Abstract
Our aim was to evaluate the association between ritodrine and magnesium sulfate (MgSO4) and the occurrence of neonatal hyperkalemia or hypoglycemia among late preterm infants in a retrospective cohort study. We used a nationwide obstetrical database from 2014. A total of 4,622 live preterm infants born at 32–36 gestational weeks participated. Fourteen risk factors based on both clinical relevance and univariate analysis were adjusted in multivariable logistic regression analyses. Neonatal hyperkalemia and hypoglycemia occurred in 7.6% (284/3,732) and 32.4% (1,458/4,501), respectively. Occurrence of hyperkalemia was associated with concomitant usage of ritodrine and MgSO4 compared with no usage (adjusted odds ratio [aOR] 1.53, 95% confidence interval [CI] 1.09–2.15). Occurrence of hypoglycemia was associated with ritodrine alone (aOR 2.58 [CI 2.21–3.01]) and with concomitant usage of ritodrine and MgSO4 (aOR 2.59 [CI 2.13–3.15]), compared with no usage, and was associated with long-term usage (≥ 48 hours) of ritodrine and cessation directly before delivery. In conclusion, in late preterm infants, usage of ritodrine together with MgSO4 was associated with occurrence of critical neonatal hyperkalemia, and long-term usage of ritodrine and cessation directly before delivery were associated with neonatal hypoglycemia.
Similar content being viewed by others
Introduction
The Cause Analysis Committee for Cerebral Palsy of the Japan Council for Quality Health Care (JCQHC) has suggested that of the nearly 1,000 cases of cerebral palsy, some may have occurred in neonates with hypoglycemia and/or hyperkalemia born to mothers receiving either ritodrine or magnesium sulfate (MgSO4)1. In addition, 14 cases of unexpected neonatal hyperkalemia from mothers using tocolytic agents2,3,4,5,6,7,8,9,10,11,12,13,14 –especially ritodrine and MgSO4 concomitantly2,3 – have been independently reported by neonatologists in Japan. At JCQHC request, the Japan Society of Perinatal and Neonatal Medicine (JSPNM) is evaluating the association between these agents and neonatal hyperkalemia or hypoglycemia occurrence. However, to the best of our knowledge, there have been no cohort studies in English suggesting a relationship between ritodrine administration and neonatal hyperkalemia. Regarding MgSO4, there has only been one case reported of a very low birth weight infant with hyperkalemia accompanied by hypermagnesemia15.
In 2013 the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) recommended against long-term tocolysis ≥48 hours with ritodrine and MgSO416,17,18. Following these restrictions, Kissei Pharmaceutical Co. Ltd. manufacturing ritodrine in Japan, published “A review of EU restrictions on short-acting beta-agonists, and guidelines regarding efficacy and safety of ritodrine hydrochloride (injection and tablet) in Japan” in Dec. 201419. Accordingly, two leading societies for obstetrical decision-making in Japan, the Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG), have not prohibited long-term tocolysis using ritodrine20,21. Therefore, long-term tocolysis using ritodrine has often been performed22.
Although MgSO4 had been used for seizure prophylaxis in women with preeclampsia or eclampsia23, its usage as a tocolytic agent became covered by insurance in Japan in 200624. Generally, maintenance therapy with MgSO4 is not recommended in the USA25; however, long-term MgSO4 tocolysis is widely performed in Japan because the package insert does not prohibit usage at 22–36 gestational weeks24. In addition, long-term tocolysis with both ritodrine and MgSO4 has been used in Japan when uterine contractions could not be controlled using ritodrine or MgSO4 alone26,27,28,29.
Ritodrine and MgSO4 have been used widely as tocolytic agents in Japan20,21. Although hyperglycemia and hypokalemia are well-known adverse events of ritodrine in pregnant women30, and hypoglycemia is a well-known adverse event in neonates born to mothers receiving ritodrine30, whether ritodrine usage in preterm labor is associated with increased risk of neonatal hyperkalemia is unknown. Furthermore, although hyperkalemia has been reported as a rare adverse event of MgSO4 in pregnant women24, it is unknown whether MgSO4 usage in women during preterm labor is also associated with increased neonatal hyperkalemia risk. To our best knowledge, association between ritodrine and/or MgSO4 usage and neonatal hyperkalemia occurrence has not been documented in a large cohort study.
We hypothesized that long-term tocolysis with either ritodrine or MgSO4 until 36 gestational weeks, or the combination of ritodrine and MgSO4 may be associated with increased risks of neonatal hypoglycemia or hyperkalemia in Japan. Moreover, we also hypothesized neonatal hypoglycemia or hyperkalemia may be associated with increased risks of abnormal neurological findings including cerebral palsy. Therefore, our main aim was to investigate hyperkalemia and hypoglycemia incidence in neonates born to mothers receiving either ritodrine or MgSO4 therapy for preterm labor. Our secondary aim was to investigate the association between neonatal hypoglycemia and hyperkalemia and later occurrence of abnormal neurological findings including cerebral palsy. Our study focused only on late preterm infants born after 32 gestational weeks. Such preterm infants are usually not severely ill at birth, and tend to be cared for in step down neonatal units or obstetrical wards without close examination, unlike neonatal intensive care units (NICUs).
Results
Maternal and infantile characteristics based on presence /absence of hypoglycemia
Hypoglycemia occurred in 32.4% at birth and at <3 hours after delivery in 94.3% of cases. Frequencies of the following were significantly higher in infants with hypoglycemia: Mother with PL/shortened CL/CI (preterm labor/shortened cervical length/cervical incompetency), placenta previa/low-lying placenta, cesarean section, twins/triplets, small-for-gestational-age (SGA) infants, and women with ritodrine alone or the concomitant usage of both ritodrine and MgSO4. However, in infants with hypoglycemia, frequencies of women with preterm premature rupture of the membranes (pPROM), and GH/PE/eclampsia/ HELLP/AFLP (gestational hypertension/ preeclampsia/eclampsia/hemolysis, elevated liver enzymes, and low platelets/acute fatty liver of pregnancy) were significantly lower (Table 1).
Effects of various risk factors on hypoglycemia occurrence
In multivariable logistic regression analyses using the “Hypoglycemia set”, independent risk factors for hypoglycemia occurrence were cesarean section, SGA infants, and delivery to a mother with ritodrine alone or concomitant usage of ritodrine and MgSO4 (Table 2). Interestingly, preterm premature rupture of the membranes (pPROM) was a negative independent risk factor for hypoglycemia occurrence.
Maternal and infantile characteristics based on presence/absence of hyperkalemia
Hyperkalemia occurred in 7.6% of cases at birth, and 24.0% at <3 hours, 9.2% at 3–5 hours, 53.0% at 6–23 hours, and 13.8% at ≥24 hours. Frequencies of hyperkalemia were significantly higher in infants born at <35 gestational weeks, with an Apgar score at 1 minute <3, and whose mother used ritodrine and MgSO4 concomitantly (Table 3)
Effects of various risk factors on hyperkalemia occurrence
In multivariable logistic regression analyses using the “Hyperkalemia set”, independent risk factors for hyperkalemia occurrence were delivery at <35 gestational weeks, an Apgar score at 1 minute <3, and delivery to a mother with concomitant usage of ritodrine and MgSO4 (Table 4).
Maternal and infantile characteristics with the combination of MgSO4 and ritodrine
Data from four usage groups – MgSO4 alone, ritodrine alone, both MgSO4 and ritodrine, and neither MgSO4 nor ritodrine – are shown in Supplementary Table S1. MgSO4 was the smallest group (n = 243, 5.3%), but frequency of GH/PE/eclampsia/ HELLP/AFLP was highest (67.1%), suggesting MgSO4 alone was mainly used eclampsia prevention during pregnancy (Supplementary Table S1). On the contrary, frequencies of PL/shortened CL/CI were significantly higher in the ritodrine alone group (79.6%) and the combined MgSO4 and ritodrine group (85.0%) compared to the MgSO4 alone group (35.8%) and the control group using neither MgSO4 nor ritodrine (30.4%). This suggests ritodrine alone or both ritodrine and MgSO4 in combination was mainly selected for PL therapy. Frequencies of hypoglycemia were significantly higher in women using ritodrine alone (41.4%) and using both MgSO4 and ritodrine (42.9%) than in controls (22.2%). Frequency of hyperkalemia was significantly higher in women using both MgSO4 and ritodrine (10.9%) than in controls (6.5%).
Duration- and dose-dependent effects of ritodrine on hypoglycemia occurrence
Next, we evaluated the association of the duration, maximum rate of administration, final rate of administration just before cessation, and time from cessation to delivery for ritodrine with the occurrence of hypoglycemia using the “Hypoglycemia: Ritodrine-alone plus control set” (Supplementary Table S2). The risk of hypoglycemia was associated with long-term tocolysis (total administration periods ≥2 days [48 hours]). However, short-term tocolysis (<2 days [48 hours]) was not a risk factor for hypoglycemia. The maximum rate of injection or final rate of injection just before cessation of ritodrine did not show dose-dependence. The risk of hypoglycemia was related to cessation just before delivery; if ritodrine was stopped ≥4 hours before delivery, the aOR of hypoglycemia was almost two thirds of when stopped <4 hours before delivery.
Duration- and dose-dependent effects of ritodrine or MgSO4 on hyperkalemia occurrence
Incidence rate of hyperkalemia in women with ritodrine alone was not different from that in women with MgSO4 alone. We then evaluated the association of tocolytic agents’ duration, maximum rate of administration, final rate of administration just before cessation, and time from cessation to delivery with hyperkalemia occurrence, using the “Hyperkalemia: Both ritodrine and MgSO4 plus control set” (Supplementary Table S3, S4). Hyperkalemia risk was associated with long-term tocolysis with ritodrine. In women in whom the maximum rate of injection or final rate of injection just before cessation of ritodrine was ≥170 μg/min, the risk was significantly higher than in those with no usage of ritodrine. Risk of hyperkalemia was related to the cessation of ritodrine just before delivery; if stopped ≥4 hours before delivery, hyperkalemia risk almost equaled no usage. As for MgSO4, there was no relationship between duration of administration, maximum rate of injection, or final rate of injection before cessation. However, risk of hyperkalemia was related to MgSO4 cessation just before delivery; hyperkalemia risk in women in whom MgSO4 was stopped ≥4 hours before delivery was not significantly different from that in women with no usage of ritodrine.
Maternal and infantile characteristics based on presence/absence of cerebral palsy occurring 3 years after birth
Cerebral palsy occurred in 23 cases (0.5%) (Supplementary Table S5). Frequencies of placental abruption, delivery at <35 weeks of gestation, and Apgar score at 1 minute <3 were significantly higher in infants with cerebral palsy. Gestational weeks at delivery was earlier, and birth weight was also smaller. Due to this small sample size, we did not perform multivariable analysis.
Maternal and infantile characteristics based on presence/absence of any neurological impairments occurring 3 years after birth
Neurological impairments occurred in 193 cases (4.5%) (Supplementary Table S6). Cerebral palsy was only 12%. Other impairments included developmental language disorder alone, low score of developmental quotient, autism spectrum disorder (autism), attention-deficit/hyperactivity disorder (ADHD), auditory disorder, visual impairment, epilepsy, or developmental coordination disorder. Frequencies of nulliparous women, GH/PE/eclampsia/ HELLP/AFLP, placenta abruption, delivery at <35 weeks of gestation, male infants, SGA infants, Apgar score at 1 minute <3, and hypoglycemia were higher in infants with neurological impairments. In addition, frequencies of PL/shortened CL/CI, or cervical incompetency, and twins/triplets were significantly lower in infants with neurological impairments. Median gestational weeks at delivery was earlier and birthweight was also smaller.
Effects of various risk factors on occurrence of any neurological impairments
Neurological impairments were evaluated in 4,279 infants (Supplementary Table S7). Excluding 989 patients with missing data for 16 variables, a total of 3,290 patients underwent multivariable analysis to assess effects on occurrence of any neurological impairments with the following 16 risk factors: combination of MgSO4 and ritodrine, obstetrical complications, cesarean section, delivery at <35 wk, twins/triplets, infantile sex, SGA infants, large-for-gestational-age infants, Apgar score at 1 min <3, hypoglycemia at <48 h after birth, and hyperkalemia at <48 h after birth. Placental abruption, delivery at <35 weeks of gestation, male sex, SGA infants, and hypoglycemia were independent risk factors for the occurrence of any neurological impairments.
Discussion
Our current large cohort study yielded three novel findings. First, neonatal hyperkalemia within 48 hours after birth was associated with the concomitant usage of ritodrine and MgSO4 among late preterm infants born at 32–36 gestational weeks. Second, neonatal hypoglycemia within 48 hours was associated with the usage of ritodrine alone or the concomitant usage of ritodrine and MgSO4; incidence of hypoglycemia was markedly higher in infants born to mothers with cessation of ritodrine just before delivery; in addition, incidence of hypoglycemia was higher in infants born to mothers with long-term tocolysis with ritodrine. Third, in infants born at 32–36 weeks of gestation, placental abruption, delivery at <35 weeks of gestation, male sex, SGA infants, and hypoglycemia within 48 hours after birth were independent risk factors for the occurrence of any neurological impairments.
In the current study, for the first time we have revealed concomitant usage of ritodrine and MgSO4 is associated with incidence of neonatal hyperkalemia within 48 hours after birth in neonates born at 32–36 gestational weeks, although we could not show an association between neonatal hyperkalemia and cerebral palsy. Suzuki2 analyzed data available in the Cause Analysis Report and demonstrated the relationship between delivery and cerebral palsy was unknown in 6.2% of nearly 800 cases of cerebral palsy. These infants showed marked characteristics: although considered normal, thereafter their condition changed suddenly and finally they developed severe cerebral palsy. Of these, 6 were cases of hypoglycemia and 3 of hyperkalemia, and in 2 cases of hyperkalemia with later occurrence of cerebral palsy, ritodrine and MgSO4 were concomitantly used. In addition, in 11 of 14 cases of unexpected neonatal hyperkalemia soon after birth, ritodrine and MgSO4 were also concomitantly used3. Our results further support the suggested association between concomitant usage of ritodrine and MgSO4 and neonatal hyperkalemia. It is well known neonatal hyperkalemia can cause electrocardiographic abnormalities including ventricular tachycardia31. Therefore, our results serve as a warning about concomitant usage of ritodrine and MgSO4 to prevent neonatal hyperkalemia. However, it is unknown why concomitant usage of ritodrine and MgSO4, but not ritodrine alone or MgSO4 alone, is associated with neonatal hyperkalemia. Hypermagnesemia might affect Na + /K + -ATPase15,32; however, to the best of our knowledge, there have been no cohort studies suggesting a relationship between ritodrine administration and neonatal hyperkalemia. Therefore, our clinical observations may suggest presence of synergy between ritodrine and hypermagnesemia to modify the Na + /K + -ATPase function.
Although we could not reveal the association between neonatal hypoglycemia and the later occurrence of cerebral palsy in this study, it is well-known that neonatal hypoglycemia increases the incidence of cerebral palsy33,34. Risk factors for cerebral palsy in infants with hypoglycemia are as follows: blood glucose levels <15 mg/dL, long duration of hypoglycemia, non-reassuring fetal status (NRFS), low Apgar score <5 at 1 min, neonatal seizure, pathological jaundice, and hypertensive disorders of pregnancy for the mother35. Ritodrine is well-known to induce hyperglycemia in mothers which can cause hypoglycemia in neonates36,37. In the current study, incidence of hypoglycemia was markedly higher in infants born to mothers with either long-term tocolysis of ritodrine or cessation just before delivery. Therefore, ritodrine might be one of the risk factors for cerebral palsy. Hyperkalemia was not associated with the occurrence of cerebral palsy. In addition, we found placental abruption, delivery at <35 weeks of gestation, and Apgar score at 1 minute <3 were associated with later occurrence of cerebral palsy. Placental abruption and early delivery are well-known risk factors for cerebral palsy38. Although the contribution of asphyxia to the overall incidence of cerebral palsy is relatively small39, metabolic acidosis in fetal umbilical cord arterial blood obtained at delivery with both pH <7.00 and base deficit ≥12 mmol/L would have been sufficient to cause cerebral palsy40.
In infants born at 32–36 weeks of gestation, placental abruption, delivery at <35 weeks of gestation, male sex, SGA infants, and hypoglycemia were independent risk factors for occurrence of any neurological impairments. Hyperkalemia was not an independent risk factor. Male sex is at higher risk of autism41,42, and may be at high risk of ADHD43; however, the association between male sex and cerebral palsy is controversal44. An SGA infant is a risk factor for cerebral palsy in moderate to late preterm infants45, and may be also associated with autism or ADHD46,47. Although our outcome of any neurological impairment was a composite outcome, and our data could be significantly biased due to a retrospective study design, our data support associations of male sex or an SGA infant on occurrence of neurological impairments including cerebral palsy, autism, or ADHD.
Our results warn about the concomitant usage of ritodrine and MgSO4 to prevent neonatal hyperkalemia, and also warn about long-term usage of ritodrine and its cessation just before delivery to prevent neonatal hypoglycemia. Furthermore, because these adverse events occurring outside NICUs are not well-recognized among obstetricians and neonatologists, this study could have a marked impact on modifying current neonatal management for infants born to mothers with tocolytic agents. Accordingly, neonatal assessment of hyperkalemia can be suggested in women with concomitant usage of ritodrine and MgSO4, and neonatal blood glucose monitoring can also be suggested in women with long-term tocolysis with ritodrine or cessation just before delivery.
The main strengths of our study include the following: (1) it is the first large cohort study evaluating associations between long-term tocolysis with both ritodrine and MgSO4, and neonatal hypoglycemia and hyperkalemia; (2) it presents the first evidence of a synergic effect between ritodrine and MgSO4 on occurrence of neonatal hyperkalemia; and (3) it is the first large cohort study investigating association of neonatal hyperkalemia and later occurrence of any neurodevelopmental impairments including cerebral palsy.
Our study has several limitations. First, it may be difficult to generalize results from this study with other countries. However, ritodrine hydrochloride and MgSO4 have a long usage history in prenatal care, and MgSO4 use is still widespread. The novel adverse events (neonatal hyperkalemia) due to concomitant use of ritodrine and magnesium sulfate should be added in the package insert of both MgSO4 and ritodrine hydrochloride. Second, this was a retrospective cohort study possibly resulting in high risk of selection bias. Although we targeted infants born at 32–36 gestational weeks, 19% of infants did not have data for either hypoglycemia or hyperkalemia possibly leading to bias. In addition, we could not confirm presence/absence of neurodevelopmental impairments in almost 7% of infants, resulting in underestimation of the associations between hypoglycemia/hyperkalemia and subsequent occurrence of neurodevelopmental impairments. However, in this large retrospective cohort study, we attempted to decrease systematic bias through secondary investigation for ritodrine and magnesium sulfate usage, and attempted to adjust possible confounding factors using multivariable logistic regression analysis. In addition, before using the nationwide database, we checked for inappropriate values and transformed them to missing values. The main outcomes of hypoglycemia and hyperkalemia were newly investigated in the secondary inquiry, and diagnoses of hypoglycemia and hyperkalemia were validated by investigating levels of glucose and potassium within 48 hours after birth. Therefore, although this study is a retrospective study using a large database, we believe reducing selection bias risk is feasible as it adjusting for possible confounding factors in the relationship between ritodrine/magnesium sulfate usage and neonatal hypoglycemia/hyperkalemia occurrence. Third, we could not collect data on hypoglycemia/hyperkalemia from 277 hospitals (78%). However, the incidences of GH/PE/eclampsia/HELLP/AFLP, placenta previa/low-lying placenta, DM, GDM, and cesarean section in infants in the current study were almost the same as those in infants not involved in the current study (Supplementary Table S8), indicating current subjects may have been appropriately extracted from a nationwide obstetrical database. Fourth, inclusion criteria for pregnant women with only late preterm infants may limit generalizability of our results. However, since long-term tocolysis with ritodrine and MgSO4 is often performed at 32–36 gestational weeks in Japan, and since tocolysis is not performed at ≥37 gestational weeks, we believe targeting at 32–36 gestational weeks may be appropriate for analyzing the relationship between tocolytic agents and occurrence of neonatal hypoglycemia and hyperkalemia.
Methods
Study design and participants
This was a retrospective cohort study of neonates born at 32–36 gestational weeks using a nationwide obstetrical database from 201448. Because we needed to collect information on the infantile prognosis until almost 3 years old, it was followed by a secondary survey conducted in Japan in 2017–2018. In previous case series of hyperkalemia neonates, 50% (7/14) were born at 32–36 gestational weeks3,4,5,6,7,8,9,10,11,12,13,14. Neonates are usually managed on obstetrical wards in Japan if they have either ≥2,000 g birthweight or are at ≥35 gestational weeks at delivery. Therefore, we speculated such neonates might have developed cerebral palsy due to possible delay of the detection of hyperkalemia, because in neonates on obstetrical wards electrolyte abnormalities are not checked routinely unless they show symptoms. In addition, hyperkalemia often occurs in neonates born at <32 gestational weeks; therefore, exclusion of such early preterm infants may facilitate analysis of the association between tocolytic agents and hyperkalemia occurrence. Thus, we decided to investigate neonates born in a relatively late preterm period (32–36 gestational weeks), to evaluate the possible relationship between tocolytic agents and neonatal hyperkalemia. For hypoglycemia, Suzuki2 analyzed data available in the Cause Analysis Report, and determined 5 of 6 neonates (83%) who had hypoglycemia suspected associated with development of cerebral palsy were born at ≥37 gestational weeks. However, we did not include neonates born at ≥37 gestational weeks in our analysis since assessment of hypoglycemia was not part of our routine examinations.
We received approval from the JSOG Clinical Research Ethics Committee to use a nationwide obstetrical database from 2014 (No. JSOG2017–51), and also received approval from the JSPNM Clinical Research Ethics Committee for execution of the current study in the survey group to study the effects of tocolytic agents on neonatal adverse events (No. JSPNM2017–1). Then, we requested the directors of Departments of Obstetrics and Gynecology in 355 hospitals that had registered in the nationwide obstetrical database from 2014 (total stillbirths and infants: n = 220,052; those born at 32–36 gestational weeks: n = 24,943) to cooperate with the current study. Finally, 78 directors consented to this study, and kindly secured the cooperation of neonatologists in each hospital. A research investigator and research team members in each hospital gained approval for this study from each Clinical Research Ethics Review Committee. All methods in this retrospective study were performed in accordance with the relevant guidelines (Ethical Guidelines for Medical and Health Research Involving Human Subjects). Because this study is a retrospective study, it was very difficult to gain appropriate informed consents from each subject. Therefore, we gained consents using opt-out, which is a way for investigators to give subjects an opportunity to refuse to participate in this study by announcing the detail of this study in each participating institute. The Survey Committee constructed input pages for survey data on the Web system. Data were collected within 1 year after the approval of the current study.
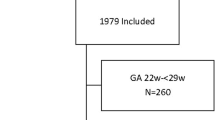
We excluded the following infants from our survey: (1) born to women with unknown data on the usage of ritodrine or MgSO4, (2) whose mothers were administered MgSO4 only after birth or at an unknown time, (3) with a birthweight of either <500 or>4,000 g, or unknown birth weight, (4) with stillbirth or major anomalies (chromosomal anomalies, neonatal abnormalities, conditions probably contributing to impaired neurodevelopment, conditions requiring emergency surgery soon after delivery, and lethal conditions), and (5) with unknown data on both hypoglycemia and hyperkalemia (Fig. 1). The remaining 4,622 infants with data on either hypoglycemia or hyperkalemia at <48 hours after delivery were analyzed.
Patient Flowchart. “Basal data set” (N = 4,622) was created from 6,136 surveyed infants born at 32–36 gestational weeks. “Hypoglysemia set” (N = 4,501) was created from “Basal data set” after excluding 121 infants with unknown data on hypoglycemia; and “Hyperkalemia set” (N = 3,732) was created from “Basal data set” after excluding 890 infants with unknown data on hyperkalemia. “Hypoglycemia set” was divided into “Hypoglycemia: Ritodrine-alone plus control set” (Ritodrine-alone [N = 1,624] and control 1 without either ritodrine or MgSO4 [N = 1,909]), “Hypoglycemia: MgSO4-alone plus control set” (MgSO4-alone [N = 233] and control 1), and “Hypoglycemia: Both ritodrine and MgSO4 plus control set” (Both ritodrine and MgSO4 [N = 735] and control 1). “Hyperkalemia set” was also divided into “Hyperkalemia: Ritodrine-alone plus control set” (Ritodrine-alone [N = 1,364] and control 2 without either ritodrine or MgSO4 [N = 1,516]), “Hyperkalemia: MgSO4-alone plus control set”, (MgSO4-alone [N = 212] and control 2), and “Hyperkalemia: Both ritodrine and MgSO4 plus control set” (Both ritodrine and MgSO4 [N = 640] and control 2).
Collection of new variables by the secondary survey
The Survey Committee used a nationwide obstetrical database from 2014, which included 314 variables on maternal and neonatal information48. The input data in all variables were initially automatically checked using internalized data check scripts, and data input staff were informed of possible incorrect data. However, since the database was built using data from 355 institutes, there were inappropriate data in the database. Then, one author (A.O.) attempted to validate the database. The initial number of cases in the database was 24,960, but we found that 17 cases were duplicated; after exclusion the remaining 24,943 cases were used. Next, we determined inappropriate values for maternal height, pre-pregnancy maternal body weight, maternal body weight at delivery, maternal age, bleeding amounts, gestational weeks at premature rupture of the membranes, disseminated intravascular coagulation (DIC) score, neonatal birth weight, neonatal birth height, neonatal head circumference, Apgar score at 1 minute (min), Apgar score at 5 min, pH of umbilical artery, placental weight, and umbilical cord length. We finally transformed the inappropriate values to missing values.
The Survey Committee extracted the following 6 variables: facility name, anonymization number, date of birth, gestational weeks and days at delivery, birth weight, and neonatal sex. The committee members collaboratively decided survey items for the secondary survey. In the survey for ritodrine they were: presence/absence of injections, medical product name, total administration days (6 codes), maximum infusion speed (8 codes), final infusion speed (8 codes), and interval (hours) from cessation of ritodrine to delivery (6 codes). In the MgSO4 survey they were: presence/absence of injections, medical product name, total administration days (6 codes), maximum infusion speed (8 codes), final infusion speed (8 codes), interval hours from cessation of MgSO4 to delivery (6 codes), and administration period (pre-delivery alone, post-delivery alone, both pre- and post-delivery, unknown). In the survey for neonatologists they were: presence/absence of admission to NICU, causes for admission to NICU, presence/absence of measurements of magnesium concentrations in umbilical cord, concentration of magnesium in umbilical cord, presence/absence of measurements of blood sugar within 48 hours after delivery, blood sugar level at the nadir (mg/dL), presence/absence of hypoglycemia defined as <40 mg/dL49, timing of the nadir blood sugar level (5 codes), presence/absence of measurements of potassium concentrations within 48 hours after delivery, potassium level at the maximum (mEq/L), presence/absence of hyperkalemia defined as >6.5 mEq/L50, timing of the maximum potassium level (5 codes), three consecutive potassium levels just after the occurrence of hyperkalemia, infantile prognosis at almost 3 years (4 codes: death, presence of abnormal neurological findings, absence of abnormal neurological findings, unknown), detailed information on the disease or condition leading to abnormal neurological findings, and date of judgment of infantile prognosis.
Primary/secondary outcomes and risk factors
Primary outcomes were the occurrence of hyperkalemia and hypoglycemia. Secondary outcomes were cerebral palsy, and any neurodevelopmental impairments including cerebral palsy occurring 3 years after birth. Cerebral palsy was judged by the senior pediatrician (S. K.) who was involved neither in data acquisition nor in database construction. Cerebral palsy was defined as a non-progressive, non-transient central nervous system disorder characterized by abnormal muscle tone in at least 1 extremity and abnormal control of movement and posture51.
Based on both clinical relevance and univariate analysis, risk factors for occurrence of hypoglycemia or hyperkalemia were: obstetrical complications, cesarean section, MgSO4 usage during pregnancy, ritodrine usage, gestational weeks at delivery, birth weight, multiple pregnancy, infantile sex, SGA and large-for-gestational-age defined as an infant with weight below the 10th percentile or ≥ the 90th percentile of gestational age52, and Apgar score at 1 min <3. The above-mentioned 14 risk factors plus hypoglycemia and hyperkalemia were also determined risk factors for cerebral palsy or other neurological impairments.
Statistical analysis
Continuous variables are shown as the median (interquartile range) because of non-normal distributions of gestational weeks and birth weight at 32–36 gestational weeks, and binary and categorical variables are shown as n (%). The associations of ritodrine/MgSO4 usage during pregnancy and the occurrence of infantile hypoglycemia/hyperkalemia within 48 hours after delivery were analyzed using Fisher’s exact test or the χ2 test, followed by univariate logistic regression analyses. Then, multivariable regression analyses were performed while adjusting for confounding variables. Because the primary outcomes of hyperkalemia and hypoglycemia occurred in >200 cases, we judged we could use a maximum of 20 risk and/or confounding factors in the multivariable models. All analyses were performed using IBM SPSS Statistics (version 25 for Windows) and R (EZR ver. 1.37)53. Level of p < 0.05 was considered significant.
Data availability
Data and materials used in this study are available upon reasonable request to the corresponding author and under a collaboration agreement.
References
Japan Council for Quality Health Care. Dai 7 kai Sanka Iryo Hosho Seido: Saihatsu boushi ni kansuru houkokusho [Japan Obstetrics Compensation System for Cerebral Palsy: Report for Preventive Measure] [in Japanese]. (ed. Japan Council for Quality Health Care) (Japan Council for Quality Health Care, 2017).
Suzuki, S. Shussei souki no shinseiji kyuuhen ni taiousuru [Strategy for the emergent serious condition of neonates] [in Japanese]. Acta Obst. Gynaec. Jpn. 68, 133–136 (2016).
Itani, Y. Yokisenu shussyou go souki no shinseiji kou K kessyou [Unexpected neonatal hyperkalemia in early neonatal period] [in Japanese]. JAOG news. 67, 10–11 (2015).
Takayanagi, T. et al. Seigo 24 jikan inai ni shoukousei no kou kariumu kessyou wo kitashita seijukuji no 2 rei [Occurrence of symptomatic hyperkalemia within 24 hours after birth in two term newborns] [in Japanese]. Acta Neonatologica Japonica. 38, 833–836 (2002).
Hosoda, N., Uchida, T., kyoba, S. & Watanabe, M. Botai heno ryuusan magunesiumu touyo ni yori kou kariumu kessyou to kin kintyou teika wo kitashita soutai [Twins with hyperkalemia and hypotonia born from mother with administration of magnesium sulfate during pregnancy] [abstract] [in Japanese]. J. Jpn. Soc. Perin. Neon. Med. 44, 654 (2008).
Yokozeki, Y. et al. Seigo 24 jikan de genin fumei no kou kariumu kessyou wo kitashita 2 syourei [A twin with hyperkalemia with unknown cause within 24 hours after birth] [abstract] [in Japanese]. J. Jpn. Soc. Perin. Neon. Med. 45, 426 (2009).
Suzuki, M., Yoshio, H., Masaki, H., Yoshinare, R. & Ito, S. Botai ni ensan ritodorin to ryuusan maguneshiumu touyo ni yori kou kariumu kessyou wo kitashita 2 syourei [Two neonates with hyperkalemia born from mother with administration of both ritodrine hydrochloride and magnesium sulfate] [abstract] [in Japanese]. J. Jpn. Soc. Perin. Neon. Med. 21, 727 (2009).
Yokota, S. et al. Botai he no ensan ritodorin to ryuusan magunesiumu no eikyou ni yori, kou K kessyou, shinshitsu sei hinpaku (VT) wo teishita shinseiji no 1 rei [Hyperkalemia followed by ventricular tachycardia affected by the administration of both ritodrine hydrochloride and magnesium sulfate to her mother] [abstract] [in Japanese]. J. Jpn. Soc. Perin. Neon. Med. 22, 684 (2010).
Taniguchi, H. et al. Kou magunesiumu kessyou ni tomonai ikkasei kou kariumu kessyou wo teishita DD soutai rei [Transient hyperkalemia with hypermagnesemia in dichorionic diamniotic twins] [abstract] [in Japanese]. Acta paediatrica Japonica. 114, 1949 (2010).
Uchida, N. et al. Kou maguneshiumu kessyou ni tomonau hi bounyou sei kou kariumu kessyou wo kurikaeshita souzan ji rei [Late preterm infant with repeated non-oligouretic hyperkalemia associated with hypermagnesemia] [in Japanese]. Japanese Journal of Pediatrics. 64, 133–136 (2011).
Kaneko, T., Kobayashi, R., Usuda, T. & Wada, M. Kou kariumu (K) kessyou, shinshitsu hinpaku (VT) wo hassyoushita seikisan ji no ichi rei [Hyperkalemia followed by ventricular tachycardia in term neonate: a case report] [abstract] [in Japanese]. J. Jpn. Soc. Perin. Neon. Med. 48, 467 (2012).
Nabeshima, K., Shigehara, K., Furukawa, N. & Tokuda, S. Kou K kesshou to RAA kei no koushin wo mitometa long-term tocolysis go no late preterm ji 2 rei [Two late preterm infants with hyperkalemia, increased levels of plasma renin and serum aldosterone born from mother with long-term tocolysis with both ritodrine hydrochloride and magnesium sulfate] [abstract] [in Japanese]. J. Jpn. Soc. Perin. Neon. Med. 48, 516 (2012).
Tanaka, K. et al. Kou maguneshiumu kessyou ni tomonai syussei tyokugo kara kou kariumu kessyou wo hassyoushita goku teisyussyou taijuu ji no 1 rei [A very low birth weight infant with hyperkalemia just after birth accompanied by hypermagnesemia: a case report] [abstract] [in Japanese]. J. Jpn. Soc. Perin. Neon. Med. 25, 514 (2013).
Aoki, K., Matsuuchi, S. & Akaba, K. Souzan ji ni okeru botai ryuusan maguneshiumu shiyou to shinseiji kou kariumu kessyou tono kankei [Effects of antenatal magnesium sulfate on early neonatal hyperkalemia in preterm infants] [in Japanese]. J. Jpn. Soc. Perin. Neon. Med. 54, 1037–1042 (2018).
Tanaka, K. et al. Early-onset neonatal hyperkalemia associated with maternal hypermagnesemia: a case report. BMC Pediatr. 18, 55 (2018).
FDA Drug Safety Communication: New warnings against use of terbutaline to treat preterm labor. https://www.fda.gov/Drugs/DrugSafety/ucm243539.htm (2011).
FDA Drug Safety Communication: FDA Recommends Against Prolonged Use of Magnesium Sulfate to Stop Pre-term Labor Due to Bone Changes in Exposed Babies. https://www.fda.gov/Drugs/DrugSafety/ucm353333.htm (2013).
Restrictions on use of short-acting beta-agonists in obstetric indications – CMDh endorses PRAC recommendations. https://www.ema.europa.eu/news/restrictions-use-short-acting-beta-agonists-obstetric-indications-cmdh-endorses-prac-recommendations (2013).
Oushu ni okeru tanjikan sayou gata beta shigeki yaku ni taisuru sochi oyobi nihon ni okeru utemerin (tyuu, jou) no yuukousei, anzensei ni tsuite [A review of EU restrictions on short-acting beta-agonists, and guidelines regarding efficacy and safety of ritodrine hydrochloride (injection and tablet) in Japan] [in Japanese]. http://di.kissei.co.jp/vcms_lf/re247001.pdf (2014).
Minakami, H. et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J. Obstet. Gynaecol. Res. 40, 1469–1499 (2014).
Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2017 edition [in Japanese]. http://www.jsog.or.jp/activity/pdf/gl_sanka_2017.pdf (2017).
Takagi, K. & Satoh, T. Is long-term tocolysis effective for threatened premature labour? J. Int. Med. Res. 37, 227–239 (2009). Multicentre Premature Labour Study Group.
Witlin, A. G. & Sibai, B. M. Magnesium sulfate therapy in preeclampsia and eclampsia. Obstet. Gynecol. 92, 883–889 (1998).
Magsent Injection Syringe 40 mL [Package insert]. https://pins.japic.or.jp/pdf/newPINS/00051281.pdf (2014).
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 171: Management of Preterm Labor. Obstet. Gynecol. 128, e155–e164 (2016).
Shinohara, S., Sunami, R., Uchida, Y., Hirata, S. & Suzuki, K. Association between total dose of ritodrine hydrochloride and pulmonary oedema in twin pregnancy: a retrospective cohort study in Japan. BMJ Open. 7, e018118 (2017).
Nakamura, M. et al. Comparison of perinatal outcomes between long-term and short-term use of tocolytic agent: a historical cohort study in a single perinatal hospital. J. Obstet. Gynaecol. Res. 42, 1680–1685 (2016).
Dudley, D., Gagnon, D. & Varner, M. Long-term tocolysis with intravenous magnesium sulfate. Obstet. Gynecol. 73, 373–378 (1989).
Kawagoe, Y., Sameshima, H., Ikenoue, T., Yasuhi, I. & Kawarabayashi, T. Magnesium sulfate as a second-line tocolytic agent for preterm labor: a randomized controlled trial in Kyushu Island. J. Pregnancy. 2011, 965060 (2011).
Utemerin injection 50 mg [Package insert]. https://di.kissei.co.jp/dst01/pdf/di_uti19.pdf (2019).
Emder, P. & Crawford, G. Ventricular tachycardia in a neonate secondary to hyperkalaemia. Aust. Paediatr. J. 19, 112–113 (1983).
Bara, M., Guiet-Bara, A. & Durlach, J. Regulation of sodium and potassium pathways by magnesium in cell membranes. Magnes. Res. 6, 167–177 (1993).
Mahajan, G., Mukhopadhyay, K., Attri, S. & Kumar, P. Neurodevelopmental outcome of asymptomatic hypoglycemia compared with symptomatic hypoglycemia and euglycemia in high-risk neonates. Pediatr. Neurol. 74, 74–79 (2017).
McIntyre, S. et al. A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev. Med. Child. Neurol. 55, 499–508 (2013).
Montassir, H. et al. Associated factors in neonatal hypoglycemic brain injury. Brain Dev. 31, 649–656 (2009).
Seltzer, H. S. Drug-induced hypoglycemia. A review of 1418 cases. Endocrinol. Metab. Clin. North. Am. 18, 163–183 (1989).
Musci, M. N. Jr., Abbasi, S., Otis, C. & Bolognese, R. J. Prolonged fetal ritodrine exposure and immediate neonatal outcome. J. Perinatol. 8, 27–32 (1988).
Trønnes, H., Wilcox, A. J., Lie, R. T., Markestad, T. & Moster, D. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: a national cohort study. Dev. Med. Child. Neurol. 56, 779–785 (2014).
Ellenberg, J. H. & Nelson, K. B. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev. Med. Child. Neurol. 55, 210–216 (2013).
Phelan, J. P., Korst, L. M. & Martin, G. I. Application of criteria developed by the Task Force on Neonatal Encephalopathy and Cerebral Palsy to acutely asphyxiated neonates. Obstet. Gynecol. 118, 824–830 (2011).
May, T., Adesina, I., McGillivray, J. & Rinehart, N. J. Sex differences in neurodevelopmental disorders. Curr. Opin. Neurol. 32, 622–626 (2019).
May, T., Sciberras, E., Brignell, A. & Williams, K. Autism spectrum disorder: updated prevalence and comparison of two birth cohorts in a nationally representative Australian sample. BMJ Open. 7, e015549 (2017).
Wang, T. et al. Prevalence of attention deficit/hyperactivity disorder among children and adolescents in China: a systematic review and meta-analysis. BMC Psychiatry. 17, 32 (2017).
Linsell, L., Malouf, R., Morris, J., Kurinczuk, J. J. & Marlow, N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: a systematic review. Dev. Med. Child. Neurol. 58, 554–569 (2016).
Zhao, M., Dai, H., Deng, Y. & Zhao, L. SGA as a risk factor for cerebral palsy in moderate to late preterm infants: a system review and meta-analysis. Sci. Rep. 6, 38853 (2016).
Lampi, K. M. et al. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J. Pediatr. 161, 830–836 (2012).
Heinonen, K. et al. Behavioural symptoms of attention deficit/hyperactivity disorder in preterm and term children born small and appropriate for gestational age: a longitudinal study. BMC Pediatr. 10, 91 (2010).
Takeda, S. et al. Houkoku: Syuusanki iinkai [Annual Report: Perinatal Medicine Committee] [in Japanese]. Acta Obstet. Gynaecol. Jpn. 68, 1381–1403 (2016).
Committee on Fetus and Newborn. Adamkin D.H. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics. 127, 575–579 (2011).
Vemgal P., Ohlsson A. Interventions for non-oliguric hyperkalaemia in preterm neonates. Cochrane Database Syst. Rev. (1), CD005257 (2007).
Ishii, N., Kono, Y., Yonemoto, N., Kusuda, S. & Fujimura, M. Neonatal Research Network, Japan. Outcomes of infants born at 22 and 23 weeks’ gestation. Pediatrics. 132, 62–71 (2013).
Itabashi, K. et al. Atarashii zaitai kikan betsu shussei ji taikaku hyoujun ti no dounyuu ni tsuite [Introduction of new gestational age-specific standards for birth size] [in Japanese]. Acta paediatrica Japonica. 114, 1271–1293 (2010).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Acknowledgements
The authors did not receive any funding relevant to this article to disclose. However, this study was supported by the JSPNM. The supporting society had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The suggestion that some cases of cerebral palsy might have occurred in neonates with hypoglycemia and/or hyperkalemia who were born to mothers with either ritodrine or MgSO4 was provided by Takashi Okai, Chairman of the Cause Analysis Committee for Cerebral Palsy of the JCQHC, who recently passed away. We are grateful to Mr. Adam Lebowitz (General Studies Department, Jichi Medical University School of Medicine, Tochigi, Japan) for checking the revised manuscript. The authors would like to thank all of the participating institutions and patients involved in the study for their valuable contributions.
Author information
Authors and Affiliations
Consortia
Contributions
Yukari Yada (Y.Y.), Akihide Ohkuchi (A.O.), Katsufumi Otsuki (K.O.), Keiji Goishi (K.G.), Mari Takahashi (M.T.), Naohiro Yonemoto (N.Y.), Shigeru Saito (S.S.), and Satoshi Kusuda (S.K.) are main authors. A.O. wrote the main manuscript text and prepared figure and all tables. Y.Y., A.O., K.O., K.G., M.T., S.S., and S.K. designed this study. Y.Y., A.O., K.O., K.G., M.T., S.S., and all researchers listed in Consortia: Authors’ list for the Survey Group Studying the Effects of Tocolytic Agents on Neonatal Adverse Events in Japan Society of Perinatal and Neonatal Medicine contributed acquisition of data. Y.Y., A.O., K.O., K.G., S.S., and S.K. firstly performed statistical analyses, N.Y. secondly checked the statistical results in view of a professional statistician, and A.O. performed the final statistical analysis using IBM SPSS Statistics version 25 and EZR version 1.37. All main authors assisted with analysis, and interpretation of data. All main authors reviewed the manuscript, and had an important role on critical revision of the manuscript. S.K. supervised this study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yada, Y., Ohkuchi, A., Otsuki, K. et al. Synergic interaction between ritodrine and magnesium sulfate on the occurrence of critical neonatal hyperkalemia: A Japanese nationwide retrospective cohort study. Sci Rep 10, 7804 (2020). https://doi.org/10.1038/s41598-020-64687-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64687-w
This article is cited by
-
Ritodrine-induced rhabdomyolysis and psychiatric symptoms: a case report and literature review
BMC Pregnancy and Childbirth (2023)
-
Neonatal rebound hyperkalemia associated with ritodrine alone: a case report
BMC Pediatrics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.