Abstract
Valuable female cattle are continuously subject to follicular puncture (ovum pick-up - OPU). This technique is commonly used for in-vitro embryo production, but may result in ovarian lesion. Mesenchymal stem cells (MSC) ameliorate the function of injured tissues, but their use to treat ovarian lesions in cattle has not been established. We investigated whether a local injection of MSC would reduce the negative effects of repeated OPU under acute and chronic scenarios in bovines. First, we performed four OPU sessions and injected 2.5 × 106 MSCs immediately after the 4th OPU procedure (n = 5). The treated organs (right ovary) were compared to their saline-treated counterparts (left), and presented superior production of oocytes and embryos in the three following OPU sessions (P < 0.05). Then, cows with progressive fertility loss went through three OPU sessions. Animals received MSC, saline, or MSC + FSH in both ovaries after the first OPU. In the two following OPU sessions, the MSC and MSC + FSH - treated groups failed to present any significant alteration in the number of oocytes and embryos compared to saline-treated animals. Thus, MSC have beneficial effects on the fertility of OPU-lesioned cows, but not in cows with cystic ovarian disease and chronic ovarian lesions.
Similar content being viewed by others
Introduction
The use of in vitro embryo technologies has grown worldwide over the past decades and, according to the International Embryo Technology Society (IETS), in 2017 more bovine embryos were produced in vitro than generated in vivo1. The development of transvaginal ultrasound-guided follicle aspiration (a.k.a. ovum pick-up or OPU) and subsequent adaptation for use in cattle2 was a key step for the development of in vitro embryo production (IVEP) procedures. This technique allowed the repeated recovery of cumulus-oocyte complexes (COC) from live donors, and genetically superior donors could be used for large-scale embryo production, boosting animal breeding programs3. The association of OPU and IVEP was successfully used to produce offspring from donors that failed to respond to exogenous FSH treatment4, pregnant cows5, prepubertal heifers, and young calves5,6. Because of its advantages, over 95% of all embryos produced in vitro are derived from COC recovered by OPU1.
When compared to the previous alternatives to recover COC from live animals, such as laparotomy or laparoscopy, OPU is less traumatic7, and can be performed repeatedly in the same donor7,8. Thus, this technique is generally considered a safe way to recover COC from cattle7,8,9. However, as with any other needle-based biopsy system, OPU inevitably causes trauma to the ovarian tissue. Ovarian damage subsequent to OPU has been largely neglected, partially due to the internal (non-apparent) nature of the lesions, and also the high regenerative capacity of the ovary, an organ where tissue remodeling occurs naturally over the cycles. Few studies have evaluated ovarian damage caused by OPU, and these reported abnormalities such as tunica albuginea thickening, inflammation, stromal fibrosis, and adhesions10,11,12. Repeated OPU procedures have also been associated with endocrine abnormalities including increased plasma FSH/LH concentrations, follicle growth rate, and incidence of codominance13, and it is also a risk factor for the development of cystic ovarian disease14.
The cumulative damage caused by successive OPU procedures is associated with a progressive decrease in COC yield and embryo production in donors undergoing repeated collections over several years. The decreased rate, however, is highly variable among donors because it depends on a range of factors including the skill of the technician, type and caliber of the needle used, and aspiration interval. Additionally, there is a significant variation in antral follicle count (AFC) among individuals, as well as among cattle breeds7,8. In general, Bos indicus breeds have a greater AFC compared with Bos taurus, and thus yield more oocytes15. OPU sessions yielding over 100 oocytes are not unusual and a record of 564 oocytes collected in a single OPU session from a Nelore donor has been reported16. Concomitantly, an OPU-related decrease in the number of COC retrieved was observed in Bos indicus, but not in Bos taurus and, within the same breed, for cows with high AFC, but not for those with low AFC. Although there are no clear statistics in this regard, field practitioners also report an increasing number of donors, some of them high-value animals, which have compromised reproductive function and failed to produce viable oocytes following repeated OPU cycles (Viana et al. personal communication17).
Treatment with mesenchymal stem cells (MSC) has been shown to be a valuable therapy in a number of conditions associated with acute and chronic inflammatory processes18,19,20,21. Stem cells produce a number of cytokines and other soluble mediators that have the ability to modulate the inflammatory and immune activation processes, and potentially minimize inflammation-associated tissue damage22,23,24. In donors undergoing repeated OPU, MSC therapy could be an effective strategy to reduce the detrimental effects of inflammation and subsequent fibrosis on ovarian function. Stem-cell therapy has been shown to improve ovarian function in laboratory animal models in which infertility was artificially induced25,26,27,28,29. However, the clinical use in large farm animals is still under discussion30,31. The present study was designed to evaluate the effect of intraovarian MSC treatment on oocyte yield and embryo production. We hypothesized that MSC treatment would reduce the negative effects of repeated OPU on donor performance, by improving oocyte quantity and/or quality. In this regard, we designed two experiments, aiming to evaluate the effects of intraovarian treatment with MSC in ovaries under acute and chronic injury processes due to follicular puncture.
Results
MSC treatment promotes higher production of oocytes, embryos and expanded blastocysts in acute OPU-induced ovarian lesions
The animals presented no difference (P > 0.05) in any of the analyzed parameters between the ovaries (right versus left) before MSC treatment (OPU sessions 1 to 4). Thus, it is possible to attribute any differences between treated (right) and untreated (left) ovaries in OPU sessions 5 to 8, to MSC infusion. Treated ovaries presented more total (P < 0.02) and viable oocytes (P < 0.01), when compared to the untreated ovaries, resulting in more embryos produced in vitro (P < 0.01), as well as superior production of early and expanded blastocysts (Fig. 1, Table 1).
Effect of intraovarian injection of mesenchymal stem cells (MSC) on total OPU/IVEP acute outcomes. Treatments were performed immediately after the 4th OPU/IVEP session. The right ovary received MSC treatment, while the left ovary remained as a control and received DMPBS (n = 5 per group). Endpoints are shown according to the ovary, before (sessions 1 to 4) and after (sessions 5 to 8) treatment. *P < 0.05.
OPU session did not present any effect on the number of total or viable oocytes recovered, nor on the number of total or expanded blastocysts produced in any of the experimental groups (Fig. 2A to D). The same lack of effect was observed for the ovary x OPU session interaction. The percentage of viable oocytes recovered was higher in treated ovaries compared to the untreated counterparts (89.1% vs 81.5%, P < 0.05). However, blastocyst rates did not differ between treated and untreated ovaries before or after treatment (50.4% vs 55.5%, P > 0.05).
Effect of intraovarian injection of mesenchymal stem cells (MSC) on OPU/IVEP acute outcomes per session. Treatments were performed immediately after the 4th OPU/IVEP session. The right ovary received MSC treatment, while the left ovary remained as a control and received DMPBS. Endpoints are shown as mean ± SEM (n = 5 per group) according to the ovary, before (sessions 1 to 4) and after (sessions 5 to 8) treatment. (A) total number of oocytes collected; (B) number of viable COC; (C) total number of embryos produced; (D) number of expanded blastocysts produced.
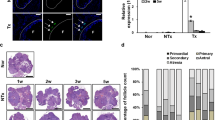
There was no difference (P > 0.05) in the abundance of transcripts of KTR8, PLAC8, SLC2A1, CASP3, PROX3, or SOD2 in the embryos produced from treated and untreated ovaries, or from slaughterhouse ovaries. However, SLC2A3 was overexpressed (P = 0.04) after MSC treatment, compared with controls (Fig. 3).
Gene expression patterns in blastocysts produced in vitro from oocytes recovered after intraovarian treatment with MSC (right ovary) or from untreated ovaries (left ovary) after acute lesion. Gene expression in embryos from slaughterhouse ovaries was used as a reference (expression value = 0). Graphs show the relative abundance of mRNA of CASP3 (A), KRT8 (B), PLAC8 (C), PRDX3 (D), SLC2A1 (E), SLC2A3 (F), and SOD2 (G) genes determined by qPCR. Data were normalized by ΔΔCt, using Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-Actin (ACTB) as endogenous controls. * Indicates statistical difference (P < 0.05).
MSC treatment doesn’t significantly alter the production of oocytes, embryos and expanded blastocysts in chronic OPU-induced lesion ovaries
Active immunization against GnRH was effective to reduce the number of large follicles present in the ovaries (from 2.6 ± 0.3 to 0.2 ± 0.1, P < 0.05 - Data not shown). Only one cow still presented a large ovarian cyst (33 mm diameter) and was removed from the experiment. In the remaining 18 cows, the average AFC after immunization was 11.2 ± 0.8 (range 3 to 19), and there was no difference among experimental groups (11.2 ± 1.4, 11.1 ± 2.0, 11.4 ± 1.3 for Control group, MSC and MSC + FSH, respectively, P > 0.05), as shown in Fig. 4, Figure S1 and Table 2. One animal from the control group died before OPU session 3.
Ovarian and OPU outcomes in Gir cows with low IVEP records associated with their use as oocyte donors for long periods (chronic lesion). Cows received DMPBS (control group), mesenchymal stem cells (MSC group), or mesenchymal stem cells followed by FSH priming (MSC + FSH group). There was no statistical difference among groups (P > 0.05).
The OPU outcomes are shown in Fig. 4(A–D), Figure S1 and Table 2. Despite individual variation in response to the MSC and MSC + FSH treatments, there was no difference (P > 0.05) among groups in AFC, number of follicles aspirated, retrieved oocytes, or viable oocytes. Due to the low number of viable oocytes, the numbers of cleaved embryos and blastocysts were low and did not differ among groups (Table 2, P > 0.05). Over the same period, blastocyst rates of the IVEP laboratory using the same maturation, fertilization, and culture media and conditions, and with the same breeds (Gir x Holstein), ranged from 16.4 to 27.0%.
Discussion
Mesenchymal stem cells have shown therapeutic efficacy for different purposes, such as immunological disorders32, variable chronic lesions33, and ischemia34. In the context of ovarian physiology, MSC were shown to be beneficial in the treatment of different disorders. Even though to date, most studies have been performed in rodents, MSC have promoted positive effects on chemotherapy-induced lesions, premature ovarian failure, and polycystic ovary syndrome35,36,37,38,39. The positive results can be explained, at least in part, by the effects of MSC on granulosa cells, as well as their anti-inflammatory, antiapoptotic, and regenerative effects, and potential paracrine actions40. Despite the positive results obtained, rodents differ significantly from humans when it comes to the female reproductive system41. In this sense, not only are cattle more similar to humans in the context of female tract physiology, but they also constitute an economically relevant model which may positively impact the economy by presenting longer reproductive life after MSC therapy.
The present study evaluated, for the first time, the use of intraovarian MSC as a treatment to reduce the negative effects of repeated OPU in donor cattle. In Experiment 1 we first tested the MSC in cows that underwent OPU for a short period (four sessions), thus reflecting the acute effects of puncture injuries. Our results demonstrated that the treated ovaries yielded more total and viable oocytes, leading to an increase in embryo production per OPU session. We then tested in Experiment 2 whether the MSC treatment would also bring any benefit to cows with a history of multiple OPU sessions and progressive failure in producing embryos and pregnancies. In spite of a positive variation in AFC and oocyte recovery in the treated cows, there was no significant difference compared with controls.
Stem cells have a positive tropism to any area undergoing an inflammatory process. Thus, to ensure cells would act specifically within the ovary, we used an intraovarian injection, a non-usual treatment route in large animal veterinary practice. A number of studies reported intrafollicular42,43,44, intraluteal45,46, or intraovarian44,47 administration of drugs, but most, if not all, were only for experimental purposes. As the aim of the present study was to test an alternative for the treatment of oocyte donors used for commercial IVEP, a previous study was performed to test the safety of this procedure and validate the amount of MSCs injected (Peixer et al. in press). The experiment was performed by injecting MSC into healthy cow ovaries and no adverse events were registered, confirming the safety of the procedure. In Experiment 1, we performed additional perforations in the ovaries before injection of saline or MSC, to reduce potential differences in inflammation between ovaries from which a greater or smaller number of oocytes was recovered.
An important potential source of variation in our results was the predictable differences in AFC among donors8. To account for this, in Experiment 1 we allocated the right and left ovaries of each animal to treatment and control groups, respectively. The lack of difference in any endpoint between the ovaries before treatment demonstrates that we succeeded in balancing individual variations, and differences after the fifth OPU sessions are likely to be a result of MSC treatment. In our experimental model we cannot ensure that MSC would not migrate from one ovary to the other. However, during migration throughout the circulatory system, these cells could be equally attracted to any other area with inflammation (e.g., other areas perforated during OPU such as the vaginal wall), and the number of cells eventually reaching the contralateral ovary is probably negligible.
In Experiment 2, most donors had varied records of chronic COD and refractoriness to conventional GnRH or P4 treatments, so they were treated with an anti-GnRH vaccine. Previous studies have shown that cows receiving either GnRH immunization or GnRH agonist, despite having suppressed follicle growth, can be used as oocyte donors and produce embryos in vitro14,48. Therefore, we decided to include a group with FSH priming, and thus we could not use the same experimental model as in Experiment 1. To minimize the potential effect of differences in AFC among donors, the cows were balanced-distributed according to AFC into the experimental groups.
In Experiment 1 (animals with acute lesions), the MSC treatment positively affected the number of total and viable oocytes, as well as the proportion of viable oocytes, suggesting a beneficial effect on follicle development. This positive effect could be due to either the increased follicle recruitment or a reduced follicle atresia. In the current study, differences were observed shortly after treatment, suggesting that the MSC had a positive effect on the population of growing antral follicles that were aspirated within a few weeks after treatment, and thus were recruited from the pool of primordial follicles before MSC treatment. This observation is in line with the anti-apoptotic effect and subsequent increase in antral follicle population observed after this type of cell therapy in other species28,29.
The inflammation associated with repeated OPU procedures may cause the presence of high concentrations of inflammatory signals in the ovary. Infections in other organs such as the uterus or mammary gland have also been associated with an increase in fibrotic tissue in the ovary, disturbed folliculogenesis, abnormal intrafollicular environment, altered gene expression in granulosa cells, and reduced oocyte levels of GDF9 and developmental potential49,50,51,52. The presence of bacterial LPS induces the accumulation of inflammatory mediators such as IL-1beta, IL-6, and IL-850,53,54, which are also likely to be increased due to the mechanical damage caused by OPU.
We can infer that the presence of MSC may have negatively modulated the production of inflammation-related cytokines, as observed in mice27, and thus minimized their detrimental effects on growing follicles, reducing atresia and improving oocyte quality. Concordantly, the increase observed in IVEP outcomes was due to the recovery of a greater number of viable COC.
The present data support the hypothesis that differences in oocyte quality may impact embryo development. We are not sure why among the genes evaluated, only SLC2A3 was overexpressed in embryos produced from the treated ovaries, compared with untreated ovaries or slaughterhouse controls. However, this result suggests that metabolism and glucose uptake by the embryo may have been affected by MSC treatment. Glucose is the main energy substrate for the embryo; however, the embryo’s glycolytic ability is low initially, but increases markedly at the blastocyst stage55. A positive correlation was observed between glucose uptake and viability of preimplantation embryos56. Since glucose is hydrophilic, it must be transported into the cell by transporters known as the solute carrier family 2 (SLC2A), among which SLC2A3 is particularly important for embryos. A study in mice reported that when the SLC2A3 gene was deleted, embryo development until the blastocyst stage was not affected, but embryo lethality was observed shortly after implantation57.
MSC treatment was not effective to increase IVEP in Experiment 2. In this case, we can speculate that the chronic inflammatory process in the ovaries due to repeated OPU over the years may have compromised follicular population or ovarian physiology in a way that could no longer be restored by MSC. For instance, repeated OPU causes accumulation of scar tissue in ovarian stroma12, resulting in ovary hardening, as clinically observed11. The changes observed in ovarian stiffness could affect preantral follicle development, as observed when preantral follicles are cultured in alginate hydrogel with different densities58, and result in a progressive reduction in AFC, as observed in the cows enrolled in Experiment 2. In fact, the average AFC values for these cows were about one third of those observed in healthy heifers of the same herd (11.2 ± 0.8 vs. 30.8 ± 1.1, respectively). Moreover, it is likely that there was also an interplay among the chronic ovarian inflammation, endocrine disbalance, and aging, which may have contributed to the very low IVEP outcome of most of these cows.
In summary, the present study presents the first evidence that the intraovarian injection of MSC has beneficial effects on AFC and on IVEP in zebu cows undergoing repeated OPU. However, such effects may be limited depending on how long cows have been used as oocyte donors, reflecting the extent of the damage in the ovarian tissue.
Methods
Unless otherwise indicated, all reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Animals and location
Multiparous, lactating Nelore cows at 60d after parturition and non-lactating Gir oocyte donors (N = 5 and N = 19, respectively, both Bos taurus indicus breeds), were used. These animals were from two commercial farms, one at Flores de Goiás, GO, and the other at Leopoldina, MG, Brazil. The cows were raised on pasture (mostly Brachiaria decumbens), with ad-libitum access to water and to vitamin and mineral supplements throughout the experiment. Nelore cows were selected based on reproductive soundness, inferred by the lack of pathological conditions in the genital tract, as determined by ultrasound scanning and rectal palpation. On the other hand, Gir cows were previously submitted to multiple sessions of OPU and were to be culled due to low performance on IVEP. According to their records, in the last fifteen OPU-IVEP sessions these cows had a drop of 47.8% in the number of viable oocytes recovered and of 77.5% in the number of embryos produced. When first examined for this study, all Gir cows presented chronic cystic ovarian disease (COD), with an average of 2.6 ± 0.3 follicles above the expected diameter of the ovulatory follicle in Gir (circa 12 mm)13, and 9 out of 19 also presented mucometra. These cows were 10.4 ± 0.5 years old when this study was performed.
This study was approved by the Committee for Ethics in the Use of Animals of the Universidade Católica de Brasília (CEUA-UCB, protocol 003/18), and all experiments were performed in accordance with relevant guidelines and regulations.
Experimental design
In experiment 1, we evaluated the effect of intraovarian injection of MSC on oocyte yield, quality, and developmental potential during in vitro embryo production. To account for the expected variability in AFC, all cows (Nelore, N = 5) were treated in one ovary, while the other was used as a control. Each cow underwent eight OPU sessions at 15-day intervals. Immediately after the fourth OPU session, an additional lesion was induced in each ovary by 30 punctures, performed with a 16 G Jelco needle, to ensure that substantial acute injury would be present, regardless of the number of punctures needed for follicle aspiration in each cow. Six hours later, the left ovary received an injection of 500 μL Dulbecco’s Modified Phosphate Buffer Saline (DMPBS) (control ovary), whereas the right ovary received 500 μL DMPBS with 2.5 × 106 allogeneic MSC (treated ovary). In both cases, the final volume was distributed across three points on the ovarian cortex. The results of oocyte yield and embryo production in the four sessions before and in the four sessions after treatment were recorded for each ovary and donor.
In experiment 2, we evaluated the potential benefits of MSC treatment in cows with low IVEP performance associated with long-term effects of OPU. The cows underwent a gynecological exam by B-mode and color Doppler ultrasonography to characterize ovarian status. To control for the potential detrimental effects of COD, cows were treated with two injections of an anti-GnRH vaccine (Bopriva, Zoetis Saúde Animal, São Paulo, Brazil), given 30 days apart, as previously described14. Twenty days later, cows were re-evaluated and were distributed in a balanced manner according to ovarian antral follicle count (AFC) into one of the following experimental groups: a) control group (CG), intraovarian injection of saline; b) intraovarian injection of MSC (treatment in both ovaries); or c) injection of MSC after priming with 150 IU FSH (Pluset, Hertape Calier Saúde Animal, Juatuba, Brazil). All cows underwent three OPU sessions, 20 days apart. MSC were injected in groups designated MSC and MSC + FSH, immediately after OPU session 1, and FSH was injected in the MSC + FSH group 48 h before OPU sessions 2 and 3. Oocyte and embryo yield were recorded for each donor and results compared among groups and OPU sessions.
In both experiments, the endpoints evaluated were the number and quality of the oocytes recovered, and number and rates of in vitro embryo production. In Experiment 1, the gene expression pattern was evaluated in the blastocysts, as described below.
Collection and characterization of mesenchymal stem cells
The method used to isolate, culture, and freeze MSC was previously described (Peixer et al. in press). Briefly, a sample of adipose tissue was recovered at a slaughterhouse from a healthy bull, washed in saline, treated with hyaluronidase, and filtered. The cells isolated were cultured in Dulbecco´s modified Eagle´s media (DMEM), at 37.5 °C and 5% CO2 atmosphere. The medium was renewed at 24 h and non-adherent cells discarded. At 80% confluence, cells were isolated with trypsin, and frozen in liquid N2 in a dimethyl sulfoxide (DMSO) solution with fetal calf serum (FCS) at a final concentration of 1 × 106 cells/mL. Characterization of MSC was carried out according to guideline 131 of the International Society for Cellular Therapy59. Immunophenotyping was performed by flow cytometry and Amnis image quantification using positive (CD29 (rat anti-human), CD44 (rat anti-equine), and CD90 (goat anti-canine)) and negative (CD34 (rat anti-human)) surface markers. The ability of MSC to differentiate into osteoblasts, chondrocytes and adipocytes was also evaluated, as described previously60,61. Samples were also screened for the presence of potential contaminants (bacteria, fungi, and mycoplasma). Cell viability after thawing was evaluated by flow cytometry using annexin-Alexa Fluor 488 and propidium iodide (Thermo-Fisher Scientific, Bremen, Germany).
Transvaginal guided follicular aspiration and MSC injection
Cumulus-oocyte complexes were recovered by OPU, as described elsewhere13. Briefly, all follicles larger than 3 mm were aspirated using a portable ultrasound device (Aloka SSD 500, Aloka Co., Tokyo, Japan; or MyLab 30 Gold, Esaote, Genova, Italy) equipped with a biopsy guide, and disposable 20 G needles (WTA, Cravinhos, Brazil). A vacuum pressure of 80 to 100 mm Hg was used. The follicular fluid was recovered in 50 mL tubes containing 15 mL of DPBS supplemented with 1% FCS and 5 IU/mL sodium heparin (Hemofol, Cristália, São Paulo, Brazil). The recovered COC were classified according to the number of cumulus cell layers and cytoplasm morphology. Only COC classified as viable were transferred to cryotubes (Corning, 1.2 mL, New York, USA) containing maturation medium that consisted of TCM199 (Gibco, New York, USA) supplemented with 0.05 IU/mL FSH (Pluset, Hertape-Calier, Barcelona, Spain), 1 mg/mL estradiol, and 10% FCS, and kept in a portable incubator at 38.5 °C until transportation to the IVEP laboratory. The total number and the grade of oocytes recovered from each ovary (right or left) and from each donor were recorded.
The intraovarian injection of MSC was performed with the same ultrasound device used for OPU. After thawing and washing twice in PBS, the MSC suspension was loaded into a 1 mL syringe, which was connected to a new aspiration line. Ultrasound imaging was used to select three regions of the ovarian cortex free of antral follicles or luteal tissue, and to guide the injection needle. Each region was then injected with approximately 1/3 of the MSC suspension volume.
In vitro embryo production
Mature COC were obtained after a 24 h incubation at 38.5 °C under an atmosphere of 5% CO2 in air, using the same media used for transportation. Expanded COC were then washed and transferred to fertilization media consisting of Tyrode’s albumin lactate pyruvate (TALP) supplemented with 10 μg/mL heparin, 20 μM D-penicillamine, 10 μM hypotaurine, and 1 μM epinephrine. For all fertilization procedures, frozen semen samples from a single bull of proven fertility (Aberdeen Angus or Holstein) were used at 1 × 106 spermatozoa/mL. Oocytes and spermatozoa were co-incubated for 20 h at 38.5 °C in a humidified incubator with 5% CO2. After coincubation (day 0), presumptive zygotes were transferred to 50 µL drops of synthetic oviduct fluid (SOFaa) supplemented with essential and non-essential amino acids, 0.34 mM sodium tricitrate, 2.77 mM myo-inositol, and 10% FBS under mineral oil. The zygotes were cultured for 7 days, when embryo production, developmental stage and quality were evaluated. The embryos were also evaluated on Day 2 (D2) post-insemination for cleavage and on Day 7 (D7) for blastocyst development.
Gene Expression Quantification by Real Time Quantitative PCR (RT-qPCR)
For gene expression analysis, the relative abundance of transcripts for eight target genes involved in embryo quality was quantified by RT-qPCR in D7 in vitro-produced embryos. The selected genes were: placenta-specific 8 (PLAC8), keratin protein 8 (KRT8), heat stress (heat shock 27-kDa protein 1 (HSPB1)); apoptosis cysteine peptidase 3 (CASP3), superoxide dismutase 2 (SOD2), solute carrier family 2 member 1 (SLC2A1), solute carrier family 2 member 3 (SLC2A3) and Peroxiredoxin 3 (PRDX3). Three pools of 29 to 32 embryos for each treatment group were used. Total RNA was isolated using the RNeasy Plus Micro Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Complementary DNA synthesis was performed as described in Leme et al.62. The RT-qPCR reactions were performed using the Fast SYBR Green Master Mix Kit (Applied Biosystems, Foster City, California, USA) in an Applied Biosystem 7500 Fast Real Time PCR System (Applied Biosystem). Each sample was analyzed in triplicates with an amplification efficiency between 90 and 110%, and the specificity of each PCR product was determined by analyzing the melting curve and size of amplicon on an agarose gel. The reactions were performed in a final volume of 25 μL, using cDNA equivalent to 0.8 embryos per reaction. The qPCR conditions were: 95 °C for 5 minutes followed by 50 cycles of denaturation at 95 °C for 10 seconds and then annealing and extension at 60 °C for 30 seconds. The primer names, sequences, fragment sizes and annealing temperatures are listed in Table 3. The average expression level of two constitutive genes, Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-Actin (ACTB), was used for data normalization. The relative quantification of each gene was calculated by the ΔΔCt method with efficiency correction63.
Statistical analysis
First, the Shapiro-Wilk test was used to assess the normality of the data. The data referring to the number of early blastocysts in OPU sessions 1 to 4 and 5 to 8 (experiment 1) were transformed into natural logarithms. The total oocytes recovered in sessions 1 to 4 were transformed into square root. All other endpoints were analyzed using non-transformed data. Analyses were performed to assess the main effects of ovary (treated versus untreated), OPU session, as well as their interactions. In order to test for natural differences between right and left ovaries, the data referring to the OPU sessions performed before (1 to 4) treatment were analyzed separately from the data obtained from OPU sessions performed after MSC treatment (5 to 8). Statistical analysis was also performed in order to compare ovaries before and after treatment. In data analyses for MSC treatment during chronic lesion (Experiment 2), the follicle population, the number of follicles aspirated, retrieved COC, and viable oocytes were normally distributed. The main effects of treatment, OPU session, and their interactions were analyzed. The SAS MIXED procedure with a REPEATED statement was used for both experiments, to account for the autocorrelation between sequential measurements (SAS University Edition; SAS Institute Inc., Cary, NC, USA). Whenever a significant main effect of ovary was detected, the Student’s t test was used to compare differences among means in Experiment 1. Whenever a significant effect of treatment, session or interaction was observed, the Tukey's post hoc test was used to compare differences among means in Experiment 2. Data are presented as mean ± standard error of the mean (SEM) of non-transformed data. In the acute inflammation experiment (Experiment 1), comparisons of gene expression among groups were performed using ANOVA and Tukey’s test or Kruskal-Wallis and Mann-Whitney tests for normally and non-normally distributed data, respectively. These analyses were performed using the software GraphPad Prism 6, and a P-value <0.05 indicated statistical significance.
References
Viana, J. H. M., Figueiredo, A. C. S., Gonçalves, R. L. R. & Siqueira, L. G. B. A historical perspective of embryo-related technologies in South America. Animal Reproduction 15, 963–970 (2018).
Pieterse, M. C., Kappen, K. A., Kruip, T. A. M. & Taverne, M. A. M. Aspiration of bovine oocytes during transvaginal ultrasound scanning of the ovaries. Theriogenology 30, 751–762 (1988).
Granleese, T., Clark, S. A., Swan, A. A. & van der Werf, J. H. J. Increased genetic gains in sheep, beef and dairy breeding programs from using female reproductive technologies combined with optimal contribution selection and genomic breeding values. Genet. Sel. Evol. 47, 70 (2015).
Thibier, M. The zootechnical applications of biotechnology in animal reproduction: current methods and perspectives. Reprod. Nutr. Dev. 45, 235–242 (2005).
Aller, J. F., Mucci, N. C., Kaiser, G. G., Callejas, S. S. & Alberio, R. H. Effect of repeated eCG treatments and ovum pick-up on ovarian response and oocyte recovery during early pregnancy in suckling beef cows. Anim. Reprod. Sci. 133, 10–15 (2012).
Majerus, V. et al. Embryo production by ovum pick up in unstimulated calves before and after puberty. Theriogenology 52, 1169–1179 (1999).
Santl, B. et al. Comparison of ultrasound-guided vs laparoscopic transvaginal ovum pick-up (OPU) in simmental heifers. Theriogenology 50, 89–100 (1998).
Boni, R., Roelofsen, M. W., Pieterse, M., Kogut, J. & Kruip, T. A. Follicular dynamics, repeatability and predictability of follicular recruitment in cows undergoing repeated follicular puncture. Theriogenology 48, 277–289 (1997).
Kruip, T. A., Boni, R., Wurth, Y. A., Roelofsen, M. W. & Pieterse, M. C. Potential use of ovum pick-up for embryo production and breeding in cattle. Theriogenology 42, 675–684 (1994).
Petyim, S., Båge, R., Forsberg, M., Rodríguez-Martínez, H. & Larsson, B. Effects of repeated follicular punctures on ovarian morphology and endocrine parameters in dairy heifers. J. Vet. Med. A Physiol. Pathol. Clin. Med. 48, 449–463 (2001).
Petyim, S., Båge, R., Forsberg, M., Rodríguez-Martínez, H. & Larsson, B. The effect of repeated follicular puncture on ovarian function in dairy heifers. J. Vet. Med. A Physiol. Pathol. Clin. Med. 47, 627–640 (2000).
Viana, J. H. M. et al. Caracterização de seqüelas subseqüentes à punção folicular em bovinos. Pesquisa Veterinária Brasileira 23, 119–124 (2003).
Viana, J. H. M., Palhao, M. P., Siqueira, L. G. B., Fonseca, J. F. & Camargo, L. S. A. Ovarian follicular dynamics, follicle deviation, and oocyte yield in Gyr breed (Bos indicus) cows undergoing repeated ovum pick-up. Theriogenology 73, 966–972 (2010).
Faria, O. A. C. et al. Effects of Active Immunization Against GnRH in Oocyte Donors with Cystic Ovarian Disease. Reproduction, Fertility and Development 30, 190 (2018).
Pontes, J. H. F. et al. Large-scale in vitro embryo production and pregnancy rates from Bos taurus, Bos indicus, and indicus-taurus dairy cows using sexed sperm. Theriogenology 74, 1349–1355 (2010).
Panattoni, J. F. et al. Quality and viability of slaughter house oocytes for ovine embryo production in vitro. Anais do XVII Congresso Brasileiro de Reprodução Animal - Anals of the XVII Brazilian Animal Reproduction Congress. http://www.cbra.org.br/portal/publicacoes/anaisxviicbra/ANAIS%20do%20XVII%20CBRA.pdf.
Gimenes, L. U. et al. The interval between the emergence of pharmacologically synchronized ovarian follicular waves and ovum pickup does not significantly affect in vitro embryo production in Bos indicus, Bos taurus, and Bubalus bubalis. Theriogenology 83, 385–393 (2015).
Lindroos, B., Suuronen, R. & Miettinen, S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev Rep 7, 269–291 (2011).
Rosado, I. R. et al. Immunomodulatory and neuroprotective effect of cryopreserved allogeneic mesenchymal stem cells on spinal cord injury in rats. Genet. Mol. Res. 16, (2017).
Carvalho, J. L. et al. Priming mesenchymal stem cells boosts stem cell therapy to treat myocardial infarction. J. Cell. Mol. Med. 17, 617–625 (2013).
Spejo, A. B., Carvalho, J. L., Goes, A. M. & Oliveira, A. L. R. Neuroprotective effects of mesenchymal stem cells on spinal motoneurons following ventral root axotomy: synapse stability and axonal regeneration. Neuroscience 250, 715–732 (2013).
Caplan, A. I. Why are MSCs therapeutic? New data: new insight. J. Pathol. 217, 318–324 (2009).
de Oliveira Bravo, M., Carvalho, J. L. & Saldanha-Araujo, F. Adenosine production: a common path for mesenchymal stem-cell and regulatory T-cell-mediated immunosuppression. Purinergic Signal. 12, 595–609 (2016).
Haddad, R. & Saldanha-Araujo, F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res. Int. 2014, 216806 (2014).
Takehara, Y. et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab. Invest. 93, 181–193 (2013).
Abd-Allah, S. H. et al. Mechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbits. Cytotherapy 15, 64–75 (2013).
Lai, D., Wang, F., Dong, Z. & Zhang, Q. Skin-Derived Mesenchymal Stem Cells Help Restore Function to Ovaries in a Premature Ovarian Failure Mouse Model. PLoS ONE 9, e98749 (2014).
Elfayomy, A. K., Almasry, S. M., El-Tarhouny, S. A. & Eldomiaty, M. A. Human umbilical cord blood-mesenchymal stem cells transplantation renovates the ovarian surface epithelium in a rat model of premature ovarian failure: Possible direct and indirect effects. Tissue and Cell 48, 370–382 (2016).
Mohamed, S. A. et al. Human Mesenchymal Stem Cells Partially Reverse Infertility in Chemotherapy-Induced Ovarian Failure. Reprod. Sci. 25, 51–63 (2018).
Chang, L.-B. et al. Therapeutic potential of amniotic fluid stem cells to treat bilateral ovarian dystrophy in dairy cows in a subtropical region. Reproduction in Domestic Animals 53, 433–441 (2018).
Grady, S. T. et al. Effect of intra-ovarian injection of mesenchymal stem cells in aged mares. J. Assist. Reprod. Genet. 36, 543–556 (2019).
Ball, L. M. et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br. J. Haematol. 163, 501–509 (2013).
Ciccocioppo, R. et al. Long-Term Follow-Up of Crohn Disease Fistulas After Local Injections of Bone Marrow-Derived Mesenchymal Stem Cells. Mayo Clin. Proc. 90, 747–755 (2015).
Gupta, P. K. et al. Administration of Adult Human Bone Marrow-Derived, Cultured, Pooled, Allogeneic Mesenchymal Stromal Cells in Critical Limb Ischemia Due to Buerger’s Disease: Phase II Study Report Suggests Clinical Efficacy. Journal of Vascular Surgery 66, 1303 (2017).
Kim, T.-H. et al. 3D-cultured human placenta-derived mesenchymal stem cell spheroids enhance ovary function by inducing folliculogenesis. Sci. Rep. 8, 15313 (2018).
Huang, B. et al. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res. Ther. 9, 216 (2018).
Xie, Q. et al. Mesenchymal Stem Cells Alleviate DHEA-Induced Polycystic Ovary Syndrome (PCOS) by Inhibiting Inflammation in Mice. Stem Cells Int. 2019, 9782373 (2019).
Li, J. et al. Human chorionic plate-derived mesenchymal stem cells transplantation restores ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. Stem Cell Research & Therapy vol. 9 (2018).
Yoon, S. et al. Recovery of ovarian function by human embryonic stem cells derived mesenchymal stem cells in cisplatin induced premature ovarian failure in mouse. Fertility and Sterility 110, e322 (2018).
Yang, M. et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab. Invest. 100, 342–352 (2020).
Treuting, P. M. & Dintzis, S. M. Comparative Anatomy and Histology: A Mouse and Human Atlas. (Academic Press, 2012).
Ginther, O. J., Bergfelt, D. R., Beg, M. A., Meira, C. & Kot, K. In vivo effects of an intrafollicular injection of insulin-like growth factor 1 on the mechanism of follicle deviation in heifers and mares. Biol. Reprod. 70, 99–105 (2004).
Li, Q., Jimenez-Krassel, F., Kobayashi, Y., Ireland, J. J. & Smith, G. W. Effect of intrafollicular indomethacin injection on gonadotropin surge-induced expression of select extracellular matrix degrading enzymes and their inhibitors in bovine preovulatory follicles. Reproduction 131, 533–543 (2006).
Gasperin, B. G. et al. FGF10 inhibits dominant follicle growth and estradiol secretion in vivo in cattle. Reproduction 143, 815–823 (2012).
Hayashi, K. et al. The cooperative action of angiotensin II with subluteolytic administration of PGF2alpha in inducing luteolysis and oestrus in the cow. Reproduction 124, 311–315 (2002).
Watanabe, S. et al. Effect of intraluteal injection of endothelin type A receptor antagonist on PGF2alpha-induced luteolysis in the cow. J. Reprod. Dev. 52, 551–559 (2006).
Oropeza, A., Wrenzycki, C., Herrmann, D., Hadeler, K.-G. & Niemann, H. Improvement of the developmental capacity of oocytes from prepubertal cattle by intraovarian insulin-like growth factor-I application. Biol. Reprod. 70, 1634–1643 (2004).
Batista, E. O. S. et al. Ovarian follicular growth suppression by long-term treatment with a GnRH agonist and impact on small follicle number, oocyte yield, and in vitro embryo production in Zebu beef cows. Theriogenology 85, 1680–1687 (2016).
Rahman, M. M. et al. Chronic mastitis is associated with altered ovarian follicle development in dairy cattle. J. Dairy Sci. 95, 1885–1893 (2012).
Bromfield, J. J. & Sheldon, I. M. Lipopolysaccharide reduces the primordial follicle pool in the bovine ovarian cortex ex vivo and in the murine ovary in vivo. Biol. Reprod. 88, 98 (2013).
Roth, Z. et al. Naturally occurring mastitis disrupts developmental competence of bovine oocytes. J. Dairy Sci. 96, 6499–6505 (2013).
Santos, G. et al. Subclinical mastitis interferes with ovulation, oocyte and granulosa cell quality in dairy cows. Theriogenology 119, 214–219 (2018).
Bromfield, J. J. & Sheldon, I. M. Lipopolysaccharide initiates inflammation in bovine granulosa cells via the TLR4 pathway and perturbs oocyte meiotic progression in vitro. Endocrinology 152, 5029–5040 (2011).
Zhao, S. et al. Detrimental effects of lipopolysaccharides on maturation of bovine oocytes. Asian-australas. J. Anim. Sci. 32, 1112–1121 (2019).
Arhin, S. K. et al. Multiple facilitated glucose transporters SLC2As are required for normal mouse preimplantation embryo development. Am. J. Transl. Res. 11, 3412–3425 (2019).
Gardner, D. K., Wale, P. L., Collins, R. & Lane, M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Hum. Reprod. 26, 1981–1986 (2011).
Schmidt, S. et al. Essential role of glucose transporter GLUT3 for post-implantation embryonic development. J. Endocrinol. 200, 23–33 (2009).
Brito, I. R. et al. Alginate hydrogel matrix stiffness influences the in vitro development of caprine preantral follicles. Mol. Reprod. Dev. 81, 636–645 (2014).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 (2006).
De Francesco, F., Ricci, G., D’Andrea, F., Nicoletti, G. F. & Ferraro, G. A. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng. Part B Rev. 21, 572–584 (2015).
Marx, C., Silveira, M. D. & Beyer Nardi, N. Adipose-derived stem cells in veterinary medicine: characterization and therapeutic applications. Stem Cells Dev. 24, 803–813 (2015).
de Oliveira Leme, L. et al. Effect of vitrification using the Cryotop method on the gene expression profile of in vitro-produced bovine embryos. Theriogenology 85, 724–33.e1 (2016).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
Acknowledgements
This work had financial support from the Brazilian National Research Council (CNPq, Project #424074/2016-8) and Fundação de Amparo à Pesquisa do Distrito Federal (FAPDF). MP, PM and TA received grants from CAPES. The authors thank Ms. Rose Gonçalves and Mr. José Luis Gonçalves for supplying the animals and infrastructure for the first experiment of this research, and thank Evandro do Carmo Guimarães and the Fazendas do Basa for the animals and infrastructure used in the second experiment.
Author information
Authors and Affiliations
Contributions
Patrícia F. Malard, Maurício A.S. Peixer, Robert Pogue, and Juliana L. Carvalho contributed to the study conception and design. Material preparation and data collection were performed by Patrícia F Malard, Maurício A.S. Peixer, João G. Grazia, Luis F. Feres, Hilana dos S.S. Brunel, Carla L. Villarroel, and Luiz G.B. Siqueira. Data analysis was performed by Margot A.N. Dode, João Henrique M. Viana, Robert Pogue, and Juliana L. Carvalho. The first draft of the manuscript was written by João Henrique M. Viana, the final version of the manuscript was produced by Juliana L. Carvalho and Robert Pogue, and all authors commented on all versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.A.S.P. and P.F.M. declare financial competing interests as owners of Bio. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Malard, P.F., Peixer, M.A.S., Grazia, J.G. et al. Intraovarian injection of mesenchymal stem cells improves oocyte yield and in vitro embryo production in a bovine model of fertility loss. Sci Rep 10, 8018 (2020). https://doi.org/10.1038/s41598-020-64810-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64810-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.