Abstract
Besides providing food and shelter to natural enemies of crop pests, plants used in conservation biological control interventions potentially provide additional ecosystem services including providing botanical insecticides. Here we concurrently tested the strength of these two services from six non-crop plants in managing cabbage pests in Ghana over three successive field seasons. Crop margin plantings of Ageratum conyzoides, Tridax procumbens, Crotalaria juncea, Cymbopogon citratus, Lantana camara and Talinum triangulare were compared with a bare earth control in a three-way split plot design such that the crop in each plot was sprayed with either a 10% (w/v) aqueous extract from the border plant species, a negative control (water) and a positive control (emamectin benzoate ‘Attack’ insecticide). Pests were significantly less numerous in all unsprayed treatments with non-crop plant margins and in corresponding sprayed treatments (with botanical or synthetic insecticide positive control) while treatments with bare earth margin or sprayed with water (negative controls) had the highest pest densities. Numbers of predators were significantly depressed by synthetic insecticide but higher in other treatments whether unsprayed or sprayed with botanical insecticide. We conclude that some plant species have utility in both conservation biological control and as source of botanical insecticides that are relatively benign to natural enemies. In this crop system, however, the additional cost associated with using botanical insecticides was not justified by greater levels of pest suppression than achieved from border plants alone.
Similar content being viewed by others
Introduction
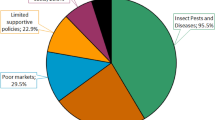
Insect pest attack causes huge crop losses1 affecting the potential availability of food for over one billion people2, and threatening global food security3. Effective pest management is critical to meeting global food demands4,5,6 and synthetic insecticides have been the principal tool in the past six decades7,8. Even with the introduction of newer and relatively safer insecticides8 and increased application per unit area and time9, crop loss resulting from insect damage has doubled in the past four decades1,10,11. Indiscriminate and excessive application has led to the elimination of important organisms in the agroecosystem that support crop production12,13. This is especially prevalent in developing countries including Ghana where farmers and the environment are exposed to high levels of synthetic insecticides, including active ingredients that have been banned in developed nations due to high toxicity and persistence14. Inadequate provision of important ecosystem services including natural enemy-mediated pest control reinforces reliance on hazardous inputs such as insecticides and threatens agricultural sustainability15,16.
Conservation biological control based on habitat manipulation has been proposed as a sustainable alternative to synthetic insecticides17. Habitat manipulation involves intentionally establishing plant species at the farm scale or landscape scale to provide conducive habitats, floral resources and alternative prey at the right time and space to support natural enemy assemblages to promote biological pest suppression18,19. This approach to conservation biological control can result in other benefits besides insect-mediated pest suppression, such as pollination15 and provisioning botanical insecticides, herbal medicines, spices and indigenous food20,21. Insecticides obtained from plants (‘botanicals’) have been documented to be cost-effective and sustainable in managing insect pests including those that have developed resistance to synthetic insecticides22,23,24,25. Botanical insecticides cause minimal harm to predators and parasitoids because they usually exhibit actions such as repellence, anti-feeding, oviposition deterrence rather than toxicity26. Whilst botanicals can exhibit toxicity, they tend to pose minimal environmental impact when used in the crude form at low concentrations26 as when used as ‘home-made’ products by smallholder farmers. Furthermore, botanicals break down easily upon exposure to air and sunlight, so are more compatible with ecologically-based pest management systems such as conservation biological control27,28,29.
Integrated pest management (IPM) combines various pest management tactics as much as practicable to maintain pest population below the economic injury level in a sustainable manner4,30. The integration of two or more pest management tactics may deliver better long term pest suppression than one tactic in isolation30. However, the combination of the various tactics must not result in major trade-offs or have lethal and/or sub-lethal effects on non-target organisms especially natural enemies that form the core of conservation biological control16,31.
Although both conservation biological control18,19 and botanicals23,24,32,33 have been successfully used in managing field pests in separate applications, combining the two tactics requires careful assessment of their respective and combined effects on pest and natural enemies34 as well as the overall impacts on crop yield and quality. In addition, there is an expected increased cost of plant protection with two pest management tactics and thus, an economic analysis must be done to ensure the viability and profitability for such interventions.
A number of studies have explored the effect of synthetic insecticides on biological control agents and report varying effects on natural enemies e.g.34,35,36. Yet, few studies have reported on impacts of synthetic insecticides on both pests and natural enemies as well as on crop yield e.g.37,38. To date, the value of conservation biological control especially where it is integrated with pesticides is unclear38 and no study has either combined habitat manipulation for conservation biological control and the use of botanicals, or analyzed the economic implications of combining these tools.
We address this gap by studying over three consecutive seasons the effects of six non-crop plant species that are native or naturalized in Ghana and readily available to smallholder farmers. Ageratum conyzoides, Tridax procumbens (Asteraceae), Crotalaria juncea (Fabaceae), Cymbopogon citratus (Poaceae), Lantana camara (Verbenaceae) and Talinum triangulare (Talinaceae) were tested in field experiments. Each was used as a crop margin habitat manipulation plant to promote conservation biological control by providing floral resources as well as shelter for natural enemies and source of botanicals in a factorial design to quantify the combined and individual effects of botanicals and conservation biological control on three major pests of cabbage, diamondback moth, Plutella xylostella L. (Lepidoptera: Plutellidae), cabbage aphid, Brevicoryne brassicae L. (Hemiptera: Aphididae) and cabbage web worm, Hellula undalis Fab. (Lepidoptera: Crambidae) as well as two predators, spiders (Araneae) and ladybird beetles (Coccinellidae).
Results
Effects of habitat manipulation and spraying on P. xylostella
In all the three seasons, habitat manipulation had significant (P < 0.05) effects on mean numbers of P. xylostella per plant. All treatments with habitat manipulation plants were significantly better than the no plant in all weeks (Tables 1, 2 and 3). Both botanicals and Attack were not different from each other in season one but were both significantly lower in P. xylostella numbers than the tap water control (Table 1). In week one of season two, botanicals significantly reduced P. xylostella numbers better than both Attack and tap water which were not different from each other (Table 2). However, in week two, botanicals and Attack performed equally but both were significantly better than the tap water control. In week three, Attack reduced P. xylostella numbers significantly better than both botanicals and tap water which were not different from each other. In week four, Attack as significantly better than both botanicals and tap water whilst botanical was also significantly better than the tap water (Table 2). In week two and four of season three, botanicals and Attack were not significantly different from each other but were both better than the tap water (Table 3). But in week one, botanicals gave significantly lower numbers of P. xylostella compared with both Attack and tap water which were not significantly different from each other. However, in week three, Attack was significantly lower than both botanicals while botanicals were also better than the tap water with mean P. xylostella numbers of 0.18, 0.46 and 0.79 per plant for Attack®, botanicals and tap water respectively (Table 3).
Effects of habitat manipulation and spraying on B. brassicae
Habitat manipulation had a significant effect on B. brassicae infestation severity in week one, three and four in season one. Habitat manipulation with T. procumbens was significantly lower (P = 0.011, F = 3.05.68, df = 6, 60) in B. brassicae score than habitat manipulation with A. conyzoides and the no plant control but not different from the remaining treatments. In week three, all habitat manipulation treatments with plants were significantly lower (P = 0.001, F = 13.12, df = 6,60) in B. brassicae score than the no plant control treatment. Habitat manipulation with T. procumbens and C. juncea were lower in B. brassicae than all other treatments. Spraying had an effect only in week three where Attack was significantly (P = 0.001, F = 13.96, df = 2,60) lower than botanical while botanical was significantly lower than tap water in B. brassicae score. Habitat manipulation had significant effect of B. brassicae score in all weeks in season two but no effect of spraying was observed. In week one, the no habitat manipulation had significantly higher (P = 0.032, F = 2.48, df = 6,60) B. brassicae score than the remaining treatments except habitat manipulation with L. camara and C. citratus. All habitat manipulation treatments with non-crop strips were significantly (P = 0.001, F = 5.44, df = 6,60) lower in B. brassicae score than the no plant manipulation except plots with C. juncea and L. camara. In weeks three and four, the no plant control was higher in B. brassicae numbers than the habitat manipulation treatments with plant strips. No effects of habitat manipulation and spraying were observed throughout season three.
Effect of both habitat manipulation and spraying on H. undalis were found only in the first week of season two. Habitat manipulation with C. juncea, C. citratus and T. triangulare were significantly (P = 0.001, F = 4.15, df = 6,60) lower than the remaining treatments.
From Table 4, there were significant interactions between week and both habitat manipulation and spraying and necessitated Tukey’s post-hoc (HSD) to differentiate which weeks combined with the factor levels differ from each other.
Interaction between habitat manipulation and spraying resulted in a significant (P < 0.05) effect on numbers of P. xylostella. The no plant, water-sprayed (‘Nopwater’) treatment was significantly (P < 0.05) higher in numbers of P. xylostella in all seasons (Fig. 1), two (Fig. 2) and three (Fig. 3) except in week four of season two where it was not significantly different from the Crotalaria-bordered, water-sprayed (‘Crotwater’) treatment.
The Ageratum-bordered, synthetic insecticide (Attack)-sprayed (‘AgeraAttack’) treatment combination was significantly (P < 0.05) higher in B. brassicae score in week one than the remaining treatments except Crotwater and Nopwater. In week two, the Nopwater treatment was significantly higher than the remaining treatments (Fig. 4). In season two week one, Nopwater was significantly higher than the remaining treatments in B. brassicae infestation score except in week two when it was not higher than Crotwater (Fig. 5).
Effect of treatments on predators
Interaction between habitat manipulation and spraying resulted in a significant effect only in week three of season two in which the Nopwater was significantly (P < 0.05) higher in spider numbers than the remaining treatments (Fig. 6). In season one Nopwater was significantly (P < 0.05) higher in ladybird beetle numbers than the remaining treatment combinations (Fig. 7).
Effect of treatments on cabbage head yield and quality
There were significant interactions between habitat manipulation and spraying. In season one, the highest yield per plant was observed in LanAttack treatment but was not significantly (P > 0.05) better than any of the habitat manipulation and botanical insecticide combinations except Agerabot (Table 5). In season two, TalAttack had the highest yield per plant and was better than eight treatments including Nopwater. In season three, Talwater had the highest yield per plant and was significantly (P < 0.05) better than only one (Crotbot) habitat manipulation and botanical insecticide combinations, four habitat manipulations plus Attack combinations, four habitat manipulations only treatments and the no habitat manipulation treatment (Nopwater) (Table 5). NopAttack had the highest yield per hectare in season one and was significantly (P < 0.05) better the remaining treatments except LanAttack, Lanbot, Cymbot and Lanwater (Table 6). The Nopwater had the highest damaged yield per hectare in seasons one and two. It was significantly (P < 0.05) higher than the remaining treatments except in season two when it was not significantly different from Crotwater (Table 6). TalAttack was the highest in undamaged yield per hectare in season two and was significantly (P < 0.05) better than ten treatments including the Nopwater which had the lowest undamaged yield per hectare (Table 6). In season three, Lanwater was the highest in yield per hectare and was significantly (P < 0.05) better than nine treatments including the Nopwater (Table 6).
Economic analyses
Cost of plant protection and income
Costs of plant protection ranged between $70.40 and $257.40 per hectare (Table 7). The habitat manipulation-only treatments were lowest whereas the habitat manipulation plus Attack treatments were the highest. In season one, the NopAttack had the highest income from undamaged yield and highest net income per hectare while the Nopwater was the lowest in income from undamaged yield per hectare. Among the habitat manipulation treatments, LanAttack was the highest in both incomes from undamaged yield and net income per hectare. In each habitat manipulation combination, the tap water spraying options were the lowest in income from undamaged income (Table 7). In season two, TalAttack was the highest in both incomes from undamaged yield and net income per hectare whilst the lowest was the Nopwater. In season three, Talwater had the highest income from undamaged heads as well as net income per hectare whereas the Nopwater was the lowest in income from undamaged heads but CrotAttack was the lowest in net income per hectare (Table 7).
Cost: benefit ratios
In season one, cost: benefit ratios ranged between 1: 8.15 and 1: 49.00 for CrotAttack and Lanwater respectively. In season two, ratios between 1: 4.01 and 1: 36.3 for Crotbot and Agerawater respectively were obtained while in season three, CrotAttack and TriAttack recorded negative ratios of 1: −0.84 and 1: −2.23 respectively. The highest cost: benefit ratio was obtained in Talwater 1: 35.67 (Table 7).
Discussion
Both habitat manipulation with the non-crop plants and spraying contributed to pest suppression in this study, with the effects cascading on improved cabbage head yield and quality, overall net income, and cost: benefit ratios in all seasons. The no plant manipulation control sprayed with Attack (‘NopAttack) suppressed pests and resulted in the highest yield in the first season but was subsequently outperformed by some of the habitat manipulation treatments. In contrast, Nopwater showed high numbers of pests resulting in reduced yield and quality of cabbage in all the three seasons. The various non-crop plants exhibited different potential in supporting conservation biological control and as botanical insecticides. Higher cost: benefit ratios were exhibited by the treatments especially habitat manipulation without spraying options. Habitat manipulation with non-crop strips plus spraying with Attack had a strong additive effect in season one but this was reduced in season two and subsequently in trade-offs in season three.
This study demonstrated the potential of exploiting the dual ecosystem services of botanical insecticides and conservation biological control for managing brassicas pests. Habitat manipulation for conservation biological control, however, was more potent in suppressing pests compared with the use of botanicals of the same plant species. Habitat manipulation with most of the plant species sprayed with tap water had lower numbers of pests comparable with habitat manipulation sprayed with botanicals or Attack. For instance, habitat manipulation with T. triangulare and spraying with both tap water and botanicals gave low numbers of pests even though aqueous extracts of T. triangulare was found to be inactive against P. xylostella and B. brassicae in an earlier lab bioassay (Amoabeng et al., unpublished report). This suggests that natural enemies might be exclusively responsible for pest suppression in those treatments and also indicating the potential of T. triangulare in habitat manipulation for conservation biological control. In contrast, habitat manipulation with C. juncea and sprayed with botanical from the same plant as well as no spraying had higher pest infestation than C. juncea plots sprayed with Attack. Attack was thus, likely responsible for the low pest numbers. Crotalaria juncea was not a good candidate for habitat manipulation for conservation biological control compared with the remaining five plant species.
Pests encountered in the present study are among the most destructive herbivores that cause problems to brassica production worldwide. Plutella xylostella, the most damaging among them is difficult to manage globally39,40. Hellula undalis and B. brassicae also pose serious challenge to brassicas crops41. For decades, these pests have been managed with the use of synthetic insecticides resulting in various ecological concerns42,43. Suppression of cabbage pests with natural enemies has been found to give better pest control, high and quality crop yield compared with application of synthetic insecticides7,37,44. A study on cabbage root fly Delia radicum (Diptera: Anthomyiidae) showed a reduced number of pupae and larvae of the pest in habitat manipulation treatments compared with the no manipulation control45. Another experiment that assessed impact of flower strips on cabbage moth Mamestra brassicae L. (Lepidoptera: Noctuidae) and cabbage white butterfly, Pieris rapae L. (Lepidoptera: Pieridae) Pfiffner et al.46 found that parasitism of M. brassicae and predation of P. rapae were enhanced in flower strip treatments compared with the no flower strip control.
Botanicals too have been successful against pests of brassica crops e.g.22,23,24,32. Concurrent use of conservation biological control with botanical insecticides may result in better pest suppression compared with each of them in isolation30. Habitat manipulation and botanical insecticides played a complementary role in achieving pest suppression. The potential of each plant in supporting natural enemies for conservation biological control and as botanical insecticide is therefore key to maximising the potential benefits of dual pest management services. Habitat manipulation with C. juncea plus Attack resulted in an effective suppression of pests compared with habitat manipulation with C. juncea and sprayed with extract of C. juncea. On the other hand, habitat manipulation with A. conyzoides sprayed with either Attack or extract of A. conyzoides both resulted in effective pest suppression and in some cases the aqueous extract spray option performed better than the Attack.
Pests suppression in this study highlights the potential role of predators in conservation biological control. Predators have been acknowledged to effectively suppress pests in managed agroecosystems7,47 even though parasitoids have often been the focus of biological control of brassica crop pests31,48. Spiders and ladybird beetles were generally more numerous in the habitat manipulation plus botanical insecticides and habitat manipulation plus tap water treatments compared with habitat manipulation plus Attack treatments indicating the potential negative impact of Attack on predators. A study in Nicaragua found that plots sprayed with synthetic insecticides had low numbers of generalist predators including spiders compared with a no insecticides application treatment7. The current study therefore supports the notion that aqueous plant extracts are relatively harmless to natural enemies49,50,51 and can be integrated with biological pest control unlike synthetic insecticides such as Attack which has deleterious effects on natural enemies. This insecticide (Attack) is a semi-synthetic derivative of the natural product abamectin in the avermectin family52,53. It is considered to have minimal effect on predators and parasitoid because it degrades rapidly through sunlight on the leaf surface resulting in shorter contact periods in the crop environment52. Some studies have found Attack® less harmful to natural enemies than other synthetic insecticides and therefore recommend its inclusion in integrated pest management programmes e.g.51,54,55. Other studies have, however, found it to have negative impacts on natural enemies e.g.32,56,57,58. In assessing the acute and sub-lethal toxicity of 14 pesticides on the generalist predator Orius laevigatus (Hemiptera: Anthocoridae), Biondi et al.59 found Attack to be moderately harmful until seven days after spraying. Khan et al.57 and Biondi et al.59 cautioned that the inclusion of insecticides such as Attack in IPM programmes should carefully be evaluated. In the present study, treatments sprayed with Attack had very low numbers of spiders and ladybird beetles.
Numbers of predators were higher in the no habitat manipulation sprayed with tap water (control) compared with the habitat manipulation treatments where floral resources and shelters were available. This might have happened because natural enemies moved between plots and showed aggregative numerical responses to where prey were most abundant. Monsrud and Toft60 reported that, aggregative numerical response occurs when predators respond to increasing number of prey or are attracted to alternate prey that aggregate around honeydew produced as a result of aphid feeding. In a three-year study on effect of floral resources on cabbage root fly D. radicum, Nilsson et al.61 found that while there was an overall increase in hymenopteran parasitoid catches in habitat manipulation treatments, parasitism was higher in the control treatment where pest infestation was higher compared with the habitat manipulation treatments. The study further explained that parasitoids may feed on floral resources but also needed prey to complete their life cycle, hence, the high parasitism rate in areas with high pest infestation. Donaldson et al.62 reported that numbers of predators increased in response to increasing population of soybean aphid and decreased when aphid population decreased. In the present study, aggregative numerical responses could have occurred as there was high B. brassicae infestation in the no plant habitat manipulation treatment.
The various plant species showed differences in supporting conservation biological control resulting in differences in crop yield. Borders of T. triangulare gave higher cabbage yields compared with, for example, C. juncea. Floral morphology, colour and duration of bloom may affect the potential of various plant species to support the activities of natural enemies for pest suppression as reported by Anderson and Dobson63 and Begum et al.64. Talinum triangulare for instance, is a perennial species with bright floral colours and persistent blooming and might have attracted natural enemies more effectively than other plant species. This result indicates that plant species selection is vital to maximising outcomes in conservation biological control interventions19.
Generally, there was improvement in yield and quality of cabbage season after season. This might be due to enhanced local densities of predators and possibly local suppression of pests. Similar results have been reported when cabbage pests were managed using IPM practices in which high marketable yields were obtained as a results of enhanced natural enemy activities7,44. In season one, yields of each habitat manipulation treatment sprayed with either botanical insecticides or Attack resulted in higher yields compared with habitat manipulation without spraying. However, in subsequent seasons, yield in some of the sole conservation biological control treatments were higher than treatments with both conservation biological control and spraying. This could be due to an additive effect of conservation biological control and spraying on cabbage yield in season one and trade-offs in latter seasons. Trade-offs between biological control and the application of insecticides have been reported7,44. Habitat manipulation for conservation biological control could be complemented with the application of biological pesticides such as botanical insecticides during the early stages of the season when numbers of natural enemies may not be enough to keep pests below what could cause economic injury to crops. Activity of natural enemies cannot, however, be ruled out in season one as all habitat manipulation treatments outperformed the no habitat manipulation treatments in both yield and quality.
Though efforts were made to reduce inter-plot interference, the 5 m alley between treatments was small compared with the movement patterns of agricultural arthropods that can readily move meters. Consequently, treatments that are close to each other might have had effect on each other. However, the marked differences in yield and quality among the various habitat manipulation with plant treatments and the no plant manipulation as well as treatment with various spraying options indicate that the closeness of the plots did not entirely mask the potential of each of the habitat manipulation or spraying treatments.
Yields observed in the present study compare favourably with those obtained in similar studies using the same crop variety at the same site e.g.32,33 and in the same locality e.g.51 indicating that habitat manipulation for conservation biological control alone or in combination with botanical insecticides have the potential to effectively manage brassica crop pests.
In this study, habitat manipulation sprayed with tap water had the lowest cost of protection while habitat manipulation sprayed with Attack had the highest cost. For a pest management tactic to be adopted, output in terms of crop yield and quality and income from the sale of produce must be appreciable enough to offset the cost of protection and result in profit. It was observed that irrespective of the high cost of protection for habitat manipulation plus spraying treatments (both botanical and Attack) they had higher net incomes compared with their corresponding habitat manipulation without spraying treatments in season one. This was the result of a high yield and quality in all habitat manipulation plus spraying treatments compared with treatments with only habitat manipulation. But in seasons two and three most of the habitat manipulation plus tap water and habitat manipulation plus botanicals recorded higher incomes than the habitat manipulation and Attack treatments. This may have resulted from the overall improvement in yield and quality of the habitat manipulation plus tap water and habitat manipulation plus botanical treatments.
Incomes were generally higher in season one for all treatments than in season two because prices of undamaged cabbage heads were 25% higher in season one than in season two. However, incomes in season three were higher than the two previous seasons even though the price of cabbage heads was the same as in season two. This was due to an increase in cabbage yields in season three compared with season two. In addition, there were no damaged yields in season three, meaning all yields attracted the normal market value thereby demonstrating that profitability is a factor of cost of production, quality and quantity of yield as well as the market value of produce at any given time65,66.
Cost: benefit ratios were higher in season one and declined towards season three. This was because income from the control treatment was lowest in season one and increased towards season three. This was due to enhanced yield and quality of cabbage which might have resulted from possible movement of natural enemies from habitat manipulation plots to control plots (Griffith45,67). Cost: benefit ratios in this study were calculated based on income from the control treatment hence, increase in yield and income of the control treatment reduced the cost: benefit ratio.
Cost: benefit ratios show the economic viability and biological effectiveness of pest management interventions in relation to the control66,68. Positive ratios denote economic viability whilst negative ratios mean the pest management tactic was not cost-effective. The highest cost: benefit ratio obtained in this study is superior to those attained in other studies while the lowest is below those obtained in those studies e.g.65,66,69. In all these studies, only cost of plant protection was used in calculating the ratios, and income from the control was the reference point.
In the present study, cost: benefit ratios ranged between 1: −2.23 for habitat manipulation with T. procumbens sprayed with Attack (TriAttack) in season three up to 1: 49 for Talwater in season one. While some treatments were strongly beneficial in all seasons, others were only beneficial in some seasons. NopAttack had cost: benefit ratios of 1: 33.50, 1: 9.81 and 1: 4.64 for seasons one, two and three respectively whereas Triwater had cost: benefit ratios of 1: 28.50, 1: 23.60 and 1: 17.84 for seasons one, two and three respectively. On the hand, TriAttack had cost: benefit ratios of 1: 20.04 and 1: 14.03 in season one and two respectively and 1: −2.23 in season three being negative, suggesting that combination of two pest management tactics may have additive effects resulting in positive cost: benefit ratios in early seasons but trade-offs may occur in latter seasons. In such instances, combination of tactics should only be encouraged when it is absolutely necessary to achieve optimum and cost-effective pest suppression.
Conclusions
The combination of habitat manipulation for conservation biological control and application of botanical insecticides can result in effective pest suppression with corresponding increase in crop yield, quality and income. The use of synthetic insecticides has always been a curative attempt to salvage crops from damage. However, it often leads to elimination of important organisms in the agroecosystem. Selection of insecticides to use alongside habitat manipulation should be done carefully to avoid negative impacts on natural enemies that are fundamental to successful conservation biological control. Similarly, the choice of plant species for habitat manipulation should cautiously be considered because not all plant species have the potential to provide floral resources for natural enemies. Further care should be taken if dual ecosystem services of botanical insecticides and conservation biological control are to be achieved. There is the possibility of trade-off between the provision of floral resources and insecticidal activity by the same plant species. There is evidence that compounds that have activity against insects may be found in the nectar of some plant species. In the current study, habitat manipulation plus tap water was more cost-effective than habitat manipulation plus spraying with botanical insecticides or Attack®. Combination of other compatible tactics with conservation biological control should be done only when it is indispensable to achieve ideal pest suppression. In such cases, inexpensive and ecologically benign insecticides such as botanicals should be preferred over synthetic insecticides such as Attack®.
Materials and methods
Location of experiments
Experiments ran over three consecutive seasons of cabbage Brassica oleracea var. capitata (Brassicaceae) production at the Crops Research Institute (CRI), Kwadaso, Kumasi, Ghana (6°43′N,1°36′W; 287 m asl) between January 2017 and March 2018. The three seasons were between June and August 2017 (major rainy season), September and November 2017 (minor rainy season) and December 2017 and March 2018 (dry season). The site was in the wet, semi-deciduous forest zone of Ghana with average rainfall of 1,484 mm in 137 wet days per annum. Average temperature is 25.6 °C in the range of 19.6 °C and 35 °C and average relative humidity of 83.2%.
Experimental design and treatments
Experiments consisted of a 7 × 3 factorial in a randomized complete block design with split plot arrangement and replicated four times. The main plot factor consisted of seven levels of habitat manipulation i.e. with six non-crop plant species and a no plant (control) i.e. 1. A. conyzoides 2. T. procumbens 3. C. juncea 4. C. citratus 5. L. camara 6. T. triangulare and 7. no plant (bare earth control) (Table 8). The sub-plot factor was three levels of spraying: 1. Botanical insecticide prepared from the same plant species as bordering the crop of that plot, 2. Conventional insecticide (Attack®, emamectin benzoate) as positive control, and 3. Negative control (tap water) (Table 8). Main plots measured 11 m × 3 m with 5m-wide bare earth alley between plots. Each main plot was divided into three sub-plots (3 m × 3 m) to accommodate the three levels of spraying with a 1m-wide bare earth alley between subplots. The ‘no plant’ habitat manipulation treatment did not have a botanical sub-plot treatment, thus there was a total of 20 treatments and 80 plots.
Habitat manipulation plants were established in the field between January and May 2017 to reach blooming stage (except C. citratus which does not flower) before transplanting of cabbage. Cabbage B. oleracea var. capitata (cv. Oxylus) seedlings were grown for five weeks after which they were transplanted to the field at a spacing of 0.5 m × 0.5 m. For the conservation biological control treatment with no border plant, seven rows of seven cabbage stands were planted per sub plot. Other treatments had six rows of seven cabbages per sub plot with the relevant border plant as a central strip with three rows of cabbage either side. This resulted in 42 plants per sub plot (49 for the control). Experiments were kept free from weeds by hand hoeing. Well decomposed poultry manure at 500 g per cabbage plant was applied 10 days after transplanting to enhance soil fertility. The experiment was rain-fed but manual irrigation was done when required, especially during the dry season.
Spraying and data collection
Each main plot had three sub-plots that received the three spraying levels i.e. botanical, Attack and tap water. Aqueous extracts of the plants were used at concentration of 10% w/v. Matured leaves of each of the six plants were collected from around the experimental area and weighed when fresh and blended using kitchen blender. Leaves were not collected from the habitat manipulation plots so as not to disturb natural enemies. In all cases, blending was done until no plant fragments were visible to the naked eye22. Mixtures were sieved using muslin cloth to obtain a particle-free extract and was transferred into a 15 L capacity Knapsack sprayer for immediate application32. A 1 ml Sunlight liquid soap per litre of plant extract was added to the aqueous extracts prior to spraying. Attack was used at the recommended rate of 1.5 ml per litre. Spraying was done weekly beginning from three weeks after transplanting cabbage seedlings and continued for four consecutive weeks in each season.
Numbers of pests including diamondback moth Plutella xylostella L. (Lepidoptera: Plutellidae), cabbage aphid Brevicoryne brassicae L. (Hemiptera: Aphididae) and cabbage webworm Hellula undalis Fab. (Lepidoptera: Crambidae), and natural enemies including spiders (Araneae) and ladybird beetles (Coccinellidae) were assessed weekly, three weeks after transplanting cabbage seedlings and a day before spray application for four consecutive weeks. Four plants were randomly selected in each sub-plot to count pest and natural enemy numbers. Numbers of B. brassicae were often difficult to count so were scored on a scale of 0–5 (0 = no infestation and 5 = highest infestation i.e. large continuous colonies)32.
Economic analysis
Field establishment of non-crop plants for habitat manipulation
The number of minutes used in gathering and planting the conservation biological control border plants for each plot was recorded and extrapolated to assume a commercial crop area of one hectare. Values varied according to how easily each was acquired and established. At the time of the study, one person-day at the location of the experiments costed US$ 8.80. Lantana camara was established using stem cuttings and required nursing before field establishment so 11 person-days were calculated to be required to establish L. camara for a cabbage crop area of one hectare resulting in US$ 96.80. Cymbopogon citratus established easily, but it was relatively difficult to get the propagules due to its popular use as anti-malaria herb in Ghana. Therefore, 10 person-days were required to gather and establish costing US$ 88.00. Seedlings were used to establish C. juncea and to ensure good stand, replacement of dead seedlings was required, hence 10 person-days at US$ 88.00 were required to establish a crop area of one hectare. Ageratum conyzoides and T. procumbens were the most abundant weed species around the experimental area and were relatively easy to gather so 8 person-days valued at US$ 70.40 for each. Talinum triangulare was the easiest to establish but was relatively difficult to gather due to its use as an indigenous vegetable in Ghana. Therefore, 9 person-days at US$ 79.20 was required to establish it. For these border plant treatments, the cost of plant protection was solely the cost of collecting and establishment.
Estimating cost of spraying
In each of the seasons, there were four spray applications of aqueous extracts and emamectin benzoate (Attack) to the respective sub plots and the costs of these interventions were extrapolated to assume a commercial crop of one hectare. Simple knapsack sprayers are used on farms in Ghana so 3 person-days were required to spray one hectare of cabbage. Thus, 12 person-days (amounting $ 105.60) were required for the four spray applications per season. The no habitat manipulation sprayed with Attack treatment required, 3.2 person-days to spray one hectare. Thus, 12.8 person-days at $112.64 was required for each season. The difference was due the higher plant stand compared with the habitat manipulation treatments. Collecting plant materials and preparing aqueous plant extracts for each habitat manipulation and botanical treatment required 2 person-days per hectare. Cost of volume of Attack required to spray one hectare was $15.00. Thus, for each season $60.00 of Attack was required to spray the no habitat manipulation plus Attack treatment whilst $55.00 worth of Attack was required to spray the habitat manipulation plus Attack treatments. The reduction in the amount required for Attack was due to the reduced plant stand in the habitat manipulation plus Attack treatments. Sunlight liquid soap at $1.00 for each habitat manipulation and botanical insecticide treatment per season was used. Thus, cost of plant protection for habitat manipulation and spraying treatment combinations = cost of establishing non-crop plants + labour cost of collecting and preparing botanical extracts + labour cost of spraying + cost of sunlight liquid soap. For habitat manipulation sprayed with Attack, cost of protection = cost of establishing non-crop plants + cost of Attack + labour cost of spraying. For no habitat manipulation sprayed with Attack, cost of protection = Cost of Attack + labour cost of spraying.
Computing cost: benefit ratios
Total income was obtained by adding incomes from both undamaged and damaged heads. Income from undamaged yield was obtained by multiplying the head yield per hectare by the selling price per kg of cabbage head. Income from damaged heads was obtained by multiplying damaged head yield by selling price per kg of damaged heads. Net benefit per hectare for each treatment was derived by subtracting the total cost of plant protection from total income of each treatment. Benefit over the no habitat manipulation and spraying control was obtained by subtracting the income of the control treatment from that of each habitat manipulation or habitat manipulation + spraying treatment. The cost: benefit ratio of each treatment was derived by subtracting the income of the control treatment from the net income of each treatment and the products were divided by total cost of plant protection for each treatment65.
Yield assessment and marketing of cabbage heads
Cabbage heads in each treatment were separated into undamaged and damaged heads and weighed. Undamaged heads were without visible signs of caterpillar feeding or holes whereas damaged heads had visible signs of insect feeding, but had reduced market value. The local currency, Ghana cedi (ɇ) exchange rate to the US$ was 1:0.22 during the period of the study and this exchange rate was used to calculate values in US$. At the first season harvest, 1 kg undamaged cabbage head was sold for US$ 0.44 and damaged was US$ 0.22 at the local (Agric Nzema) market. At the harvest of both second and third seasons, price per kg of undamaged and damaged heads were US$ 0.33 and US$ 0.22, respectively. Income from the sale of cabbage was converted to per hectare by extrapolating plant population of the habitat manipulation treatments to 35,000 plants per hectare assuming planting distances of 0.5 m × 0.5 m and considering the area occupied by the non-crop plants for manipulation and spaces for easy movement while the no habitat manipulation treatments had 35,500 plants per hectare considering only spaces for easy movement.
Statistical analysis
Mixed model repeated measure analysis was performed using the statistical package for social scientists (SPSS) IBM version 24 (IBM, 2016). Where significant differences were observed (i.e. P-value <0.05), mean separation was done using Tukey’s post-hoc, Honestly Significant Difference (HSD) test.
References
Oerke, E.-C. Crop losses to pests. The Journal of Agricultural Science 144, 31–43 (2006).
Losey, J. E. & Vaughan, M. The economic value of ecological services provided by insects. Bioscience 56, 311–323 (2006).
Poppy, G. M. et al. Food security in a perfect storm: using the ecosystem services framework to increase understanding. Philosophical Transactions of the Royal Society of London B: Biological Sciences 369, 20120288 (2014).
Roubos, C. R., Rodriguez-Saona, C. & Isaacs, R. Mitigating the effects of insecticides on arthropod biological control at field and landscape scales. Biological Control 75, 28–38, https://doi.org/10.1016/j.biocontrol.2014.01.006 (2014).
E. Birch, A. N., Begg, G. S. & Squire, G. R. How agro-ecological research helps to address food security issues under new IPM and pesticide reduction policies for global crop production systems. Journal of Experimental Botany 62, 3251–3261 (2011).
Bruce, T. J. A. Tackling the threat to food security caused by crop pests in the new millennium. Food Security 2, 133–141, https://doi.org/10.1007/s12571-010-0061-8 (2010).
Bommarco, R., Miranda, F., Bylund, H. & Björkman, C. Insecticides suppress natural enemies and increase pest damage in cabbage. Journal of Economic Entomology 104, 782–791 (2011).
Guedes, R., Smagghe, G., Stark, J. & Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annual review of entomology 61, 43–62 (2016).
Chagnon, M. et al. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environmental Science and Pollution Research 22, 119–134, https://doi.org/10.1007/s11356-014-3277-x (2015).
Pimentel, D. In Integrated Pest Management: Innovation-Development Process: Volume 1 (eds. Rajinder Peshin & Ashok K. Dhawan) 89–111 (Springer Netherlands, 2009).
Pimentel, D. & Burgess, M. In Integrated Pest Management: Pesticide Problems, Vol. 3 (eds. David Pimentel & Rajinder Peshin) 47–71 (Springer Netherlands, 2014).
Borel, B. When the pesticides run out. Nature 543, 302–304 (2017).
Li, H., Cheng, F., Wei, Y., Lydy, M. J. & You, J. Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: An overview. Journal of Hazardous Materials 324, 258–271, https://doi.org/10.1016/j.jhazmat.2016.10.056 (2017).
Affum, A. O., Acquaah, S. O., Osae, S. D. & Kwaansa-Ansah, E. E. Distribution and risk assessment of banned and other current-use pesticides in surface and groundwaters consumed in an agricultural catchment dominated by cocoa crops in the Ankobra Basin, Ghana. Science of The Total Environment 633, 630–640, https://doi.org/10.1016/j.scitotenv.2018.03.129 (2018).
Potts, S. G. et al. Safeguarding pollinators and their values to human well-being. Nature 540, 220 (2016).
Bommarco, R., Vico, G. & Hallin, S. Exploiting ecosystem services in agriculture for increased food security. Global Food Security 17, 57–63, https://doi.org/10.1016/j.gfs.2018.04.001 (2018).
Wyckhuys, K. A. G. et al. Current status and potential of conservation biological control for agriculture in the developing world. Biological Control 65, 152–167, https://doi.org/10.1016/j.biocontrol.2012.11.010 (2013).
Gurr, G. M. et al. Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nature Plants 2, 16014 (2016).
Gurr, G. M., Wratten, S. D., Landis, D. A. & You, M. in Annual Review of Entomology 62, 91–109 (2017).
Finney, D. M., White, C. M. & Kaye, J. P. Biomass production and carbon/nitrogen ratio influence ecosystem services from cover crop mixtures. Agronomy Journal 108, 39–52 (2016).
Finney, D. M. et al. Ecosystem services and disservices are bundled in simple and diverse cover cropping systems. Agricultural & Environmental Letters 2 (2017).
Amoabeng, B. W., Stevenson, P. C., Pandey, S., Mochiah, M. B. & Gurr, M. G. Insecticidal activity of a native Australian tobacco, Nicotiana megalosiphon Van Heurck & Muell. Arg. (Solanales: Solanaceae) against key insect pests of brassicas. Crop Protection 106, 6–12, https://doi.org/10.1016/j.cropro.2017.11.018 (2018).
Mkenda, P. et al. Extracts from field margin weeds provide economically viable and environmentally benign pest control compared to synthetic pesticides. PLoS ONE 10, https://doi.org/10.1371/journal.pone.0143530 (2015).
Mkindi, A. et al. Invasive weeds with pesticidal properties as potential new crops. Industrial Crops and Products 110, 113–122, https://doi.org/10.1016/j.indcrop.2017.06.002 (2017).
Rioba, N. B. & Stevenson, P. C. Ageratum conyzoides L. for the management of pests and diseases by small holder farmers. Industrial Crops and Products 110, 22–29 (2017).
Isman, M. B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66 (2006).
Isman, M. B. Botanical insecticides: for richer, for poorer. Pest management science 64, 8–11 (2008).
Miresmailli, S. & Isman, M. B. Botanical insecticides inspired by plant–herbivore chemical interactions. Trends in Plant Science 19, 29–35, https://doi.org/10.1016/j.tplants.2013.10.002 (2014).
Zapata, N., Vargas, M., Latorre, E., Roudergue, X. & Ceballos, R. The essential oil of Laurelia sempervirens is toxic to Trialeurodes vaporariorum and Encarsia formosa. Industrial Crops and Products 84, 418–422, https://doi.org/10.1016/j.indcrop.2016.02.030 (2016).
Gentz, M. C., Murdoch, G. & King, G. F. Tandem use of selective insecticides and natural enemies for effective, reduced-risk pest management. Biological Control 52, 208–215, https://doi.org/10.1016/j.biocontrol.2009.07.012 (2010).
Gurr, G. M. et al. Landscape ecology and expanding range of biocontrol agent taxa enhance prospects for diamondback moth management. A review. Agronomy for Sustainable Development 38, 23 (2018).
Amoabeng, B. W. et al. Tri-trophic insecticidal effects of African plants against cabbage pests. PloS one 8, e78651 (2013).
Fening, K. et al. Sustainable management of two key pests of cabbage, Brassica oleracea var. capitata L.(Brassicaceae), using homemade extracts from garlic and hot pepper. Organic agriculture 3, 163–173 (2013).
Stemele, M. A. Comparative effects of a selective insecticide, Bacillus thuringiensis var. kurstaki and the broad-spectrum insecticide cypermethrin on diamondback moth and its parasitoid Cotesia vestalis (Hymenoptera; Braconidae). Crop Protection 101, 35–42, https://doi.org/10.1016/j.cropro.2017.07.015 (2017).
Anjum, F. & Wright, D. Relative toxicity of insecticides to the crucifer pests Plutella xylostella and Myzus persicae and their natural enemies. Crop Protection 88, 131–136, https://doi.org/10.1016/j.cropro.2016.06.002 (2016).
Fagan, W. F., Hakim, A. L., Ariawan, H. & Yuliyantiningsih, S. Interactions between Biological Control Efforts and Insecticide Applications in Tropical Rice Agroecosystems: The Potential Role of Intraguild Predation. Biological Control 13, 121–126, https://doi.org/10.1006/bcon.1998.0655 (1998).
Furlong, M. J. et al. Integration of endemic natural enemies and Bacillus thuringiensis to manage insect pests of Brassica crops in North Korea. Agriculture, Ecosystems and Environment 125, 223–238, https://doi.org/10.1016/j.agee.2008.01.003 (2008).
Naranjo, S. E., Ellsworth, P. C. & Frisvold, G. B. in Annual Review of Entomology 60, 621–645 (2015).
Nahusenay, D. G. & Abate, G. A. Evaluation of selected botanical aqueous extracts against cabbage aphid (Brevicoryne brassicae L.(Hemiptera: Aphididae) on cabbage (Brassicae oleraceae L.) under field condition in Kobo District, North Wollo, Ethiopia. Journal of Horticulture and Forestry 10, 69–78 (2018).
Nouri-Ganbalani, G., Borzouei, E., Shahnavazi, M. & Nouri, A. Induction of resistance against Plutella xylostella (L.)(Lep.: Plutellidae) by jasmonic acid and mealy cabbage aphid feeding in Brassica napus L. Frontiers in Physiology 9, 859 (2018).
Javier, A. M. V., Ocampo, V. R., Ceballo, F. A. & Javier, P. A. Insecticidal Activity of Crude Ethanolic Extracts of Five Philippine Plants against Cabbage Worm, Crocidolomia pavonana Fabricius (Lepidoptera: Crambidae). Philippine Journal of Science 147, 513–521 (2018).
Furlong, M. J., Wright, D. J. & Dosdall, L. M. Diamondback moth ecology and management: problems, progress, and prospects. Annual review of entomology 58, 517–541 (2013).
Li, Z. et al. Management and population dynamics of diamondback moth (Plutella xylostella): planting regimes, crop hygiene, biological control and timing of interventions. Bulletin of Entomological Research, 1–9, https://doi.org/10.1017/S0007485318000500 (2018).
Furlong, M. J. et al. Experimental analysis of the influence of pest management practice on the efficacy of an endemic arthropod natural enemy complex of the diamondback moth. Journal of Economic Entomology 97, 1814–1827 (2004).
Nilsson, U., Rännbäck, L. M., Anderson, P. & Rämert, B. Herbivore response to habitat manipulation with floral resources: a study of the cabbage root fly. Journal of Applied Entomology 136, 481–489 (2012).
Pfiffner, L., Luka, H., Schlatter, C., Juen, A. & Traugott, M. Impact of wildflower strips on biological control of cabbage lepidopterans. Agriculture, Ecosystems &. Environment 129, 310–314, https://doi.org/10.1016/j.agee.2008.10.003 (2009).
Symondson, W., Sunderland, K. & Greenstone, M. Can generalist predators be effective biocontrol agents? Annual review of entomology 47, 561–594 (2002).
Grzywacz, D. et al. Current control methods for diamondback moth and other brassica insect pests and the prospects for improved management with lepidopteran-resistant Bt vegetable brassicas in Asia and Africa. Crop Protection 29, 68–79, https://doi.org/10.1016/j.cropro.2009.08.009 (2010).
Campos, E. V. R. et al. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecological Indicators, https://doi.org/10.1016/j.ecolind.2018.04.038 (2018).
Charleston, D. S., Kfir, R., Dicke, M. & Vet, L. E. M. Impact of botanical extracts derived from Melia azedarach and Azadirachta indica on populations of Plutella xylostella and its natural enemies: A field test of laboratory findings. Biological Control 39, 105–114, https://doi.org/10.1016/j.biocontrol.2006.05.012 (2006).
Fening, K., Adama, I. & Tegbe, R. On-farm evaluation of homemade pepper extract in the management of pests of cabbage, Brassica oleraceae L., and french beans, Phaseolus vulgaris L., in two agro-ecological zones in Ghana. African Entomology 22, 552–560 (2014).
Ioriatti, C. et al. Toxicity of emamectin benzoate to Cydia pomonella (L.) and Cydia molesta (Busck)(Lepidoptera: Tortricidae): laboratory and field tests. Pest management science 65, 306–312 (2009).
Jansson, R. et al. In Proceedings of the 3rd International Workshop on Management of Diamondback Moth and Other Crucifer Pests. MARDI, Kuala Lumpur, Malaysia (1997).
Depalo, L., Lanzoni, A., Masetti, A., Pasqualini, E. & Burgio, G. Lethal and Sub-lethal Effects of Four Insecticides on the Aphidophagous Coccinellid Adalia bipunctata (Coleoptera: Coccinellidae). Journal of economic entomology 110, 2662–2671 (2017).
Khan, S. et al. Toxicity of selected insecticides against the zig zag ladybird beetle Menochilus Sexmaculatus. Journal of Zooological Studies 3, 143–147 (2015).
Fening, K. O. et al. Sustainable management of two key pests of cabbage, Brassica oleracea var. capitata L. (Brassicaceae), using homemade extracts from garlic and hot pepper. Organic. Agriculture 3, 163–173, https://doi.org/10.1007/s13165-014-0058-2 (2013).
Khan, M. M., Nawaz, M., Hua, H., Cai, W. & Zhao, J. Lethal and sublethal effects of emamectin benzoate on the rove beetle, Paederus fuscipes, a non-target predator of rice brown planthopper, Nilaparvata lugens. Ecotoxicology and Environmental Safety 165, 19–24, https://doi.org/10.1016/j.ecoenv.2018.08.047 (2018).
Parsaeyan, E., Safavi, S. A., Saber, M. & Poorjavad, N. Effects of emamectin benzoate and cypermethrin on the demography of Trichogramma brassicae Bezdenko. Crop Protection 110, 269–274, https://doi.org/10.1016/j.cropro.2017.03.026 (2018).
Biondi, A., Desneux, N., Siscaro, G. & Zappalà, L. Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: Selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 87, 803–812, https://doi.org/10.1016/j.chemosphere.2011.12.082 (2012).
Monsrud, C. & Toft, S. The aggregative numerical response of polyphagous predators to aphids in cereal fields: attraction to what? Annals of Applied Biology 134, 265–270 (1999).
Nilsson, U. et al. Effects of conservation strip and crop type on natural enemies of D elia radicum. Journal of applied entomology 140, 287–298 (2016).
Donaldson, J. R., Myers, S. W. & Gratton, C. Density-dependent responses of soybean aphid (Aphis glycines Matsumura) populations to generalist predators in mid to late season soybean fields. Biological Control 43, 111–118, https://doi.org/10.1016/j.biocontrol.2007.06.004 (2007).
Andersson, S. & Dobson, H. E. M. Behavioral Foraging Responses by the Butterfly Heliconius melpomene to Lantana camara Floral Scent. Journal of Chemical Ecology 29, 2303–2318, https://doi.org/10.1023/a:1026226514968 (2003).
Begum, M., Gurr, G. M., Wratten, S. D. & Nicol, H. I. Flower color affects tri-trophic-level biocontrol interactions. Biological Control 30, 584–590, https://doi.org/10.1016/j.biocontrol.2004.03.005 (2004).
Amoabeng, B. W., Gurr, G. M., Gitau, C. W. & Stevenson, P. C. Cost:benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop Protection 57, 71–76, https://doi.org/10.1016/j.cropro.2013.11.019 (2014).
Aziz, M. A., Hasan, M. U., Ali, A. & Iqbal, J. Comparative efficacy of different strategies for management of spotted bollworms, Earias spp. on Okra, Abelmoschus esculentus (L). Moench. Moench. Pakistan. J. Zool 44, 1203–1208 (2012).
Griffiths, G. J. K., Holland, J. M., Bailey, A. & Thomas, M. B. Efficacy and economics of shelter habitats for conservation biological control. Biological Control 45, 200–209, https://doi.org/10.1016/j.biocontrol.2007.09.002 (2008).
Chepchirchir, R. T., Macharia, I., Murage, A. W., Midega, C. A. O. & Khan, Z. R. Ex-post economic analysis of push-pull technology in Eastern Uganda. Crop Protection 112, 356–362, https://doi.org/10.1016/j.cropro.2018.07.001 (2018).
Shabozoi, N., Abro, G., Syed, T. & Awan, M. Economic appraisal of pest management options in Okra. Pakistan Journal of Zoology 43, 869–878 (2011).
Acknowledgements
We are grateful to Mrs. Annie C. Johnson, Charles Sturt University, Orange campus for her assistance in manuscript preparation.
Author information
Authors and Affiliations
Contributions
B.W.A. and G.M.G. conceived the experiments, B.W.A. conducted the experiments, B.W.A. and K.P.A. analysed the data. All authors interpreted the results, wrote and reviewed the manuscript. Conceived and designed experiments: A.B.W., G.M.G., S.C.P. Performed experiments: A.W.B. Analyzed data: A.W.B., A.K.P., M.B.M. Wrote manuscript: A.B.W., G.M.G., S.C.P., M.B.M. and A.K.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amoabeng, B.W., Stevenson, P.C., Mochiah, B.M. et al. Scope for non-crop plants to promote conservation biological control of crop pests and serve as sources of botanical insecticides. Sci Rep 10, 6951 (2020). https://doi.org/10.1038/s41598-020-63709-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-63709-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.