Abstract
The biology of bacterial cells is, in general, based on information encoded on circular chromosomes. Regulation of chromosome replication is an essential process that mostly takes place at the origin of replication (oriC), a locus unique per chromosome. Identification of high numbers of oriC is a prerequisite for systematic studies that could lead to insights into oriC functioning as well as the identification of novel drug targets for antibiotic development. Current methods for identifying oriC sequences rely on chromosome-wide nucleotide disparities and are therefore limited to fully sequenced genomes, leaving a large number of genomic fragments unstudied. Here, we present gammaBOriS (Gammaproteobacterial oriC Searcher), which identifies oriC sequences on gammaproteobacterial chromosomal fragments. It does so by employing motif-based machine learning methods. Using gammaBOriS, we created BOriS DB, which currently contains 25,827 gammaproteobacterial oriC sequences from 1,217 species, thus making it the largest available database for oriC sequences to date. Furthermore, we present gammaBOriTax, a machine-learning based approach for taxonomic classification of oriC sequences, which was trained on the sequences in BOriS DB. Finally, we extracted the motifs relevant for identification and classification decisions of the models. Our results suggest that machine learning sequence classification approaches can offer great support in functional motif identification.
Similar content being viewed by others
Introduction
Before every cell division, bacteria need to duplicate their genetic material to ensure that this information can faithfully be passed on to both daughter cells. This essential process, called DNA replication, initiates in a highly regulated manner at chromosomal sites called oriC and is coordinated with many other cellular processes1,2. With notable exceptions as e.G. Vibrionales, usually, bacteria contain one or multiple copies of a single chromosome, which carries a single oriC sequence3,4.
Since many different proteins need to bind to and act upon oriC for initiation to occur, oriC contains many protein binding sites and DNA motifs5,6. While there is a high level of variation between oriC sequences of different organisms, there are also nearly universally occurring DNA motifs in oriC sequences7,8,9. Central among these are 9 bp short DNA motifs called DnaA boxes, which act as binding sites for the initiator protein DnaA, and exhibit differing protein binding characteristics depending on the exact sequence. Starting from these motifs, DnaA polymerizes and spreads across multiple DnaA boxes and DnaA trio motifs10, which then, in interplay with the protein IHF11, leads to double helix unwinding at a closely positioned AT-rich region called DNA unwinding element (DUE) so that the replication machinery can be loaded onto the DNA12,13. As oriC contains binding sites for proteins that relay information on the status of the cell, it can be considered as a biological information compiler and processor14,15. Taken together, these properties make oriC sequences an outstanding object for the study of DNA motifs.
All currently available computational methods for the identification of oriC sequences in bacterial chromosomes rely on nucleotide disparities on the leading and lagging strand of the DNA double helix16,17,18,19,20. As replication usually extends from oriC bidirectionally, it is one of two chromosomal sites where the leading and lagging strand switch places. The most frequently used disparity, the GC skew, usually assumes a V- or inverted V-shape with its minimum or maximum, respectively, indicating the presence of oriC21,22. However, due to natural variation, the shape of the skew can only be reliably asserted when analyzing whole chromosomal sequences or large fragments thereof. Combining the GC skew with the location of DnaA boxes, Ori-Finder23 was successfully used to identify a wide range of bacterial oriC seqences and, subsequently, create the current state-of-the-art oriC database DoriC24,25.
Deep neural networks (DNNs) have been employed for tasks similar to the identification of oriC sequences26,27,28,29,30,31,32. However, these methods are notorious for needing big amounts of data and computing power. Support vector machines (SVMs) that perform classification based on k-mer (i.e., n-gram) counts represent a less data-intensive alternative and have even been shown to outperform DNNs for smaller datasets33,34. Some k-mer-SVMs use models of DNA models that allow mismatches or gaps while performing k-mer counting, taking into account the effect of natural variation35,36,37. Furthermore, most of these machine learning models can produce a list of features important for the classification task, which is, in this case, a list of most relevant motifs.
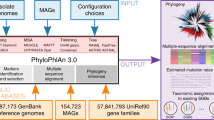
In the current study, we present a machine-learning based approach for the study of bacterial oriC sequences in four parts, exemplified on Gammaproteobacteria. This class of organisms contains many model organisms (e.g., Escherichia coli, Vibrio cholerae, and Pseudomonas putida), and causative agents for serious illnesses (such as cholera, plague, and enteritis), which makes this taxon a highly relevant study object. First, we present gammaBOriS (Gammaproteobacterial oriC Searcher), a tool that identifies oriC sequences in full chromosomes as well as chromosomal fragments of Gammaproteobacteria. Secondly, using publicly available Gammaproteobacterial chromosomal fragments as input for gammaBOriS, we gathered the largest dataset of bacterial oriC sequences available to date, BOriS DB. Based on this, we thirdly trained a set of machine learning models to classify these sequences according to their respective order, family, and genus. Finally, we present a list of motifs that were important for the identification and classification and show that the machine learning models presented here were able to learn biologically relevant information from the DNA sequences presented to them.
Results
Implementation of gammaBOriS
gammaBOriS is implemented in R and requires a Linux operating system. The front end of the website is written in jQuery in order to make gammaBOriS accessible without specific software requirements. As input file, gammaBOriS takes a fasta-formatted file containing one or more DNA sequences of any length and returns two fasta-formatted text files: One contains fragments gammaBOriS identified as oriC and the other contains DNA fragments for which the classifier abstained from a decision (see Methods).
gammaBOriS is composed of three modules that were adjusted for and trained on a training set of Gammaproteobacterial oriC sequences (Fig. 1). The core module consists of a k-mer-SVM, whose parameters were chosen to maximize the AUC of discrimination between oriC and non-oriC sequences for a balanced test dataset (for details see Methods, Fig. 1). To this end, we trained a total of 12,877 LS-GKM and spectrum kernel SVMs33,35,36 with varying parameters and sequence fragment sizes. We chose LS-GKM and spectrum kernel SVMs since these models can use DNA input natively, and model variations in motifs while retaining a fast runtime. The highest performance (an AUC of 0.958 on the validation dataset) was achieved with a LS-GKM model trained with 1250 bp fragments as input, a word length of 10 bp with 6 informative columns, and at most 4 mismatches (see Supplementary Figure 1, Supplementary Table 1).
To turn this sequence classifier into a sequence identifier, the first module of gammaBOriS splits the input sequence into a manageable number of candidate fragments by picking only fragments centered around an occurrence of a so-called seed sequence. A list of seed sequences was created by extracting the central 9bp sequences from each sequence in the oriC training dataset. This choice of seed sequences was validated by showing that all oriC sequences in the test dataset are centered around one of the seed sequences.
The third module of gammaBOriS assigns a class label to every fragment based on the classification value obtained for this sequence in the second module. As, for one input sequence, the number of candidate sequences is expected to be much higher than the number of correct oriC sequences, this can be viewed as a highly imbalanced classification problem. To mitigate high numbers of false-positive classifications, we make use of the concept of classification with abstaining38. To this end, two cutoff values are employed; below the lower cutoff, fragments are labeled “negative” and discarded, above the upper cutoff, fragments are labeled “positive”, and between the cutoffs, the classifier abstains from labeling the fragments. In the choice of cutoffs, we maximized the value of the F1 metric and minimized the number of correct oriC sequences for which this module abstained from classification, leading to a Pareto-optimal state. We found that normalizing the classification values of the fragments extracted from one sequence to a range between [0, 1] and employing cutoffs of 0.99 and 0.41 lead to the best result on the test dataset (F1 of 0.943 with 0.7% of correct oriCs in the abstained space; Fig. 2, see Supplementary Figure 2). This cutoff set was also chosen to minimize the possibility of false negative classification, taking into account a slightly higher number of false-positive classifications, as false-positive sequences can still be filtered out based on domain knowledge while false negative classifications usually cannot be reverted. Similarly, sequences for which gammaBOriS abstains from classification are also returned to the user, so that other methods as well as domain knowledge can be used to reach a decision on whether a fragment contains an oriC sequence or not.
Taxonomic distribution and gammaBOriS prediction results for oriC sequences present in the training and test dataset (see main text for more details). Color codification, from outer to inner ring, separated by semicolons, with numbers of organisms in each category in parentheses: dark blue signifies chromosomes that contain two (105), light blue those that contain a single oriC sequence (355); lime green indicates that two (13) sequences were correctly identified, pale green that one (392) was correctly identified, spring green indicates that a sequence was identified that overlaps with the correct sequence (48), white indicates incorrect identification (7); red, yellow, and white indicate that two (2), one (4), and zero (454) sequences were misidentified as oriC sequence (false-positive), respectively; darker color indicates a higher number of candidate fragments that fall between the two cutoffs, so that gammaBOriS abstained from classification for these (min.: 1, max.: 2463, mean: 129.38).
Construction of BOriS DB
We then applied gammaBOriS to all chromosomes and chromosomal fragments present in the RefSeq database39 (restricted to sequences with the release type “Major”) as well as the genomes in the Uncultivated Bacteria and Archaea (UBA) dataset40. Both datasets contain a high number of incompletely sequenced chromosomes and chromosomal fragments, and therefore cannot be analyzed using previous oriC identification methods. After discarding sequences present in both databases, we retained 25,827 oriC sequences from 1,217 different gammaproteobacterial species, most of which were not identified before. These sequences constitute the first version of BOriS DB.
Since only very few oriC sequences are experimentally confirmed for Gammaproteobacteria, and there is no established oriC benchmark dataset, a direct, computational comparison of oriC identification tools is infeasible. In theory, it is possible to assess whether a DNA sequence functions as oriC using wet lab experiments, however, especially for non-model organisms, these are prohibitively costly and error-prone. Instead, we compared a subset of BOriS DB to the current state-of-the-art oriC database, DoriC24,25 (for details, see supporting information). In 67, 90% of the chromosomes, both databases contain the same oriC sequence. In many of the cases of disagreement, the sequences identified BOriS DB show a slightly higher degree of consistency when compared to closely related sequences (see Supplementary Figures 3 and 4), although this result is hard to interpret due to a lack of experimentally confirmed oriC sequences and an established oriC benchmark dataset.
We conclude that BOriS DB, while only containing sequences from Gammaproteobacteria, is the largest database of oriC sequences to date and is at least as exact as DoriC.
Taxonomic Classification of oriC sequences
To make use of the information gathered in BOriS DB, we constructed a machine learning model that classifies gammaproteobacterial oriC sequences taxonomically at the levels of order, family, and genus. To this end, we employed nine different machine learning models, which include LS-GKM and Random Forests (RFs)36,41. For the latter, sequences were encoded using one-hot encoding, k-mer counting with 1 ≤ k ≤ 6, and a word2vec model42. Word2vec aims at deriving semantic information from the syntactic position of words (here: k-mers) and has shown great promise for natural language. Since taxonomic classification is an imbalanced multi-class classification problem, we chose macroAUPR as evaluation metric, which is calculated by averaging over the AUPR values of all classes of a taxonomic level.
The results show that, consistent across the different taxonomic levels, LS-GKM outperforms the other methods and achieves macroAUPR values of ≥0.9. RFs trained on word2vec-derived encodings, on the other hand, show the worst performance (Fig. 3). This might be because, in contrast to Random Forests, neural network-based machine learning approaches require a high amount of training data. Furthermore, there are structural differences between DNA sequences and natural languages, for which word2vec was originally designed. The fact that all models perform better at the level of order than at the level of family or genus suggest that the information present in oriC sequences is more specific at the genus level than at the others. It is also noteworthy that RFs trained on one-hot encoded sequences outperformed those trained on k-mer counting encodings, as this suggests that the position of certain motifs in the sequence is important for taxonomic classification.
Evaluation of different models for taxonomic classification of oriC sequences present in BOriS DB. MacroAUPR designates the average of the area under the precision-recall curve, a common metric for imbalanced multi-class classification tasks. RF stands for Random Forest. Error bars represent the standard deviation of the AUPR values achieved for the different taxa.
Since LS-GKM models also outperformed RF models when evaluated in regards to single taxa (see Supplementary Figures 5–7), we decided to use the former for gammaBOriTax, a tool for taxonomic classification of gammaproteobacterial oriC sequences. gammaBOriTax is available at boris.heiderlab.de both as a stand-alone tool as well as for automatic post-processing of the output of gammaBOriS.
Motif extraction
Like many other machine learning approaches, LS-GKM allows for the extraction of feature importance values that describe the relevance of motifs for the classification decision. By extracting the feature importance values from the LS-GKM model at the core of gammaBOriS, and discarding all motifs with a negative importance value, we obtained 74 motifs (see Supplementary Table 2 for a full list). Grouping these motifs by sequence similarity and common substrings results in four motif classes:
- (i)
28 motifs can be summarized to AAAGATCTTT. This sequence contains the motif GATC, which has many different functions in Gammaproteobacteria43,44,45; also, substrings of this consensus sequence are present in most of the sequences in BOriS DB,
- (ii)
27 motifs belong to a group of AT-rich motifs that form the consensus TAATAATAA (or, if allowing for ambiguous bases, ATAWWHATA), which constitutes the DnaA trio motif10,
- (iii)
15 motifs belong to a group of motifs that is reverse-complementary to those in class (ii),
- (iv)
8 motifs can be summarized to the consensus sequence TTCTGTGGATA, which is the sequence of a high affinity DnaA box (R1 and R4 in the oriC sequence of E. coli)46,47.
As these four motifs cover most of the known functional classes of motifs in oriC sequences, this result shows that the LS-GKM models can learn biologically relevant motifs during training, even if the models has had no access to knowledge about the biological function of the motifs.
We then employed the motif extraction process to the LS-GKM models trained for taxonomic classification. However, only for 13 models we obtained any positively valued k-mers (see Supporting Table 3 for a full list). We noticed that many of these displayed large overlaps (i.e., common substrings without any mutations at the end of one and the beginning of another k-mers) with other k-mers extracted from the same taxon and therefore decided to assemble them according to these overlaps. The resulting motifs are presented in Table 1, together with functional annotations derived by comparison to motifs of known function present in oriC. The fact that these motifs represent many of the known functional components of gammaproteobacterial oriC sequences further supports the finding that LS-GKM models can learn biologically relevant information from sequence alone.
It is noteworthy that the tetramer GATC is only present in motifs extracted from taxa that contain a Dam methyltransferase gene48,49. Dam methylates GATC motifs and is implicated in many processes that regulate replication initiation and DNA repair43. Surprisingly, for Vibrionales and Vibrionaceae, we obtained a motif that closely resembles the binding site consensus sequence for the initiator protein for the secondary chromosomes of these organisms, RctB50. The presence of a RctB binding site in oriC from Vibrionaceae has not been described before. Albeit the deviations in sequence might lead to a lower binding affinity, this motif might play a part in the coordination of the replication initiation between the two chromosomes51,52. Taken together, the results presented in this section show that, generally, LS-GKM models can learn biologically significant patterns and might be used to identify novel, functionally important motifs.
Because we were able to obtain important k-mers for only a few taxa, we also extracted the 20 highest-valued motifs for each taxon (for a full list see Supplementary Table 4). In these motifs, we observe a significantly higher amount of GATC motifs in taxa that do contain a Dam gene compared to those that do not (see Supplementary Figure 8). As there are no reliable databases for DNA binding sites of proteins involved in DNA replication initiation (i.e. mostly non-transcription regulatorory proteins), functional annotation of these motifs is infeasible. However, based on the results presented above, we expect that many of the motifs discovered here are specific for the respective taxon and associated with proteins essential for replication initiation.
Discussion
The application of methods from the field of machine learning to biology hold great promise, especially for the classification and identification of DNA sequences and the effect of variants in them53,54,55. However, only few studies have used machine learning approaches to illuminate the biology of prokaryotes. E. g., while machine learning methods have already been employed for the identification of origins of replication in yeast56, oriC identification in bacterial chromosomes is based on chromosome-wide nucleotide disparities such as the GC-skew57. The latter, however, cannot be used for the huge number of fragmentarily sequenced genomes currently present in public databases. Furthermore, methods developed for eukaryotic chromosomes cannot be applied to bacterial chromosomes as the composition of these sequences and the processes in replication initiation are very different58.
In this study, we present a set of machine learning-based tools (available at http://boris.heiderlab.de) for the analysis of gammaproteobacterial chromosomes and, more specifically, the oriC sequences therein. We chose to focus on one class of organisms because oriC sequences from different taxonomic classes exhibit a high level of variance7,59. Moreover, as most secondary chromosomes use particular initiation proteins for replication initiation52,60, and as they are rather rare3, we also excluded these from the scope of this study and focused on primary chromosomes.
Firstly, we introduce gammaBOriS, which is able to identify oriC sequences on fragmentary as well as full chromosomes of Gammaproteobacteria using a motif-based machine learning method. The general approach of gammaBOriS can easily be adapted for other groups of organisms if trained on their oriC sequences. Suitable datasets, however, are currently not easily available in the necessary amount and quality (e.g., equal-sized, centered, and co-oriented). The method used to create an initial oriC dataset in this study requires manual decision-making and is thus not scaleable, but also ensures that the weight of implicit assumptions can be balanced and adjusted for every case. The assumptions of the method are that (I) oriC is intergenic, (II) close to the global GC skew minimum, and (III) defined by the DUE, as well as (IV) the presence of DnaA boxes. We consider this method highly accurate, which is supported by the fact that some oriC sequences identified with it have been confirmed experimentally9,61,62. Being trained on this dataset, gammaBOriS can be seen as scalable automation of the semi-automatic method, which makes it possible to use it for the analysis of large-scale metagenomic datasets63,64.
By applying gammaBOriS on fragments deposited in public sequence databases, we created BOriS DB, the largest database of oriC sequences to date. BOriS DB currently contains 25,827 sequences from 1,217 species from the class of Gammaproteobacteria, most of which were not identified yet. We exemplify the use of BOriS DB by training machine learning methods to perform taxonomic classifications of the sequences in it. Based on the best performing classifiers, we created gammaBOriTax (Fig. 3). Furthermore, we show that it is possible to extract sets of biologically relevant motifs from the models used in gammaBOriTax. While many of the motifs identified this way have a known function, there are also motifs that we cannot annotate functionally yet (Table 1). Due to their statistical properties, models employed in gammaBOriTax will only identify motifs as relevant for classification that are both specific for a given taxon and evolutionarily conserved in this taxon. Therefore, the proteins that bind to and interact with these motifs ideal potential targets for novel antibiotics65.
The motifs identified as relevant by gammaBOriS and gammaBOriTax are representative of all the motif classes known to be functionally important for oriC of Gammaproteobacteria, including the only recently identified DnaA trio motifs10. Furthermore, gammaBOriTax identified a motif that resembles the RctB consensus sequence and might act as a low-affinity binding site, thus playing a role in coordination between the primary and secondary chromosome in Vibrionales. Based on this, we suspect that the motifs annotated as DUE in Table 1 might not simply be AT-rich sequences but functionally important protein binding sites. While further experiments are needed to confirm these hypotheses, the results presented here show that it is possible to derive sets of biologically relevant motifs from machine learning methods trained without explicit domain knowledge.
Methods
Data curation and creation
A basic “ground truth” oriC dataset was compiled using a semi-automated method described in9,61,62. A given chromosome is first split into 2.5 kb fragments that are centered around intergenic regions and then, for those fragments close to the minimum of the chromosome’s cumulative GC-skew, their respective probability of unwinding is calculated using WebSIDD66. Default values (37 °C, 0.1 M salt, circular DNA, copolymeric) were chosen for the predictions, and negative superhelicity values were tested in the range of σ. In the following, the sequences identified this way are regarded as “positive” examples, i.e., sequences that do contain an oriC sequence.
A dataset of seed sequences was created by extracting the central 9 bp from oriC sequences in the ground truth dataset. Negative sequences, i.e., DNA sequences that do not contain an oriC sequence, were collected by picking sequences from each chromosome present in the positive dataset. These sequences were chosen to have (I) the same size as and (II) the same seed sequence as the positive sequence from the respective chromosome. To be able to identify the optimal fragment length for classification, the length of the sequences in the datasets were varied from 150 to 1500 bp in steps of 50 bp. Seed sequence and sequence datasets with a fragment length of 1250 bp are available at Figshare (see Additional Information).
For cutoff selection, a highly imbalanced sequence dataset was created by extracting all fragments of a given length around each of the seed sequences from each of the chromosomes present in the positive dataset. Both the balanced “ground truth” as well as the imbalanced datasets were split into training and test datasets using a 70–30% split, leading to 318 chromosomes in the former and 141 chromosomes in the latter. For validation of the models, we used a separate validation dataset of 100 sequences created the same way.
Chromosomes were downloaded from the NCBI RefSeq ftp server39. For BOriS DB, a list of RefSeq organisms was taken from ftp://ftp.ncbi.nlm.nih.gov/genomes/refseq/bacteria/assembly_ summary.txt, a list of chromosomes for the UBA genomes was taken from Supplementary 2 of Parks et al.40.
Sequence classification using LS-GKM models
The support vector machines used as classifiers in this study derive distance matrices from a set of input sequences by counting substrings and comparing their numbers in sequence pairs directly, making these approaches fast and memory efficient. The Spectrum Kernel is based on simple k-mer composition differences33. LS-GKM and gkm-SVM models calculate differences between k-mers by allowing for mismatches and small differences between the k-mers36,67. The optimal parameter set for each of the models was assessed after model training on the validation dataset.
Taxonomic classification of oriC sequences
Different machine learning approaches were used to classify DNA sequences taken from BOriS DB with regard to their respective taxonomic orders, families and genera. The test-train-validation data split used to train gammaBOriS was also used here. LS-GKM models were trained for binary classification for each of the taxa with more than 4 examples in BOriS DB using the same parameters that showed the best classification performance for oriC/non-oriC classification. Random Forest classifiers41 taken from R package caret68 (one-hot and k-mer counting encodings) and the Python package scikit-learn69 (word2vec encoding) were trained on one-hot encoded sequences as well as on k-mer counting encoded DNA sequences (for 1 ≤ k ≤ 6) and encodings taken from a word2vec model. Pseudo-sentences, that were used as input for Gensim-based word2vec models70, were generated by splitting the sequences into all possible sets of consecutive 10-mers.
Motif extraction from LS-GKM models
The importance of motifs for LS-GKM classification decision was assessed by scoring a list of all 10-mers via the gkmpredict method provided by LS-GKM as described by the original authors of LS-GKM36. Motifs were deemed important if their score was larger than or equal to 0.
Accession codes
Datasets used for model training and validation for gammaBOriS and BOriS DB v1 are available under the DOIs https://doi.org/10.6084/m9.figshare.8108357.v1, https://doi.org/10.6084/m9.figshare.10079753, and https://doi.org/10.6084/m9.figshare.9919145.v1.
References
Jacob, F., Brenner, S. & Cuzin, F. On the regulation of DNA replication in bacteria. Cold Spring Harbor Symposia on Quantitative Biology 28, 329–348, https://doi.org/10.1101/sqb.1963.028.01.048 (1963).
Messer, W. The bacterial replication initiator DnaA. DnaA and oriC, the bacterial mode to initiate DNA replication. FEMS Microbiology Reviews 26, 355–374, https://doi.org/10.1111/j.1574-6976.2002.tb00620.x (2002).
Harrison, P. W., Lower, R. P., Kim, N. K. & Young, J. P. W. Introducing the bacterial ‘chromid’: not a chromosome, not a plasmid. Trends in Microbiology 18, 141–148, https://doi.org/10.1016/j.tim.2009.12.010 (2010).
Gao, F. Bacteria may have multiple replication origins. Front. Microbiol. 6, https://doi.org/10.3389/fmicb.2015.00324 (2015).
Zakrzewska-Czerwińska, J., Jakimowicz, D., Zawilak-Pawlik, A. & Messer, W. Regulation of the initiation of chromosomal replication in bacteria. FEMS Microbiology Reviews 31, 378–387, https://doi.org/10.1111/j.1574-6976.2007.00070.x (2007).
Leonard, A. C. & Grimwade, J. E. The orisome: structure and function. Front. Microbiol. 6, https://doi.org/10.3389/fmicb.2015.00545 (2015).
Krause, M., Rückert, B., Lurz, R. & Messer, W. Complexes at the replication origin of Bacillus subtilis with homologous and heterologous DnaA protein. Journal of Molecular Biology 274, 365–380, https://doi.org/10.1006/jmbi.1997.1404 (1997).
Brilli, M. et al. The diversity and evolution of cell cycle regulation in alpha-proteobacteria: a comparative genomic analysis. BMC Systems Biology 4, 52, https://doi.org/10.1186/1752-0509-4-52 (2010).
Jaworski, P. et al. Unique and universal features of epsilonproteobacterial origins of chromosome replication and DnaA-DnaA box interactions. Frontiers in Microbiology 7, 1555, https://doi.org/10.3389/fmicb.2016.01555 (2016).
Richardson, T. T., Harran, O. & Murray, H. The bacterial DnaA-trio replication origin element specifies single-stranded dna initiator binding. Nature 534, 412–416, https://doi.org/10.1038/nature17962 (2016).
Ryan, V. T., Grimwade, J. E., Camara, J. E., Crooke, E. & Leonard, A. C. Escherichia coli prereplication complex assembly is regulated by dynamic interplay among fis, IHF and DnaA. Molecular Microbiology 51, 1347–1359, https://doi.org/10.1046/j.1365-2958.2003.03906.x (2004).
Bramhill, D. & Kornberg, A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell 52, 743–755, https://doi.org/10.1016/0092-8674(88)90412-6 (1988).
Kowalski, D. & Eddy, M. J. The DNA unwinding element: a novel, cis-acting component that facilitates opening of the Escherichia coli replication origin. EMBO J. 8, 4335–4344 (1989).
Marczynski, G. T., Rolain, T. & Taylor, J. A. Redefining bacterial origins of replication as centralized information processors. Front. Microbiol. 6, https://doi.org/10.3389/fmicb.2015.00610 (2015).
Song, C., Zhang, S. & Huang, H. Choosing a suitable method for the identification of replication origins in microbial genomes. Front. Microbiol. 6, https://doi.org/10.3389/fmicb.2015.01049 (2015).
Song, J., Ware, A. & Liu, S.-L. Wavelet to predict bacterial ori and ter: a tendency towards a physical balance. BMC Genomics 4, 17, https://doi.org/10.1186/1471-2164-4-17 (2003).
Gao, F. & Zhang, C.-T. Ori-finder: A web-based system for finding oriCs in unannotated bacterial genomes. BMC Bioinformatics 9, 79, https://doi.org/10.1186/1471-2105-9-79 (2008).
Kundal, S., Lohiya, R. & Shah, K. iCorr: Complex correlation method to detect origin of replication in prokaryotic and eukaryotic genomes. arXiv (2016).
Maderankova, D., Sedlar, K., Vitek, M. & Skutkova, H. The identification of replication origin in bacterial genomes by cumulated phase signal. In 2017 IEEE Conference on Computational Intelligence in Bioinformatics and Computational Biology (CIBCB), https://doi.org/10.1109/cibcb.2017.8058561 (IEEE, 2017).
Zhang, G. & Gao, F. Quantitative analysis of correlation between AT and GC biases among bacterial genomes. PLOS ONE 12, e0171408, https://doi.org/10.1371/journal.pone.0171408 (2017).
Lobry, J. A simple vectorial representation of DNA sequences for the detection of replication origins in bacteria. Biochimie 78, 323–326, https://doi.org/10.1016/0300-9084(96)84764-x (1996).
Mackiewicz, P. Where does bacterial replication start? Rules for predicting the oriC region. Nucleic Acids Research 32, 3781–3791, https://doi.org/10.1093/nar/gkh699 (2004).
Luo, H., Zhang, C.-T. & Gao, F. Ori-finder 2, an integrated tool to predict replication origins in the archaeal genomes. Frontiers in Microbiology 5, https://doi.org/10.3389/fmicb.2014.00482 (2014).
Gao, F. & Zhang, C.-T. DoriC: a database of oriC regions in bacterial genomes. Bioinformatics 23, 1866–1867, https://doi.org/10.1093/bioinformatics/btm255 (2007).
Gao, F., Luo, H. & Zhang, C.-T. DoriC 5.0: an updated database of oriC regions in both bacterial and archaeal genomes. Nucleic Acids Research 41, D90–D93, https://doi.org/10.1093/nar/gks990 (2012).
Khawaldeh, S., Pervaiz, U., Elsharnoby, M., Alchalabi, A. & Al-Zubi, N. Taxonomic classification for living organisms using convolutional neural networks. Genes 8, 326, https://doi.org/10.3390/genes8110326 (2017).
Min, X. et al. Predicting enhancers with deep convolutional neural networks. BMC Bioinformatics 18, https://doi.org/10.1186/s12859-017-1878-3 (2017).
Umarov, R. K. & Solovyev, V. V. Recognition of prokaryotic and eukaryotic promoters using convolutional deep learning neural networks. PLOS ONE 12, e0171410, https://doi.org/10.1371/journal.pone.0171410 (2017).
Arango-Argoty, G. et al. DeepARG: a deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome 6, https://doi.org/10.1186/s40168-018-0401-z (2018).
Budach, S. & Marsico, A. pysster: Classification of biological sequences by learning sequence and structure motifs with convolutional neural networks. Bioinformatics 34, 3035–3037, https://doi.org/10.1093/bioinformatics/bty222 (2018).
Du, X. et al. DeepSS: Exploring splice site motif through convolutional neural network directly from DNA sequence. IEEE Access 6, 32958–32978, https://doi.org/10.1109/access.2018.2848847 (2018).
Fiannaca, A. et al. Deep learning models for bacteria taxonomic classification of metagenomic data. BMC Bioinformatics 19, https://doi.org/10.1186/s12859-018-2182-6 (2018).
Leslie, C., Eskin, E. & Noble, W. S. The Spectrum Kernel: A String Kernel For Svm Protein Classification. In Biocomputing 2002, DOI: 10.1142/9789812799623_0053 (WORLD SCIENTIFIC, 2001).
Rätsch, G. & Sonnenburg, S. Accurate Splice Site Detection for Caenorhabditis elegans. In B & P., V. (eds.) Kernel Methods in Computational Biology, 277–298 (MIT Press, 2004).
Ghandi, M. et al. gkmSVM: an r package for gapped-kmer SVM. Bioinformatics 32, 2205–2207, https://doi.org/10.1093/bioinformatics/btw203 (2016).
Lee, D. LS-GKM: a new gkm-SVM for large-scale datasets. Bioinformatics 32, 2196–2198, https://doi.org/10.1093/bioinformatics/btw142 (2016).
Elmas, A., Wang, X. & Dresch, J. M. The folded k-spectrum kernel: A machine learning approach to detecting transcription factor binding sites with gapped nucleotide dependencies. PLOS ONE 12, e0185570, https://doi.org/10.1371/journal.pone.0185570 (2017).
Balsubramani, A. The utility of abstaining in binary classification. arXiv (2015).
O’Leary, N. A. et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Research 44, D733–D745, https://doi.org/10.1093/nar/gkv1189 (2015).
Parks, D. H. et al. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nature Microbiology, https://doi.org/10.1038/s41564-017-0012-7 (2017).
Breiman, L. Random forests. Machine Learning 45, 5–32, https://doi.org/10.1023/a:1010933404324 (2001).
Mikolov, T., Chen, K., Corrado, G. & Dean, J. Efficient estimation of word representations in vector space. CoRR abs/1301.3781 (2013).
Løbner-Olesen, A., Skovgaard, O. & Marinus, M. G. Dam methylation: coordinating cellular processes. Current Opinion in Microbiology 8, 154–160, https://doi.org/10.1016/j.mib.2005.02.009 (2005).
Waldminghaus, T. & Skarstad, K. The Escherichia coli SeqA protein. Plasmid 61, 141–150, https://doi.org/10.1016/j.plasmid.2009.02.004 (2009).
Waldminghaus, T., Weigel, C. & Skarstad, K. Replication fork movement and methylation govern SeqA binding to the Escherichia coli chromosome. Nucleic Acids Research 40, 5465–5476, https://doi.org/10.1093/nar/gks187 (2012).
Schaper, S. & Messer, W. Interaction of the initiator protein DnaA of Escherichia coli with its DNA target. Journal of Biological Chemistry 270, 17622–17626, https://doi.org/10.1074/jbc.270.29.17622 (1995).
Weigel, C. DnaA protein binding to individual DnaA boxes in the Escherichia coli replication origin, oriC. The EMBO Journal 16, 6574–6583, https://doi.org/10.1093/emboj/16.21.6574 (1997).
Brezellec, P., Hoebeke, M., Hiet, M.-S., Pasek, S. & Ferat, J.-L. DomainSieve: a protein domain-based screen that led to the identification of dam-associated genes with potential link to DNA maintenance. Bioinformatics 22, 1935–1941, https://doi.org/10.1093/bioinformatics/btl336 (2006).
Sobetzko, P. et al. DistAMo: A web-based tool to characterize DNA-motif distribution on bacterial chromosomes. Front. Microbiol. 7, https://doi.org/10.3389/fmicb.2016.00283 (2016).
Egan, E. S. & Waldor, M. K. Distinct replication requirements for the two Vibrio cholerae chromosomes. Cell 114, 521–530, https://doi.org/10.1016/s0092-8674(03)00611-1 (2003).
Val, M.-E. et al. A checkpoint control orchestrates the replication of the two chromosomes of Vibrio cholerae. Science Advances 2, no. 4, e1501914, https://doi.org/10.1126/sciadv.1501914 (2016).
Fournes, F., Val, M.-E., Skovgaard, O. & Mazel, D. Replicate once per cell cycle: Replication control of secondary chromosomes. Frontiers in Microbiology 9, https://doi.org/10.3389/fmicb.2018.01833 (2018).
Larrañaga, P. et al. Machine learning in bioinformatics. Briefings in Bioinformatics 7, 86–112, https://doi.org/10.1093/bib/bbk007 (2006).
Heider, D. et al. A computational approach for the identification of small GTPases based on preprocessed amino acid sequences. Technology in Cancer Research & Treatment 8, 333–341, https://doi.org/10.1177/153303460900800503 (2009).
Min, S., Lee, B. & Yoon, S. Deep learning in bioinformatics. Briefings in Bioinformatics bbw068, https://doi.org/10.1093/bib/bbw068 (2016).
Liu, B., Weng, F., Huang, D.-S. & Chou, K.-C. iRO-3wPseKNC: identify DNA replication origins by three-window-based PseKNC. Bioinformatics 1, bty312, https://doi.org/10.1093/bioinformatics/bty312 (2018).
Luo, H., Quan, C.-L., Peng, C. & Gao, F. Recent development of Ori-Finder system and DoriC database for microbial replication origins. Briefings in Bioinformatics, https://doi.org/10.1093/bib/bbx174 (2018).
Leonard, A. C. & Mechali, M. DNA replication origins. Cold Spring Harbor Perspectives in Biology 5, a010116–a010116, https://doi.org/10.1101/cshperspect.a010116 (2013).
Wolański, M., Donczew, R., Zawilak-Pawlik, A. & Zakrzewska-Czerwińska, J. oriC-encoded instructions for the initiation of bacterial chromosome replication. Front. Microbiol. 5, https://doi.org/10.3389/fmicb.2014.00735 (2015).
Schallopp, N. et al. Establishing a system for testing replication inhibition of the Vibrio cholerae secondary chromosome in Escherichia coli. Antibiotics 7, 3, https://doi.org/10.3390/antibiotics7010003 (2017).
Donczew, R., Weigel, C., Lurz, R., Zakrzewska-Czerwinska, J. & Zawilak-Pawlik, A. Helicobacter pylori oriC–the first bipartite origin of chromosome replication in gram-negative bacteria. Nucleic Acids Research 40, 9647–9660, https://doi.org/10.1093/nar/gks742 (2012).
Jaworski, P. et al. Structure and function of the Campylobacter jejuni chromosome replication origin. Frontiers in Microbiology 9, 1533, https://doi.org/10.3389/fmicb.2018.01533 (2018).
Lund, J. B., List, M. & Baumbach, J. Interactive microbial distribution analysis using BioAtlas. Nucleic Acids Research 45, W509–W513, https://doi.org/10.1093/nar/gkx304 (2017).
Zeng, Y. et al. Metagenomic evidence for the presence of phototrophic gemmatimonadetes bacteria in diverse environments. Environmental Microbiology Reports 8, 139–149, https://doi.org/10.1111/1758-2229.12363 (2016).
Grimwade, J. E. & Leonard, A. C. Targeting the bacterial orisome in the search for new antibiotics. Frontiers in Microbiology 8, https://doi.org/10.3389/fmicb.2017.02352 (2017).
Bi, C. & Benham, C. J. WebSIDD: server for predicting stress-induced duplex destabilized (SIDD) sites in superhelical DNA. Bioinformatics 20, 1477–1479, https://doi.org/10.1093/bioinformatics/bth304 (2004).
Ghandi, M., Lee, D., Mohammad-Noori, M. & Beer, M. A. Enhanced regulatory sequence prediction using gapped k-mer features. PLoS Computational Biology 10, e1003711, https://doi.org/10.1371/journal.pcbi.1003711 (2014).
Kuhn, M. Building predictive models in R using the caret package. Journal of Statistical Software 28, https://doi.org/10.18637/jss.v028.i05 (2008).
PedregosaF. et al. Scikit-learn: Machine learning in Python. Journal of Machine Learning Research 12, 2825–2830 (2011).
Řehůřek, R. & Sojka, P. Software Framework for Topic Modelling with Large Corpora. In Proceedings of the LREC 2010 Workshop on New Challenges for NLP Frameworks, 45–50 http://is.muni.cz/publication/884893/en (ELRA, Valletta, Malta, 2010).
Grimwade, J. E., Ryan, V. T. & Leonard, A. C. IHF redistributes bound initiator protein, DnaA, on supercoiled oriC of Escherichia coli. Molecular Microbiology 35, 835–844, https://doi.org/10.1046/j.1365-2958.2000.01755.x (2000).
Acknowledgements
Calculations on the MaRC2 high performance computer of the University of Marburg were conducted for this research. We would like to thank Mr. Sitt of HPC-Hessen, funded by the State Ministry of Higher Education, Research and the Arts, for installation and maintenance of software on the MaRC2 high performance computer. We would also like to acknowledge the work of the reviewers and editors that improved the quality of this manuscript.
Author information
Authors and Affiliations
Contributions
T.S. conceived the project and drafted the manuscript. T.S. and L.M. designed gammaBOriS and performed the machine learning analyses. L.M. implemented gammaBOriS. T.S. implemented gammaBOriTax. C.W. provided the “ground truth” dataset. R.M. implemented the web server. T.S., T.W. and C.W. annotated the motifs. T.S., T.W., C.W., and D.H. discussed the results. D.H. supervised the project. All authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sperlea, T., Muth, L., Martin, R. et al. gammaBOriS: Identification and Taxonomic Classification of Origins of Replication in Gammaproteobacteria using Motif-based Machine Learning. Sci Rep 10, 6727 (2020). https://doi.org/10.1038/s41598-020-63424-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-63424-7
This article is cited by
-
The association of group IIB intron with integrons in hypersaline environments
Mobile DNA (2021)
-
A deep learning framework combined with word embedding to identify DNA replication origins
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.