhamnosus GG on saliva-derived microcosms. Arch. Oral Biol. 56, 136–147 (2011).
Biswas, S., Turner, L. & Biswas, I. Lactobacillus rhamnosus LRB mediated inhibition of oral streptococci. Mol. Oral Microbiol 33, 396–405 (2018).
Caglar, E., Kargul, B. & Tanboga, I. Bacteriotherapy and probiotics’ role on oral health. Oral Dis. 11, 131–137 (2005).
Chakraborty, B. & Burne, R. A. Effects of Arginine on Streptococcus mutans Growth, Virulence Gene Expression, and Stress Tolerance. Appl. Environ. Microbiol. 83, e00496–17 (2017).
Sharma, S. et al. Nanoscale characterization of effect of L-arginine on Streptococcus mutans biofilm adhesion by atomic force microscopy. Microbiology 160, 1466–1473 (2014).
Kolderman, E. et al. L-arginine destabilizes oral multi-species biofilm communities developed in human saliva. Plos One 10, 1–18 (2015).
Näse, L. et al. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on. Caries Res. 35, 412–420 (2001).
Stecksén-Blicks, C., Sjöström, I. & Twetman, S. Effect of long-term consumption of milk supplemented with probiotic lactobacilli and fluoride on dental caries and general health in preschool children: A cluster-randomized study. Caries Res. 43, 374–381 (2009).
Juneja, A. & Kakade, A. Evaluating the Effect of Probiotic Containing Milk on Salivary mutans streptococci Levels. J. Clin. Pediatr. Dent. 37, 9–14 (2015).
Keller, M. K., Hasslöf, P., Stecksén-Blicks, C. & Twetman, S. Co-aggregation and growth inhibition of probiotic lactobacilli and clinical isolates of mutans streptococci: An in vitro study. Acta Odontol. Scand. 69, 263–268 (2011).
Lodi, C. S. et al. Evaluation of fermented milk containing probiotic on dental enamel and biofilm: In situ study. Arch. Oral Biol. 55, 29–33 (2010).
Çaglar, E., Cildir, S. K., Ergeneli, S., Sandalli, N. & Twetman, S. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol. Scand. 64, 314–318 (2006).
Worthington, R. J., Richards, J. J. & Melander, C. Small molecule control of bacterial biofilms. Org. Biomol. Chem. 10, 7457–7474 (2012).
Dobrea, A. A. et al. The Influence of pH on The Growth of Some Lactobacillus Strains with Different Origins. Bull. UASVM Vet. Med. 71, 270–271 (2014).
Laakso, K. et al. Growth phase-associated changes in the proteome and transcriptome of Lactobacillus rhamnosus GG in industrial-type whey medium. Microb. Biotechnol 4, 746–766 (2011).
Kankainen, M. et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl. Acad. Sci. USA 106, 17193–17198 (2009).
Ceapa, C. et al. The variable regions of lactobacillus rhamnosus genomes reveal the dynamic evolution of metabolic and host-adaptation repertoires. Genome Biol. Evol 8, 1889–1905 (2016).
Huang, X. et al. A Highly Arginolytic Streptococcus Species That Potently Antagonizes Streptococcus mutans. Appl. Environ. Microbiol. 82, 2187–2201 (2016).
Lin, Y. T. J., Chou, C. C. & Hsu, C. Y. S. Effects of Lactobacillus casei Shirota intake on caries risk in children. J. Dent. Sci. 12, 179–184 (2017).
Nascimento, M. M. Potential Uses of Arginine in Dentistry. Adv. Dent. Res 29, 98–103 (2018).
Zheng, X. et al. Ecological Effect of Arginine on Oral Microbiota. Sci. Rep 7, 1–10 (2017).
Ledder, R., Mistry, H., Sreenivasan, P. K., Humphreys, G. & McBain, A. J. Arginine Exposure Decreases Acidogenesis in Long-Term Oral Biofilm Microcosms. mSphere 2, e00295–17 (2017).
Velsko, I. M., Chakraborty, B., Nascimento, M. M., Burne, R. A. & Richards, V. P. Species Designations Belie Phenotypic and Genotypic Heterogeneity in Oral Streptococci. mSystems 3, 1–14 (2018).
Burne, R. A. & Marquis, R. E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 193, 1–6 (2000).
Huang, X., Exterkate, R. A. & Ten Cate, J. M. Factors associated with alkali production from arginine in dental biofilms. J. Dent. Res. 91, 1130–1134 (2012).
Saha, S., Tomaro-Duchesneau, C., Tabrizian, M. & Prakash, S. Probiotics as oral health biotherapeutics. Expert Opin. Biol. Ther. 12, 1207–1220 (2012).
Marsh, P. D. & Bradshaw, D. J. Physiological approaches to the control of oral biofilms. Adv Dent Res 11, 176–185 (1997).
Stockert, J. C., Horobin, R. W., Colombo, L. L. & Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 120, 159–167 (2018).
Stepanovic, S., Vukovic, D., Dakic, I., Savic, B. & Svabic-Vlahovic, M. A modified microtiter - plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40, 175–179 (2000).
Merritt, J. H., Kadouri, D. E. & O’Toole, G. A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 01, Unit 1B.1 (2005).
Reyes, E. et al. Caries-free subjects have high levels of urease and arginine deiminase activity. J. Appl. Oral Sci 22, 235–240 (2014).
Borshchevskaya, L. N., Gordeeva, T. L., Kalinina, A. N. & Sineokii, S. P. Spectrophotometric determination of lactic acid. J. Anal. Chem 71, 755–758 (2016).
Acknowledgements
This research project was supported Research Grants Council, Hong Kong (Grant No: 17118519). The funders have no role in this study.
Author information
Authors and Affiliations
Contributions
Mohammed Nadeem Bijle - Contributed to the conception, design, data acquisition and interpretation, performed all statistical analyses, drafted and critically revised all drafts of the manuscript. Prasanna Neelakantan - Co-contributed to the conception, design and critically revised the manuscript. Manikandan Ekambaram - Co-contributed to the conception, design and critically revised the manuscript. Edward C.M. Lo – Co-contributed to the conception and critically revised the manuscript. Cynthia Kar Yung Yiu - Co-contributed to conception, design, analysis and critically revised all the drafts of the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bijle, M.N., Neelakantan, P., Ekambaram, M. et al. Effect of a novel synbiotic on Streptococcus mutans. Sci Rep 10, 7951 (2020). https://doi.org/10.1038/s41598-020-64956-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64956-8
This article is cited by
-
How probiotics, prebiotics, synbiotics, and postbiotics prevent dental caries: an oral microbiota perspective
npj Biofilms and Microbiomes (2024)
-
Synbiotics as potent functional food: recent updates on therapeutic potential and mechanistic insight
Journal of Food Science and Technology (2024)
-
Antimicrobial activity of Limosilactobacillus fermentum strains isolated from the human oral cavity against Streptococcus mutans
Scientific Reports (2023)
-
The Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Oral Cancer Management
Probiotics and Antimicrobial Proteins (2023)
-
Antibacterial property of a gradient Cu-bearing titanium alloy by laser additive manufacturing
Rare Metals (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.