Abstract
Although the pathogenesis of Alzheimer’s disease (AD) is unclear, neuroinflammation appears to play a role in its development. Psoriasis is a chronic inflammatory skin disease that has recently been found to genetically overlap with AD. We aimed to investigate the risk of AD in patients with psoriasis. Subjects with psoriasis (n = 535,927) and age- and sex-matched controls without psoriasis (at a 5:1 ratio; n = 2,679,635) who underwent ≥3 health examinations between 2008 and 2014 were included, drawn from the Korean National Health Insurance System database. There were 50,209 cases of AD (1.87%) in controls without psoriasis and 11,311 cases (2.11%) in patients with psoriasis, and the median follow-up was 3.35 years. In a multivariable-adjusted model, patients with psoriasis showed a significantly increased risk of AD (hazard ratio, 1.09; 95% CI, 1.07–1.12, p < 0.0001) compared to controls without psoriasis. Among patients with psoriasis, the risk of AD was significantly increased in psoriasis patients not receiving systemic therapy compared to those receiving systemic therapy (hazard ratio, 1.10; 95% CI, 1.08–1.12 vs. hazard ratio, 0.99; 95% CI: 0.90–1.09, p < 0.0001). The incidence of AD was significantly increased in patients with psoriasis compared to control subjects without psoriasis. Of note, systemic treatment for psoriasis was associated with a reduced risk of AD.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD), the most common cause of dementia in the elderly, is a chronic neurodegenerative disease affecting approximately 30 million people worldwide in 20151,2. Although the pathogenesis of AD remains unclear, it is thought that genetic susceptibility, brain inflammation, and various environmental stimuli interact with each other to cause the disease. Recently, increasing evidence has implied that aberrant immune responses, particularly involving T-helper (Th)1/Th17 cells and cytokines, are involved in the inflammation associated with neurodegeneration in AD3,4,5.
Psoriasis is a Th1/Th17-mediated chronic inflammatory skin disease, and often develops between the ages of 15 and 30 years6. Genome-wide association studies (GWASs) have demonstrated genetic overlap between AD and psoriasis, and implied that inflammation could influence the pathogenesis and progression of AD7. Additionally, recent studies have suggested that psoriasis is associated with an increased risk of mental health disorders8 and Parkinson’s disease6. Since psoriasis occur much younger than AD, it seems plausible the effects of psoriasis could influence AD. These findings raise an important question concerning whether psoriasis and AD are linked. Previously, several studies have reported an association between AD and psoriasis, however, these were restricted to specific populations and included small sample sizes, or only assessed certain outcomes9,10.
Therefore, we conducted a population-based case–control cohort study using the Korean National Health Insurance System (NHIS) database to determine whether the incidence of AD is increased in patients with psoriasis.
Results
Baseline demographics of the study population
Baseline characteristics of participants are described in Table 1. Among the 535,927 patients with psoriasis, 499,488 (93.2%) patients (no systemic therapy group) had no evidence of receiving systemic agents for psoriasis, while 36,439 (6.8%) patients (systemic therapy group) had received systemic treatment for psoriasis.
Risk of AD in patients with psoriasis compared to controls without psoriasis
After a mean follow-up period of 3.35 ± 2.01 years, the incidence of AD was 5.6 per 1,000 person-years in controls without psoriasis and 6.3 per 1,000 person-years in patients with psoriasis (no systemic therapy group: 6.5 per 1,000 person-years; systemic therapy group: 3.7 per 1,000 person-years, p for trend < 0.0001) (Table 2). The mean duration between the diagnosis of psoriasis and AD after psoriasis was 2.45 ± 1.67 years in controls and 2.42 ± 1.67 years in patients with psoriasis (Table 2). The duration between psoriasis and AD diagnosis actually reflect the time between psoriasis and AD diagnosis. In the control group, the control subject was followed up from the first diagnosis of psoriasis in psoriasis patient matched with each control subject.
As illustrated in a Kaplan–Meier plot, subjects with psoriasis showed a greater tendency to suffer from AD compared to controls without psoriasis. However, the risk of AD was decreased in the systemic therapy group compared to controls without psoriasis (log-rank test; p < 0.0001, Fig. 1). After adjusting for age, sex, income level, diabetes, hypertension, dyslipidemia and depression (model 3), patients with psoriasis showed a significantly increased risk of developing AD (HR, 1.09, 95% CI: 1.07–1.12, p < 0.0001) compared to controls without psoriasis (Table 2).
Alzheimer’s disease (AD) in patients with psoriasis. The estimated cumulative incidence of AD is significantly higher in psoriasis patients than in control subjects without psoriasis. In addition, psoriasis patients who received systemic therapy showed a lower incidence of AD than psoriasis patients without any systemic therapy for psoriasis by Kaplan-Meier analysis. (Log-rank test, p < 0.0001).
Subgroup analyses
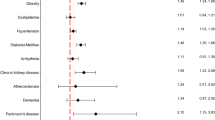
An analysis stratified by age, sex, and presence or absence of diabetes, hypertension, or dyslipidemia was conducted. Higher adjusted HRs for developing AD were observed among males, and among those without diabetes or hypertension, and in those with dyslipidemia; there were no significant differences in analyses comparing incidence rates between the sexes, diabetes, or dyslipidemia status (Table 3). However, there was a significantly higher incidence of AD in individuals without hypertension (HR = 1.13 vs 1.07, p-value = 0.0021) (Fig. 2). Of note, younger patients (40–64 years) with psoriasis had a significantly higher incidence of AD than that of older patients (≥65 years) (HR, 1.30, 95% CI: 1.23–1.39 vs. HR, 1.08, 95% CI: 1.06–1.11, p for interaction <0.0001) (Fig. 2).
Discussion
In this nationwide study, we found a significantly increased risk of newly diagnosed AD among patients with psoriasis compared to age- and sex-matched controls without psoriasis. This association was significantly stronger in middle-aged patients than in elderly patients (≥65 years) with psoriasis (HR: 1.30 vs. HR: 1.08). We also observed that those patients with psoriasis who were treated with systemic therapy had a lower risk of AD than that of controls without psoriasis.
Although the exact mechanism of AD has not been fully elucidated, increasing evidence has implied that neuroinflammation plays an important role in its development11,12,13. In AD, the activation of microglial cells, the key inflammatory cells in the brain, induces the release of proinflammatory mediators, resulting in neuronal damage14. In addition, IL-12/IL-23 signaling has been implicated in the development of amyloid-induced neurodegeneration4. Indeed, blocking the common p40 subunit of IL-12 and IL-23 reduced the number of amyloid β plaques, an important pathology of AD, and appeared to improve the cognitive deficits in a mouse model of AD3. The IL-23/T helper 17 axis is considered to be the most important factor in the development of psoriasis, and anti-IL-12/23 p40 monoclonal antibody, a drug targeting this axis, is used worldwide as a treatment of psoriasis15. Furthermore, GWASs have revealed a genetic overlap between AD and psoriasis, suggesting that an immunological mechanism plays a role in the pathogenesis of AD7,16,17. In a cross-sectional pilot study that assessed 41 patients with psoriasis and 37 controls using neuropsychological tests, Gisondi et al. reported that the incidence of mild cognitive impairment was higher in patients with chronic plaque psoriasis than in the controls, implying that patients with psoriasis are at an increased risk of developing AD10. In line with these overlapping inflammatory pathways and shared genetic risk loci, we observed that patients with psoriasis have a higher risk of AD compared to the general population using the NHIS database.
Notably, we observed a significant reduction in the incidence of AD among patients with psoriasis prescribed systemic medications (acitretin, methotrexate, cyclosporine, and biologic agents) compared to controls without psoriasis. The pathophysiological concept of CVD is widely accepted as a chronic inflammatory condition, and epidemiologic studies have shown that the systemic anti-inflammatory drugs such as biological drugs and methotrexate can attenuate the risk of CVD in patients with severe psoriasis compared to other anti-psoriatic treatments18,19,20,21,22,23,24. Recently, Lee et al. reported that a significantly increased risk of Parkinson’s disease was observed in the systemic therapy exposed-psoriasis group compared to the unexposed-psoriasis group6. In addition, it has been reported that acitretin, which is frequently used as a treatment for psoriasis in Korea25, enhances the gene expression of the metalloproteinase ADAM 10, thereby inhibiting Aβ peptide production and preventing the formation of amyloid plaques in a mouse model of AD26,27. Several clinical trials to examine the effect of TNF inhibitors in patients with AD have demonstrated their considerable beneficial effects on cognitive and behavioral improvement28,29. In this sense, we assume that systemic anti-inflammatory drugs reduce the inflammation of neuronal cells, lowering overall inflammation in the body, which has a preventive effect on AD.
In this study, age-stratified analyses (age and sex matched) revealed that the association between psoriasis and AD was more pronounced in middle-aged (40–64 years) compared to elderly (≥65 years) patients (HR 1.30 vs. 1.08, p < 0.0001). Although the absolute number and incidence of events were high above 65 years and low under 65, individuals who develop psoriasis at younger ages (40–60 years) are more likely to develop AD than age-matched individuals who are not diagnosed with psoriasis. In this regard, previous studies have shown that aging promotes a decrease in T cell immune function thereby reducing inflammation and leads to suppress the development of skin inflammatory diseases, which may support our hypothesis30,31,32. Additionally, since elderly people have various chronic diseases such as diabetes mellitus, hypertension, hyperlipidemia and depression and take many kinds of drugs, the effect of psoriasis on the development of AD may be more sensitive in middle age than in older age33,34. However, our results were meaningful after adjusting for these confounding variables, implying that reduced T cell immunity due to aging and weakened disease activity in psoriasis itself leads to the weak association between psoriasis and AD.
There were several limitations to our study. First, the subjects were identified for study inclusion based only on registered diagnostic codes. Nevertheless, the diagnoses are relatively accurate, because a diagnosis is covered by the NHIS in Korea only after the diagnosis is confirmed by the Mini-Mental State Examination (MMSE). Second, the nationwide database lacks detailed data on the severity and type of psoriasis, as patients with psoriasis were only classified as having received a systemic treatment or not. Third, because our study was a retrospective epidemiological study, it was not possible determine a causal relationship accurately. To avoid the possible effects of reverse causality, we established a 12-month washout period and excluded subjects with pre-existing psoriasis or AD. Fourth, we did not have information that could affect the incidence of AD such as education status, smoking, alcohol consumption and family history of AD. Fifth, it is well known that pathogenic variants in genes such as the APP, PSEN1, and PSEN2 are associated with early onset familial AD (ref)35. However, since the patients’ genetic information is not included in this study, it is difficult to know whether the genetic predisposition related to early AD may have affected our findings that younger patients (age <65 years) with psoriasis are more prone to AD than older patients (≥65 years). Further research is needed to explore the relationship between psoriasis and early versus late onset AD. Lastly, since our research is based on nationwide data representative of the Korean population, it is difficult to generalize this result to other racial/ethnic populations. Despite these limitations, this is the large-scale, population-based cohort study to evaluate the association between AD and psoriasis. The NHIS database covers nearly 100% of the Korean population, and our study provides the first evidence that the use of systemic anti-inflammatory drugs can reduce the risk of AD.
In conclusion, we found a significantly increased risk of AD in Korean patients with psoriasis compared to a matched control group. These results indicate that chronic inflammatory conditions in psoriasis may have an important impact on the nervous system and thus in increasing the risk of developing AD. Further studies are needed to explore the potential causes of the increased risk of AD and whether these effects vary depending on the type of systemic treatment used in psoriasis.
Methods
Data sources
The NHIS database used in this study covers approximately 97.2% of the population of Korea36. This system reviews inpatient and outpatient claims sent from healthcare institutions. The database includes information on patient age, sex, diagnoses and comorbidities based on the International Classification of Disease, 10th revision (ICD-10), and records of prescriptions, procedures, and prescribed drugs37. Enrollees in the NHIS are assigned unique identification codes, and are recommended to have a health checkup at least every 2 years38. This study was approved by the institutional review boards of the Catholic University of Korea (no. KC17ZESI0311) and Seoul St. Mary’s Hospital (No. KC17ZESI0505). The informed consent was waived because all data were anonymized and de-identified.
Study population
From the NHIS database, we enrolled patients aged 40 year or older who of age who underwent health screenings from January 2008 to December 2014. Among these subjects, patients who were diagnosed at least once with psoriasis (ICD-10 codes: L40) at clinics or hospitals were selected from the database. We excluded subjects with missing data on at least one variable. To avoid confounding by pre-existing diseases, those who had a history of AD (ICD-10 codes: F00 or G30) before the index year were also excluded. Ultimately, the study population consisted of 535,927 subjects.
We analyzed subjects with psoriasis (n = 535,927) and controls without psoriasis (n = 2,679,635) who were randomly selected according to a 5:1 ratio with age- and sex-matched subjects during the same period. Patients with psoriasis were divided into two groups; systemic therapy group: patients who received systemic treatment such as acitretin, methotrexate, cyclosporine and biologics, no systemic therapy group: patients who were not treated with any systemic agent.
Study outcomes
This study investigated newly diagnosed patients with AD. The definition of AD was based on the presence of relevant ICD-10 codes (F00 or G30) and the prescription of medication for AD (galantamine, rivastigmine, donepezil, or memantine), as determined using the NHIS claims database. When codes for AD both and other types of dementia were recorded, we followed the principal diagnosis. If both code types were included in additional diagnoses up to the second claim database, the subject was classified as having ‘other’ dementia. The patients diagnosed with AD prior to this study enrollment were excluded. This study was initiated after a 12-month washout period between 2006 and 2007 to reduce the confounding effect of previously diagnosed AD on the study outcome. The study population was followed from baseline to the date of incident AD or until December 31, 2014, whichever came first.
Statistical analysis
Baseline demographic characteristics are presented as means ± standard deviation or numbers (%). The incidence of AD was calculated by dividing the total number of incident cases by the entire follow-up duration (person-years). The cumulative incidence of AD according to the presence of psoriasis and systemic therapy for psoriasis was calculated using Kaplan–Meier curves, and the log-rank test was used to analyze differences between the groups. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the risk of AD were determined using Cox proportional hazards models after adjusting for multiple confounding variables. Model 2 was adjusted for age and sex, and model 3 was further adjusted for income level, diabetes, hypertension, dyslipidemia, and depression. We performed subsequent subgroup analyses and interaction testing using a likelihood ratio test to evaluate the difference in the risk of AD according to subgroups. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC, USA).
Ethics approval and consent to participate
This study was approved by the institutional review boards of the Catholic University of Korea (no. KC17ZESI0311) and Seoul St. Mary’s Hospital (No. KC17ZESI0505). Informed consent was not obtained because anonymous and de-identified information was used for the analysis.
References
Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England) 388, 1545–1602, https://doi.org/10.1016/s0140-6736(16)31678-6 (2016).
Wolfsgruber, S. et al. Prevalence of abnormal Alzheimer’s disease biomarkers in patients with subjective cognitive decline: cross-sectional comparison of three European memory clinic samples. Alzheimer’s research & therapy 11, 8, https://doi.org/10.1186/s13195-018-0463-y (2019).
Vom Berg, J. et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nature medicine 18, 1812–1819, https://doi.org/10.1038/nm.2965 (2012).
Griffin, W. S. Neuroinflammatory cytokine signaling and Alzheimer’s disease. The New England journal of medicine 368, 770–771, https://doi.org/10.1056/NEJMcibr1214546 (2013).
Tan, M. S. et al. IL12/23 p40 inhibition ameliorates Alzheimer’s disease-associated neuropathology and spatial memory in SAMP8 mice. Journal of Alzheimer’s disease: JAD 38, 633–646, https://doi.org/10.3233/jad-131148 (2014).
Lee, J. H., Han, K. & Gee, H. Y. The Incidence Rates and Risk Factors of Parkinson’s Disease in Patients with Psoriasis: A Nationwide Population-based Cohort Study. Journal of the American Academy of Dermatology, https://doi.org/10.1016/j.jaad.2019.07.012 (2019).
Yokoyama, J. S. et al. Association Between Genetic Traits for Immune-Mediated Diseases and Alzheimer Disease. JAMA neurology 73, 691–697, https://doi.org/10.1001/jamaneurol.2016.0150 (2016).
Bang, C. H. et al. Association of Psoriasis With Mental Health Disorders in South Korea. JAMA dermatology 155, 747–749, https://doi.org/10.1001/jamadermatol.2019.0315 (2019).
Abuabara, K. et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. The British journal of dermatology 163, 586–592, https://doi.org/10.1111/j.1365-2133.2010.09941.x (2010).
Gisondi, P. et al. Mild cognitive impairment in patients with moderate to severe chronic plaque psoriasis. Dermatology (Basel, Switzerland) 228, 78–85, https://doi.org/10.1159/000357220 (2014).
Heneka, M. T. et al. Neuroinflammation in Alzheimer’s disease. The Lancet. Neurology 14, 388–405, https://doi.org/10.1016/s1474-4422(15)70016-5 (2015).
Lu, K. et al. Association between autoimmune rheumatic diseases and the risk of dementia. BioMed research international 2014, 861812, https://doi.org/10.1155/2014/861812 (2014).
Heizmann, C. W., Fritz, G. & Schafer, B. W. S100 proteins: structure, functions and pathology. Frontiers in bioscience: a journal and virtual library 7, d1356–1368 (2002).
Calsolaro, V. & Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 12, 719–732, https://doi.org/10.1016/j.jalz.2016.02.010 (2016).
Rich, P. et al. Ustekinumab improves nail disease in patients with moderate-to-severe psoriasis: results from PHOENIX 1. The British journal of dermatology 170, 398–407, https://doi.org/10.1111/bjd.12632 (2014).
Arreola, R. et al. Immunomodulatory effects mediated by serotonin. Journal of immunology research 2015, 354957, https://doi.org/10.1155/2015/354957 (2015).
Manuel, S. L., Rahman, S., Wigdahl, B., Khan, Z. K. & Jain, P. Dendritic cells in autoimmune diseases and neuroinflammatory disorders. Frontiers in bioscience: a journal and virtual library 12, 4315–4335 (2007).
Ahlehoff, O. et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. Journal of internal medicine 273, 197–204, https://doi.org/10.1111/j.1365-2796.2012.02593.x (2013).
Ahlehoff, O. et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. Journal of the European Academy of Dermatology and Venereology: JEADV 29, 1128–1134, https://doi.org/10.1111/jdv.12768 (2015).
Solomon, D. H. et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. Jama 305, 2525–2531, https://doi.org/10.1001/jama.2011.878 (2011).
Mazzoccoli, G. et al. Anti-tumor necrosis factor-alpha therapy and changes of flow-mediated vasodilatation in psoriatic and rheumatoid arthritis patients. Internal and emergency medicine 5, 495–500, https://doi.org/10.1007/s11739-010-0458-6 (2010).
Cordiali-Fei, P. et al. Effective therapy with anti-TNF-alpha in patients with psoriatic arthritis is associated with decreased levels of metalloproteinases and angiogenic cytokines in the sera and skin lesions. Annals of the New York Academy of Sciences 1110, 578–589, https://doi.org/10.1196/annals.1423.062 (2007).
Micha, R. et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. The American journal of cardiology 108, 1362–1370, https://doi.org/10.1016/j.amjcard.2011.06.054 (2011).
Lee, S. H. et al. Variability in metabolic parameters and risk of dementia: a nationwide population-based study. Alzheimer’s research & therapy 10, 110, https://doi.org/10.1186/s13195-018-0442-3 (2018).
Han, J. H. et al. Epidemiology and Medication Trends in Patients with Psoriasis: A Nationwide Population-based Cohort Study from Korea. Acta dermato-venereologica 98, 396–400, https://doi.org/10.2340/00015555-2877 (2018).
Sodhi, R. K. & Singh, N. Retinoids as potential targets for Alzheimer’s disease. Pharmacology, biochemistry, and behavior 120, 117–123, https://doi.org/10.1016/j.pbb.2014.02.016 (2014).
Tippmann, F., Hundt, J., Schneider, A., Endres, K. & Fahrenholz, F. Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 23, 1643–1654, https://doi.org/10.1096/fj.08-121392 (2009).
Tobinick, E. L. & Gross, H. Rapid improvement in verbal fluency and aphasia following perispinal etanercept in Alzheimer’s disease. BMC neurology 8, 27, https://doi.org/10.1186/1471-2377-8-27 (2008).
Tobinick, E., Gross, H., Weinberger, A. & Cohen, H. TNF-alpha modulation for treatment of Alzheimer’s disease: a 6-month pilot study. MedGenMed: Medscape general medicine 8, 25 (2006).
Abuabara, K., Lee, H. & Kimball, A. B. The effect of systemic psoriasis therapies on the incidence of myocardial infarction: a cohort study. The British journal of dermatology 165, 1066–1073, https://doi.org/10.1111/j.1365-2133.2011.10525.x (2011).
Larbi, A., Dupuis, G., Douziech, N., Khalil, A. & Fulop, T. Jr. Low-grade inflammation with aging has consequences for T-lymphocyte signaling. Annals of the New York Academy of Sciences 1030, 125–133, https://doi.org/10.1196/annals.1329.016 (2004).
Kassi, K., Djeha, D., Gbery, I. P., Kouame, K. & Sangare, A. Psoriasis in elderly patients in the Cote d’Ivoire: socio-demographic, clinical, and therapeutic aspects, and follow-up. International journal of dermatology 55, e83–86, https://doi.org/10.1111/ijd.13138 (2016).
Phan, C. et al. Psoriasis in the elderly: epidemiological and clinical aspects, and evaluation of patients with very late onset psoriasis. Journal of the European Academy of Dermatology and Venereology: JEADV 30, 78–82, https://doi.org/10.1111/jdv.12850 (2016).
Piaserico, S. et al. Efficacy and safety of systemic treatments for psoriasis in elderly patients. Acta dermato-venereologica 94, 293–297, https://doi.org/10.2340/00015555-1719 (2014).
Loy, C. T., Schofield, P. R., Turner, A. M. & Kwok, J. B. Genetics of dementia. Lancet (London, England) 383, 828–840, https://doi.org/10.1016/s0140-6736(13)60630-3 (2014).
Song, S. O. et al. Trends in Diabetes Incidence in the Last Decade Based on Korean National Health Insurance Claims Data. Endocrinology and metabolism (Seoul, Korea) 31, 292–299, https://doi.org/10.3803/EnM.2016.31.2.292 (2016).
Koo, B. K., Lee, C. H., Yang, B. R., Hwang, S. S. & Choi, N. K. The incidence and prevalence of diabetes mellitus and related atherosclerotic complications in Korea: a National Health Insurance Database Study. PloS one 9, e110650, https://doi.org/10.1371/journal.pone.0110650 (2014).
Lee, Y. H., Han, K., Ko, S. H., Ko, K. S. & Lee, K. U. Data Analytic Process of a Nationwide Population-Based Study Using National Health Information Database Established by National Health Insurance Service. Diabetes & metabolism journal 40, 79–82, https://doi.org/10.4093/dmj.2016.40.1.79 (2016).
Acknowledgements
This study was performed using the database from the National Health Insurance System (NHIS-2017-1-285), and the results do not necessarily represent the opinion of the National Health Insurance Corporation. This research was supported by grant of the Institute of Clinical Medicine Research in the Yeouido St. Mary’s hospital, Catholic University of Korea.
Author information
Authors and Affiliations
Contributions
J.H.L., K.H. and M.K. designed the study. K.H. performed statistical analyses. J.H.L., K.H., and M.K. interpreted the data. J.K.L. and S.H.L. contributed to discussion. J.H.L. and M.K. wrote the manuscript. H.E.P. involved in the data collection and paper proofing needed for the revision process. J.H.L. is the study guarantor. The authors take responsibility for the integrity and accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, M., Park, H.E., Lee, SH. et al. Increased risk of Alzheimer’s disease in patients with psoriasis: a nationwide population-based cohort study. Sci Rep 10, 6454 (2020). https://doi.org/10.1038/s41598-020-63550-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-63550-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.