Abstract
Cylindrical silk gland (CY) spigots distinguish a large clade of modern spiders, the CY spigot clade, which includes all entelegyne spiders and their closest relatives. Following a widespread paradigm, CYs and their spigots are only known to occur in female spiders and they produce silk used in the construction of egg sacs. Here we report the occurrence of a CY spigot or CY nubbin on each posterior median spinneret (PMS) in males (5th stadium and later) of the spider Australomimetus maculosus. Late juvenile males had a CY spigot on each PMS, whereas adult males either had a CY spigot or, more often, a non-functional CY nubbin. This indicates that potential CY use by males is at least largely limited to late juvenile instars and is not involved with egg sac construction. Despite the presence of CY spigots in both sexes, sexual dimorphism with respect to CYs was still evident since males lacked the CY spigot on each posterior lateral spinneret present in late juvenile and adult females, and CY spigots of males never had the wide shaft and opening of adult females. This study adds to our knowledge of spinning apparatus variability in modern spiders and demonstrates an exception to the paradigm that, in the CY spigot clade, such spigots are restricted to female spiders.
Similar content being viewed by others
Introduction
Recent, extensive phylogenetic analyses of spiders have recognized a clade that includes all “modern” (araneomorph) spiders except the Synspermiata, Filistatidae, and Hypochilidae1,2,3. A synapomorphy of this “CY spigot clade”2,4 is the possession of spinneret spigots that are outlets for a type of silk gland known for many years as either tubuliform or cylindrical silk glands (CYs)5,6. CY spigots generally occur on both the posterior median spinnerets (PMSs) and the posterior lateral spinnerets (PLSs)7,8,9. Silk drawn from them is used primarily, if not solely, in the construction of egg sacs and so far CYs are only known to occur in females7,8,9,10,11,12,13. Indeed, this restriction to females can often be used to help distinguish CYs and their spigots from other silk gland types that produce silks for other functions14,15,16. During development in some members of the CY spigot clade, including pirate spiders (family Mimetidae)17,18,19, CY spigots make their first appearance in juvenile females9,20,21,22,23,24,25,26,27 though dissections and histological observations have indicated it is not until CY luminal contents are amassed in adults, synchronized to yolk accumulation in the eggs, that CY silk is drawn7,8,28.
Here, we document the consistent occurrence of CY spigots or their nubbins (i.e., vestiges of CY spigots) on PMSs in males (late juvenile and adult) of Australomimetus maculosus (Rainbow, 1904) (Fig. 1A–D); a widespread mimetid spider in forest habitats from eastern Australia that preys extensively on other spiders29,30,31. To our knowledge, neither CY spigots nor CY nubbins have previously been observed in males of a ‘CY spigot clade’ spider. Furthermore, A. maculosus seems to be unique in this regard among Australomimetus Heimer, 1986 species: our observations were made during an extensive survey of spinning field structures in Australasian pirate spiders18,19, when more than 30 species and 300 mimetid specimens (including close relatives of A. maculosus) were examined to generate an overview of spinning apparatus ontogeny and variability in spiders that have secondarily given up the web. In none of these other species did males have CY spigots or CY nubbins. Note that CY spigots/nubbins of male A. maculosus were restricted to PMSs: they were never observed on PLSs. Note also that we are not suggesting that male A. maculosus assist with egg sac construction. Indeed, observations presented below argue against such a role for CYs in these males.
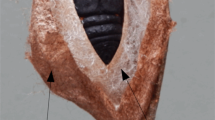
The pirate spider Australomimetus maculosus and an overview of its spinnerets. Instar number, sex, and maturity [juvenile (J) or adult (A)] given in upper or lower right of each image. (A) Specimen ZMH-A0002038 (see Supplementary Appendix S1), Mt. Colah, New South Wales; spinnerets of this specimen shown in Fig. 2C. (B) Specimen ZMH-A0002037, Launceston, Tasmania; note swollen palps on this penultimate male; spinnerets of this specimen shown in Figs. 2B,E, 3C. (C) Specimen ZMH-A0002035, Jesmond, NSW; PMS of this specimen shown in Fig. 3A. (D) Specimen ZMH-A0002048, Julatten, Queensland. (E) Complete set of spinnerets from an adult male. Unlabeled arrows point to CY nubbins on the PMS. Anterior at left. (F) Portion of the right PMS from same specimen (image flipped), showing CY nubbin (CY) from lateral perspective. Five unlabeled spigots closest to nubbin are AC spigots. Anterior at right. AT, anal tubercle; Col, colulus; TS, tracheal spiracle. Photos (A–D) taken by Greg J Anderson.
To provide comparisons to previously examined Australomimetus17,19,30,31, aspects of the spinning apparatus of A. maculosus beyond the CY spigots/nubbins of males are also briefly described below.
Results and Discussion
Spinning apparatus overview and molts to maturity
Overall, the spinnerets of A. maculosus (Fig. 1E) resembled those of related species19,30,31, but they were unique among members of the CY spigot clade2,7,8,9,10,11,12,13,14,15,16 due to the presence of a clearly visible CY spigot (Figs. 2B,D–F, 3A–F) or CY nubbin (Fig. 1E,F) on each PMS in all examined males in the 5th stadium or later (n = 9). PLSs of these males lacked any CY structures, which is standard and remains without exception among male spiders of the CY spigot clade, but differs from the general pattern of CY spigot occurrence on both PMSs and PLSs in female spiders7,8,9. An inventory of those spinning structures that vary within the Mimetidae16,17,18,19,31,32,33,34 is presented for the spinnerets of A. maculosus in Table 1. Additional structures, thus far invariant among mimetids, included the following: In juveniles, each anterior lateral spinneret (ALS) contained one primary (1°) major ampullate silk gland (MaA) spigot17,19,35,36, one secondary (2°) MaA spigot, and one 2° MaA tartipore, with a primordium of this tartipore presumably present in 1st instars17,19,36 (none examined). Adults of both sexes differed from juveniles in possessing only a nonfunctional vestige of the 2° MaA spigot, called a 2° MaA nubbin. 1° and 2° minor ampullate silk gland (MiA) structures on PMSs, including replacement of juvenile 2° MiA spigots by 2° MiA nubbins in adults (cf. Fig. 2E,F), matched their MaA counterparts exactly.
Posterior spinnerets (PLS, PMS) of Australomimetus maculosus. Instar number, sex, and maturity [juvenile (J) or adult (A)] given in upper right of each image. Unlabeled arrows in (A–D) indicate CY spigots. Females, late juvenile (A) and adult (C), have one CY spigot per PMS and PLS. Late juvenile males (B) have one CY spigot per PMS only. Adult males may also have one CY spigot per PMS (D), but more often, one or both of these are represented by CY nubbins (see Fig. 1E,F). (E) Right PMS of same specimen shown in (B) at higher magnification (image flipped). (F) Left PMS of same specimen shown in (D) at higher magnification. Specimen shown in (B,E) also shown in Figs. 1B, 3C. Specimen shown in (C) also shown in Fig. 1A. Specimen shown in (D,F) also shown in Fig. 3D. (A–D) Anterior at bottom; (E,F) anterior at left. 1° MiA, 1° MiA spigot; 2° MiA, 2° MiA spigot; AC, AC spigots; AT, anal tubercle; CY, CY spigot; N, 2° MiA nubbin; PLSL, left PLS; PLSR, right PLS; T, 2° MiA tartipore.
CY spigots (CY) in male (A–F) and female (G–L) Australomimetus maculosus. Instar number, sex, and maturity [juvenile (J) or adult (A)] given in lower left corner of each image. Male CY spigots are on PMS only. Female CY spigots are on PMS (G,I,K) and PLS (H,J,L). (F) CY spigot shaft from (E) at higher magnification and distal perspective: no opening apparent. Unidentified spigots near CY spigots are AC spigots. Specimen shown in (A) also shown in Fig. 1C. Specimen shown in (C) also shown in Figs. 1B, 2B,E. Specimen shown in (D) also shown in Fig. 2D,F. (G,H) from same specimen. (I,J) from same specimen. (A–C, E,F) left PMS; (D,G,I,K) right PMS (images flipped); (J) left PLS; (H,L) right PLS (images flipped). (A–G,I,K) Anterior at right; (H,J,L) anterior at left.
In some adult male mimetid species, the morphology of two piriform silk gland (PI) spigots on each ALS is conspicuously different from that of all other PI spigots16,17,18,33,34; hence, their designation as ‘modified PI (MoPI) spigots’. In other adult male mimetids, including some Australomimetus species18, careful inspection likewise reveals a morphologically distinct pair of PI spigots, but these do not differ so obviously from other PI spigots and have been called ‘subtle MoPI spigots’17. Neither MoPI nor subtle MoPI spigots were observed on the six adult male A. maculosus examined here.
Stadium estimates based on spigot/tartipore numbers and 2° MiA tartipore position (see Methods) indicated variation in the number of molts to maturity in A. maculosus. Additional observations may expand the range of stadia that potentially include adults, but at a minimum, females may reach adulthood after six or seven molts, males after six, seven, or eight molts (Table 1). Variation in the number of molts to maturity has been observed in a wide range of araneomorph spiders22,26,37,38,39,40, including other pirate spiders (Mimetus17, Australomimetus19), while in other taxa constancy has been reported in one or both sexes37,38,39,41. Both genetic and environmental factors may be responsible for variation37,38,42,43,44.
CY spigot occurrence
The earliest females of A. maculosus examined, two 5th instars, had the full complement of four CY spigots typical for most pirate spiders (except for the genus Gelanor Thorell, 186932,33): 1 per PMS and 1 per PLS (Fig. 2A), as did all later females examined, both juvenile and adult (Fig. 2C; Table 1). All these CY spigots were fully formed, consisting of a base and shaft (Fig. 3G–L), but only in adult females (Fig. 1A) did CY spigots have the distinctive morphology that is typical for these taxa: enlarged and rotund with wide-aperture, dome-shaped shafts32 (Fig. 3K,L). Thus, CY spigots of juvenile females were narrower than those of adult females, absolutely as well as relative to adjacent aciniform silk gland (AC) spigots. Moreover, differences in diameters of CY spigot openings, through which silk is drawn, were considerable: diameters ranged from 0.3–0.4 µm (5th instars, n = 2) and 0.5–0.8 µm (6th instar, n = 1) in juvenile females, while the range in adult females was 4.1–4.9 µm (6th instar, n = 1) and 3.8–5.4 µm (7th instars, n = 4) (Fig. 3G–L). For comparison, openings on AC and 1° MiA spigots across all 5th−8th stadia specimens in Table 1 (both sexes) ranged from 0.1–0.4 µm and from 0.9–2.1 µm, respectively (n = 17); in 5th−7th stadia juveniles, openings on 2° MiA spigots ranged from 0.5–1.0 µm (n = 6). CY spigot shafts in juvenile and adult females had finely striated sculpturing but lacked deep longitudinal grooves that can be observed in many mimetids, especially those of the Northern Hemisphere16,17,18,32,45 (Fig. 3G–L).
The earliest males examined, two 4th instars (Fig. 1D), were without CY spigots. However, all nine later males (5th stadium and beyond) had either one CY spigot (Fig. 2B,D) or one CY nubbin (Fig. 1E) per PMS, matching the locations of CY spigots on female PMSs (Fig. 2A,C), though none on the PLS (Table 1). These nine males were from seven localities in New South Wales or Tasmania, with about 1100 km between the most widely separated collection sites (Supplementary Appendix S1). Three of these were juveniles (one each: 5th, 6th, 7th instars; Fig. 1B,C) and they all had a fully formed CY spigot on each PMS (Fig. 2B,E) with a clearly discernible base and shaft (Fig. 3A–C). Openings on these CY spigots were comparable to those of juvenile females, with diameters of 0.3 µm (5th instar), 0.4 µm (6th instar), and 0.8 µm (7th instar). In contrast, four of six adult males had a CY nubbin only, essentially a base without a shaft (Fig. 1F), on each PMS, and these included one 6th instar, two 7th instars (Fig. 1E), and one 8th instar (Table 1). A third adult male 7th instar had a CY nubbin on the right PMS and a CY spigot on the left PMS (Fig. 3E), albeit without an obvious opening (Fig. 3F); possibly an artifact of storage and/or preparation conditions. Openings were present on spigots of all other silk gland types in this specimen and our observations in this and other Australomimetus species have indicated that CY spigot shafts are especially susceptible to degradation (e.g. by enzyme cleaning) and distortion. Indeed, in the one adult male (of six), an 8th instar, that had a complete CY spigot on each PMS (Figs. 2D,F, 3D, Table 1), partial degradation was evident near the openings of these spigots and this was almost certainly an artifact, not shared by other spigots.
There was a trend for CY spigot shafts to become stouter (decreased height/width) from one stadium to the next (cf. Fig. 3A–C). In females, this trend ended with a flourish, the final molt producing an especially dramatic transformation to a large and domed shape (cf. Fig. 3I–L). By contrast, in adult males, shafts (if present) retained a basically juvenile morphology, comparable to females before the final molt (cf. Figs. 2F, 3D–J). This included an absence of wide openings: despite some degradation potentially inflating measurements, diameters of 0.9 µm and 1.1 µm were obtained for openings in the one adult male (8th instar) with measurable PMS CY spigot shafts (Figs. 2D,F, 3D), only modestly wider than those of late juvenile females and far from those of adult females (see above).
In summary, CY spigots or their vestiges were present in all male specimens we examined after the 4th stadium, but while three such juveniles displayed an intact CY spigot (base and shaft) on each PMS (Figs. 2B,E, 3A–C), only 25% of the PMSs on six adult males (i.e., 3 of 12 PMSs) were so endowed (Figs. 2D,F, 3D,E); the remaining 75% had a CY nubbin (base only), incapable of acting as silk conduits (Fig. 1E,F). Though not conclusive, CY spigots in males are strong external indicators of internal CYs, and CY nubbins are indicators of fully formed CY spigots earlier in ontogeny.
CY spigot sexual dimorphisms
The usual sexual dimorphism regarding CYs that exists among members of the CY spigot clade is that females possess CY and their spigots whereas males lack them altogether. In males of A. maculosus, CY spigots were clearly observed. Despite this, CY sexual dimorphism was still apparent in the form of CY spigot distribution, morphology, and, possibly, ontogeny. Females within the CY spigot clade usually have CY spigots distributed on both PMSs and PLSs7,8,9. Females of A. maculosus, like other Australomimetus17,19,30,31, were no exception and one CY spigot was present on each of these four spinnerets (Fig. 2A,C). Males instead had one CY spigot/nubbin on each of the PMSs, but none on the PLSs (Figs. 1E, 2B,D).
Adult females of most mimetids (except Gelanor) are known for having enlarged and stout CY spigots with dome-shaped shafts displaying wide apertures16,17,18,19,30,31,32,45. Again, adult females of A. maculosus conformed to this description (Fig. 3K,L) while adult males did not. In some instances, this was because only a CY nubbin formed on an adult male PMS (Fig. 1F). However, even when a complete CY spigot formed, its morphology was essentially like that of a juvenile (male and female), with the shaft lacking a wide aperture (Fig. 3D,E). An interesting parallel to this atypical CY spigot distribution and morphology exists in another mimetid genus, albeit in the opposite sex. In females of some species of the African genus Anansi Benavides and Hormiga, 2017, one CY spigot is present on each PMS only and these spigots lack an enlarged, rotund, wide-aperture morphology34. Male Anansi, like all examined mimetids except for A. maculosus, appear to lack CYs34.
Less certain is a sexual dimorphism concerning the stadium in which CY spigots first appear in the ontogeny of A. maculosus. In this study, because no females earlier than 5th instars were examined, we can only say with confidence that full sets of CY spigots (1/PMS, 1/PLS) were present in juvenile females by the 5th stadium (Fig. 2A, Table 1). However, observations from other species suggest their first appearance likely preceded this stadium. In studies of Mimetus puritanus Chamberlin, 192317, Mimetus notius Chamberlin, 192317, and Australomimetus spinosus Heimer, 198619, CY spigots first appeared in female 3rd instars, though often not the full set of four CY spigots in A. spinosus. By the 4th stadium, however, all four CY spigots were invariably present, as they were in the only examined female 4th instar of Australomimetus djuka Harms and Harvey, 200919 and in female 4th instars of six other species of Australomimetus we have examined. In contrast, assuming our identifications of two 4th stadium A. maculosus as males are correct (see Methods), it appears that the PMS CY spigots of males do not make their first appearance until the 5th stadium (Table 1, Fig. 3A). More juveniles will need to be examined to confirm or disprove a sexual dimorphism in CY spigot ontogeny.
Role of CY in male A. maculosus
The older literature contains opposing or indistinct views regarding the presence or absence of CYs in male spiders46. Consequently, roles played by apparently non-existent CYs in males were sometimes hypothesized, including contributing fibres to sperm webs47 and producing the core fibres of orb web sticky spirals48; the latter later shown to be products of flagelliform silk glands49,50. The presence of PMS CY spigots in male A. maculosus prompts us to consider anew potential CY function in males, if only for this single species at present. Relevant to such a consideration, we emphasize that a majority of CY structures on PMSs of adult male A. maculosus were non-functional nubbins (Fig. 1E,F, Table 1) whereas intact and apparently functional CY spigots were invariably present on both PMSs on the admittedly small number (3) of late juvenile males examined (Figs. 2B,E, 3A–C). Moreover, in the minority of instances in which an adult male PMS was equipped with an intact CY spigot, it retained a basically juvenile morphology (Figs. 2D,F, 3D–F), not the rotund, wide-aperture morphology of adult females (Figs. 2C, 3K,L). Thus, if CYs in male A. maculosus do play a role, these observations suggest they are largely, if not exclusively, used by late juvenile males. This conclusion argues strongly against male A. maculosus assisting in any capacity with egg sac construction.
Certainly, A maculosus does not differ drastically in behaviour and morphology from other Australian pirate spiders despite its adaptability to habitats ranging from natural rainforests to urbanized areas, and to an apparently wide prey spectrum that may include comb-footed spiders (family Theridiidae), nursery web spiders (Pisauridae), ecribellate orb weavers (Araneidae), cribellate orb weavers (Uloboridae), daddy-long-leg spiders (Pholcidae), and sheet web builders (Desidae)29. Nothing is known about behaviours specific to late juvenile or adult males of this species and the possible prey spectrum is based on host webs in which the spiders have been observed in the field29. Behavioural studies and controlled laboratory experiments may clarify the use of CYs by males, revealing behaviours that may involve CY silk production.
Drawing of CY silk by juveniles
We cannot assume that CY spigots in juvenile females are used in the drawing of CY silk19 since CYs exhibit little silk synthesis prior to the onset of vitellogenesis in adults7,8,21,27,28,51,52. Likewise, we cannot assume a priori that silk is drawn from CY spigots of juvenile male A. maculosus, though it does at least seem more probable than in juvenile females. This is because CYs in females perform a definite role in adults, contributing silk fibres to egg sacs, and explanations proposed for CY spigots in juvenile females do not imply use of CYs by these juveniles: namely, that such spigots reflect earlier-maturing ancestors24 or act as placeholders for the functioning CY spigots of adult females23. In contrast, the non-functional CY nubbins observed in a majority of adult males argues against a significant role for CYs in adult males, making the drawing of silk by late juvenile males, for a yet unknown purpose, the most likely explanation for CY spigot occurrence in males. It will be of interest to determine if late juvenile males differ from late juvenile females of A. maculosus in their CY development, especially with respect to quantities of silk dope accumulated in the lumen, and whether morphological or histological changes occur in the CYs following the final molt in males. As noted earlier19, if juvenile females do draw CY silk, the fibres are presumably considerably narrower than those drawn by adult females during egg sac construction given differences in diameters of their CY spigot openings (see CY spigot occurrence). For the same reason, any CY silk drawn by males is likewise expected to be much narrower.
Male CY spigots are unique to A. maculosus
As the type species of the genus, A. maculosus is in most respects a fitting representative; its somatic and genital morphology is typical30,31,53 despite its relatively large body size30. Conversely, the possession of PMS CY spigots by males is clearly a unique feature. We have examined spinnerets by SEM from males of 17 other species in this genus (A. annulipes Heimer, 1986, A. audax (Hickman, 1929), A. aurioculatus (Hickman, 1929)17,31, A. catulli (Heimer, 1989), A. daviesianus Heimer, 1986, A. diabolicus Harms and Harvey, 200917, A. djuka Harms and Harvey, 200919, A. hartleyensis Heimer, 1986, A. hirsutus Heimer, 1986, A. japonicus (Uyemura, 1938), A. kioloensis Heimer, 1986, A. mendax Harms and Harvey, 2009, A. mendicus (O. Pickard-Cambridge, 1880), A. pseudomaculosus Heimer, 198617, A. spinosus Heimer, 198619, A. sydneyensis Heimer, 1986, A. tasmaniensis (Hickman, 1929)17) (Supplementary Fig. S1A–M if no reference given), as well as spinnerets from males of another eight as yet undescribed species (Supplementary Fig. S1N–U), and in none of these were CY spigots or CY nubbins observed.
Our results mark this species as a unique representative of the CY spigot clade that deviates from a widely held paradigm for modern spiders, but the secrets underlying its unique male morphology have yet to be revealed. We suggest behavioural, anatomical, and developmental studies on this species to explore the basis of this unique arrangement in spider evolution.
Methods
Spinneret examination
Spinnerets from 19 specimens of Australomimetus maculosus (Table 1), preserved in 75% ethanol, were prepared and examined by scanning electron microscopy (SEM) as described previously19. Collection and repository data for these specimens is given in Supplementary Appendix S1. All were collected in eastern Australia, from Far North Queensland to Tasmania. Eleven of the 19 specimens were males from eight localities, though only one male, a juvenile in the 4th stadium (Fig. 1D), was collected in Far North Queensland. The other ten males, including nine that were in the 5th stadium or later (i.e., those with CY spigots or nubbins) (Fig. 1B,C), were collected in New South Wales (five locations) or Tasmania (two locations).
Diameters of spigot openings were obtained from SEM images using the measurement tool in Tescan Lyra3 Control Software. Each spigot aperture was measured twice: at its widest point and perpendicular to this, with the mean then taken.
To facilitate comparisons among spinnerets of the same type (ALS, PMS, or PLS), any single spinneret scans from the right side of the opisthosoma were flipped in Microsoft PowerPoint version 1808 so they appear to be from the left side: actual handedness (right, left) is stated in the figure legends.
Terminology
Spinneret terminology follows previous research19. Briefly, a nubbin is a vestigial silk gland spigot while a tartipore is a short conduit that forms during proecdysis (the preparatory period before ecdysis) within the developing exoskeleton, surrounding a silk gland duct. This opening or pore allows the duct to remain attached to a spigot on the older, overlying exoskeleton so that silk can still be drawn from the spigot despite the intervening new exoskeleton. After ecdysis, the tartipore, though no longer functional, is still visible in the new exoskeleton.
Note that silk gland abbreviations such as ‘CY’ and ‘AC’ stand for ‘cylindrical silk gland’ and ‘aciniform silk gland’ rather than just ‘cylindrical’ and ‘aciniform’. Thus, a term like ‘CY spigot’ should be understood as meaning ‘cylindrical silk gland spigot’. In figures, to reduce labeling, CY spigots/nubbins are labeled ‘CY’ alone, but the legend states that this label stands for CY spigot or CY nubbin.
No early instars of A. maculosus were examined during this study but for instar/stadium assignments to be most meaningful we need to define the 1st instar and 1st stadium, an instar being the spider itself and a stadium being the period between ecdyses or following the final ecdysis. We follow Downes54 in calling the spider that hatches from the egg a postembryo. The 1st instar emerges and the 1st stadium begins after the postembryo has molted and discarded its old exoskeleton. At least among most araneoids, a functioning spinning apparatus is available to 1st instars, but not to postembryos.
Species, instar/stadium, and sex determinations
Juvenile specimens of A. maculosus were distinguished from other Australomimetus species on the basis of somatic characters30; adult specimens on the basis of characteristic genital structures: prominent, complex palpal bulbs in males, sclerotized epigynum and spermathecae in females30,53,55. The presence or absence of these genital structures was unequivocally ascertained and, when present, guaranteed the specimen had attained full maturity and completed its final molt.
To estimate the stadium an individual was in at the time of death, we counted PI spigots, PI tartipores, and AC spigots (Table 1) since these increase in number, albeit with variation, during ontogeny. Stadium estimates were further refined by noting the position of the post-functional 2° MiA tartipore relative to the 2° MiA spigot (if a juvenile) or 2° MiA nubbin (if an adult). If this tartipore was (postero)medial to the 2° MiA spigot/nubbin (see Fig. 2F), the most likely even-numbered stadium was assigned; if anterolateral (see Fig. 2E), the most likely odd-numbered stadium was assigned. This pattern has been observed consistently in seven other species of Australomimetus that, like A. maculosus, possess 2° MiA, as opposed to others that have lost these silk glands19 (See Supplementary Fig. S1A–C,I,O,Q,R for examples of species without 2° MiA). Moreover, the same pattern has previously been observed in two Mimetus Hentz, 1832 species17 and two araneid species (Neoscona theisi (Walckenaer, 1841)22, Araneus cavaticus (Keyserling, 1881)17,23), though Larinioides cornutus (Clerck, 1757) may not be consistent (Yu and Coddington22 figs. 8–11, note though that their ‘2nd instar’ is a ‘1st instar’ by our definition).
The sex of juveniles was determined by examining palps for distal swelling, indicative of forthcoming male palpal bulbs. Though swelling is most pronounced in penultimate males (Fig. 1B), it can be discerned earlier than this. Indeed, two estimated 4th instars identified as males exhibited a greater degree of palp swelling than was observed in late juveniles and adults that were unquestionably female.
Data availability
An additional figure and an appendix have been uploaded as part of the electronic Supplementary Material. Higher resolution spinneret SEM files are available from M.A.T.
References
Garrison, N. L. et al. Spider phylogenomics: untangling the Spider Tree of Life. PeerJ 4, e1719, https://doi.org/10.7717/peerj.1719 (2016).
Wheeler, W. C. et al. The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics 33, 574–616, https://doi.org/10.1111/cla.12182 (2017).
Fernández, R. et al. Phylogenomics, diversification dynamics, and comparative transcriptomics across the Spider Tree of Life. Curr. Biol. 28, 1489–1497; https://doi.org/10.1016/j.cub.2018.03.064; corrections 28, 2190–2194; https://doi.org/10.1016/j.cub.2018.06.018 (2018).
Griswold, C. E. & Ramírez, M. J. Phylogeny of spiders in Spiders of North America: An Identification Manual, 2nd Ed. (eds. Ubick, D., Paquin, P., Cushing, P. E. & Roth, V.) 17–29 (American Arachnological Society, 2017).
Meckel, H. Mikrographie einiger Drüsenapparate der niederen Thiere. Arch. Anat. Physiol. wissenschaft. Med. (Müller’s Arch.) 1–73, Plates I–III; https://www.biodiversitylibrary.org/item/50102#page/295/mode/1up (1846).
Oeffinger, H. Der feinere Bau der Spinnorgane von Epeira: Eine vergleichend histologische Untersuchung. Arch. mikroskop. Anat. 2, 1–12, Plate 1, https://www.biodiversitylibrary.org/item/49504#page/11/mode/1up (1866).
Kovoor, J. La soie et les glandes séricigènes des arachnides. Ann. Biol. 16, 97–171 (1977).
Kovoor, J. Comparative structure and histochemistry of silk-producing organs in arachnids in Ecophysiology of Spiders (ed. Nentwig, W.) 160–186 (https://doi.org/10.1007/978-3-642-71552-5_12 Springer, 1987).
Ramírez, M. J. The morphology and phylogeny of dionychan spiders (Araneae: Araneomorphae). Bull. Amer. Mus. Nat. Hist. 390, 1–374, https://doi.org/10.1206/821.1 (2014).
Ramakrishna & Tikader, B. K. Role of spinning apparatus in non orb-weaving and orb-weaving spiders from India. Rec. Zool. Surv. India, Occas. Pap. 101, 1–132 (1988).
Tillinghast, E. K. & Townley, M. A. Silk glands of araneid spiders: selected morphological and physiological aspects in Silk Polymers: Materials Science and Biotechnology, ACS Symp. Ser. 544 (eds. Kaplan, D., Adams, W. W., Farmer, B. & Viney, C.) 29–44, (https://doi.org/10.1021/bk-1994-0544.ch003 American Chemical Society, 1994).
Blackledge, T. A., Kuntner, M. & Agnarsson, I. The form and function of spider orb webs: evolution from silk to ecosystems. Adv. Insect Physiol. 41, 175–262, https://doi.org/10.1016/B978-0-12-415919-8.00004-5 (2011).
Garb, J. Spider silk: An ancient biomaterial for 21st century research in Spider Research in the 21st Century: trends & perspectives (ed. Penney, D.) 252–281 (Siri Scientific Press, 2013).
Coddington, J. A. Spinneret silk spigot morphology: evidence for the monophyly of orbweaving spiders, Cyrtophorinae (Araneidae), and the group Theridiidae plus Nesticidae. J. Arachnol. 17, 71–95, http://www.americanarachnology.org/JoA_free/JoA_v17_n1/JoA_v17_p71.pdf (1989).
Platnick, N. I., Coddington, J. A., Forster, R. R. & Griswold, C. E. Spinneret morphology and the phylogeny of haplogyne spiders (Araneae, Araneomorphae). Amer. Mus. Novit. 3016, 1–73, http://hdl.handle.net/2246/5043 (1991).
Griswold, C. E., Ramírez, M. J., Coddington, J. A. & Platnick, N. I. Atlas of phylogenetic data for entelegyne spiders (Araneae: Araneomorphae: Entelegynae) with comments on their phylogeny. Proc. Calif. Acad. Sci., 4th Ser. 56(Suppl. II), 1–324 (2005).
Townley, M. A. & Tillinghast, E. K. Developmental changes in spider spinning fields: a comparison between Mimetus and Araneus (Araneae: Mimetidae, Araneidae). Biol. J. Linn. Soc. 98, 343–383, https://doi.org/10.1111/j.1095-8312.2009.01297.x (2009).
Townley, M. A., Harms, D. & Benjamin, S. P. Phylogenetic affinities of Phobetinus to other pirate spider genera (Araneae: Mimetidae) as indicated by spinning field morphology. Arthropod Struct. Dev. 42, 407–423, https://doi.org/10.1016/j.asd.2013.04.003 (2013).
Townley, M. A. & Harms, D. Comparative study of spinning field development in two species of araneophagic spiders (Araneae, Mimetidae, Australomimetus). Evol. Syst. 1, 47–75, https://doi.org/10.3897/evolsyst.1.14765 (2017).
Machado, A. de Barros Observations inédites sur le colulus et les filières de quelques Aranéides, accompagnées de notes critiques sur la morphologie compare des filières. Arquiv. Mus. Bocage 15, 13–52 (1944).
Sekiguchi, K. The spinning organs in sub-adult geometric spiders and their changes accompanying the last moulting. Sci. Rep. Tokyo Kyoiku Daigaku. Sect. B 8, 33–40 (1955).
Yu, L. & Coddington, J. A. Ontogenetic changes in the spinning fields of Nuctenea cornuta and Neoscona theisi (Araneae, Araneidae). J. Arachnol. 18, 331–345; http://www.americanarachnology.org/JoA_free/JoA_v18_n3/arac_18_3_0331.pdf (1990).
Townley, M. A., Horner, N. V., Cherim, N. A., Tugmon, C. R. & Tillinghast, E. K. Selected aspects of spinning apparatus development in Araneus cavaticus (Araneae, Araneidae). J. Morph. 208, 175–191 & C5–C7; https://doi.org/10.1002/jmor.1052080204 (1991).
Müller, M. C. & Westheide, W. Comparative morphology of the sexually dimorphic orb-weaving spider Argiope bruennichi (Araneae: Araneidae). Mem. Queensland Mus. 33, 615–620, https://www.biodiversitylibrary.org/page/40476084#page/236/mode/1up (1993).
Moon, M.-J. Organization of the spinnerets and spigots in the orb web spider, Argiope bruennichi (Araneae: Araneidae). Entomol. Res. 42, 85–93, https://doi.org/10.1111/j.1748-5967.2011.00440.x (2012).
Alfaro, R. E., Griswold, C. E. & Miller, K. B. Comparative spigot ontogeny across the spider tree of life. PeerJ 6, e4233, https://doi.org/10.7717/peerj.4233 (2018).
Atanasiu-Dumitresco, M. Contributions a l’étude anatomique et cytologique de l’appareil séricigène des araignées: I-ère partie: Anatomie de l’appareil séricigène de quelques espèces d’araignées. Anal. Acad. Rom., Mem. Secţ. Ştiinţ., Ser. 3 16, 773–840, Plates I–XI; http://www.digibuc.ro/colectii/aarmss–analele-academiei-romane-memoriile-sectiunii-stiintifice-c7321 (1941).
Richter, C. J. J. Morphology and function of the spinning apparatus of the wolf spider Pardosa amentata (Cl.) (Araneae, Lycosidae). Z. Morph. Tiere 68, 37–68, https://doi.org/10.1007/BF00277422 (1970).
Jackson, R. R. & Whitehouse, M. E. A. The biology of New Zealand and Queensland pirate spiders (Araneae, Mimetidae): aggressive mimicry, araneophagy and prey specialization. J. Zool., Lond. Ser. A 210, 279–303, https://doi.org/10.1111/j.1469-7998.1986.tb03635.x (1986).
Harms, D. & Harvey, M. S. A review of the pirate spiders of Tasmania (Arachnida: Mimetidae: Australomimetus) with description of a new species. J. Arachnol. 37, 188–205, https://doi.org/10.1636/A08-35.1 (2009).
Harms, D. & Harvey, M. S. Australian pirates: systematics and phylogeny of the Australasian pirate spiders (Araneae: Mimetidae), with a description of the Western Australian fauna. Invert. Syst. 23, 231–280, https://doi.org/10.1071/IS08015 (2009).
Platnick, N. I. & Shadab, M. U. A review of the pirate spiders (Araneae, Mimetidae) of Chile. Amer. Mus. Novit. 3074, 1–30; http://hdl.handle.net/2246/4971 (1993).
Benavides, L. R. & Hormiga, G. Taxonomic revision of the Neotropical pirate spiders of the genus Gelanor Thorell, 1869 (Araneae, Mimetidae) with the description of five new species. Zootaxa 4064, 1–72, https://doi.org/10.11646/zootaxa.4064.1.1 (2016).
Benavides, L. R., Giribet, G. & Hormiga, G. Molecular phylogenetic analysis of “pirate spiders” (Araneae, Mimetidae) with the description of a new African genus and the first report of maternal care in the family. Cladistics 33, 375–405, https://doi.org/10.1111/cla.12174 (2017).
Townley, M. A., Tillinghast, E. K. & Cherim, N. A. Moult-related changes in ampullate silk gland morphology and usage in the araneid spider Araneus cavaticus. Phil. Trans. Roy. Soc. Lond. B 340, 25–38, https://doi.org/10.1098/rstb.1993.0046 (1993).
Townley, M. A. & Tillinghast, E. K. On the use of ampullate gland silks by wolf spiders (Araneae, Lycosidae) for attaching the egg sac to the spinnerets and a proposal for defining nubbins and tartipores. J. Arachnol. 31(2), 209–245, https://doi.org/10.1636/0161-8202(2003)031[0209:OTUOAG]2.0.CO; (2003).
Levy, G. The life cycle of Thomisus onustus (Thomisidae: Araneae) and outlines for the classification of the life histories of spiders. J. Zool., Lond. 160, 523–536, https://doi.org/10.1111/j.1469-7998.1970.tb03095.x (1970).
Higgins, L. E. & Rankin, M. A. Different pathways in arthropod postembryonic development. Evolution 50, 573–582, https://doi.org/10.2307/2410832 (1996).
Dolejš, P., Buchar, J., Kubcová, L. & Smrž, J. Developmental changes in the spinning apparatus over the life cycle of wolf spiders (Araneae: Lycosidae). Invert. Biol. 133, 281–297, https://doi.org/10.1111/ivb.12055 (2014).
Mallis, R. E. & Miller, K. B. Natural history and courtship behavior in Tengella perfuga Dahl, 1901 (Araneae: Zoropsidae). J. Arachnol. 45, 166–176, https://doi.org/10.1636/15-004.1 (2017).
Uhl, G., Schmitt, S., Schäfer, M. A. & Blanckenhorn, W. Food and sex-specific growth strategies in a spider. Evol. Ecol. Res. 6, 523–540, https://doi.org/10.5167/uzh-172731 (2004).
Higgins, L. Female gigantism in a New Guinea population of the spider Nephila maculata. Oikos 99, 377–385, https://doi.org/10.1034/j.1600-0706.2002.990220.x (2002).
Higgins, L. & Goodnight, C. Developmental response to low diets by giant Nephila clavipes females (Araneae: Nephilidae). J. Arachnol. 39, 399–408, https://doi.org/10.1636/B11-18.1 (2011).
Higgins, L., Coddington, J., Goodnight, C. & Kuntner, M. Testing ecological and developmental hypotheses of mean and variation in adult size in nephilid orb-weaving spiders. Evol. Ecol. 25, 1289–1306, https://doi.org/10.1007/s10682-011-9475-9 (2011).
Schütt, K. The limits of the Araneoidea (Arachnida: Araneae). Austral. J. Zool. 48, 135–153, https://doi.org/10.1071/ZO99050 (2000).
Sekiguchi, K. Differences in the spinning organs between male and female adult spiders. Sci. Rep. Tokyo Kyoiku Daigaku. Sect. B 8, 23–32 (1955).
Apstein, C. Bau und Function der Spinndrüsen der Araneida. Arch. Naturgesch. 55, 29–74, Plates III–V, https://www.biodiversitylibrary.org/item/29775#page/37/mode/1up (1889).
Warburton, C. The spinning apparatus of geometric spiders. Quart. J. Microsc. Sci., New Ser. 31, 29–39, Plate V, http://jcs.biologists.org/content/joces/s2-31/121/29.full.pdf (1890).
Sekiguchi, K. On a new spinning gland found in geometric spiders and its functions. Annot. Zool. Japon. 25, 394–399, Plate 4 (1952).
Peters, H. M. Über den Spinnapparat von Nephila madagascariensis (Radnetzspinnen, Fam. Argiopidae). Z. Naturforsch. 10b, 395–404, https://www.degruyter.com/downloadpdf/j/znb.1955.10.issue-7/znb-1955-0708/znb-1955-0708.pdf (1955).
Atanasiu-Dumitresco, M. Contributions a l’étude anatomique et cytologique de l’appareil séricigène des araignées: II-ème partie: Histologie et cytologie des glandes séricigènes des araignées. Anal. Acad. Rom., Mem. Secţ. Ştiinţ., Ser. 3 17, 263–349, Plates I–IX, http://www.digibuc.ro/colectii/aarmss–analele-academiei-romane-memoriile-sectiunii-stiintifice-c7321 (1941).
Wąsowska, S. Studies on the spinning apparatus in spiders. Postembryonic morphogeny of the spinning apparatus. Zool. Polon. 26, 355–407 (1977).
Heimer, S. Notes on the spider family Mimetidae with description of a new genus from Australia (Arachnida, Araneae). Entomol. Abhand.,Staat. Mus. Tier., Dres. 49, 113–137, https://wsc.nmbe.ch/reference/6234 (1986).
Downes, M. F. A proposal for standardization of the terms used to describe the early development of spiders, based on a study of Theridion rufipes Lucas (Araneae: Theridiidae). Bull. Brit. Arachnol. Soc. 7, 187–193, http://britishspiders.org.uk/bulletin/070609.pdf (1987).
Rainbow, W. J. Studies in Australian Araneidae. No. 3. Rec. Austral. Mus. 5, 326–336, Plate XLVI, https://doi.org/10.3853/j.0067-1975.5.1904.1072 (1904).
Acknowledgements
We are especially indebted to Greg J Anderson for collecting specimens and graciously allowing us to reproduce his photos of A. maculosus. Specimens were also collected by Ingi Agnarsson, Mara E Blosfelds, Lisa J Boutin, Stefan Eberhard, Brian M Fitzgerald, Michael R Gray, Elen Harvey, Frances SB Harvey, Mark S Harvey, John L Hickman, Vernon V Hickman, C Horseman, Jean E Jackson, S Johnson, Jun-ichi Kojima, Y Konishi, John F Lawrence, Bryce N McQuillan, Rosa Menéndez, Graham A Milledge, Geoffrey B Monteith, Michael G Rix, Helen M Smith, and Thomas A Weir, to all of whom we extend our sincere thanks. We also thank Graham A Milledge and Helen M Smith (Australian Museum), Robert Raven and Owen Seeman (Queensland Museum), and Mark S Harvey (Western Australian Museum) for the loan of critical specimens, and Cor Vink for sending the New Zealand specimens included in the Supplementary Information. The SEM used is managed by the University Instrumentation Center at UNH and was purchased with funds from the US National Science Foundation (MRI grant 1337897) awarded to Todd Gross (Mechanical Engineering, UNH), PI, and from UNH.
Author information
Authors and Affiliations
Contributions
M.A.T. prepared and examined spinnerets by SEM, prepared figures/table, and wrote the manuscript. D.H. designed the Australasian mimetid study, of which this study is a part, collected specimens in the field, obtained loans of other specimens, and co-wrote this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Townley, M.A., Harms, D. Hers and his: Silk glands used in egg sac construction by female spiders potentially repurposed by a ‘modern’ male spider. Sci Rep 10, 6663 (2020). https://doi.org/10.1038/s41598-020-63521-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-63521-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.